One-Step Carbonization Synthesis of Magnetic Biochar with 3D Network Structure and Its Application in Organic Pollutant Control
Abstract
:1. Introduction
2. Results and Discussion
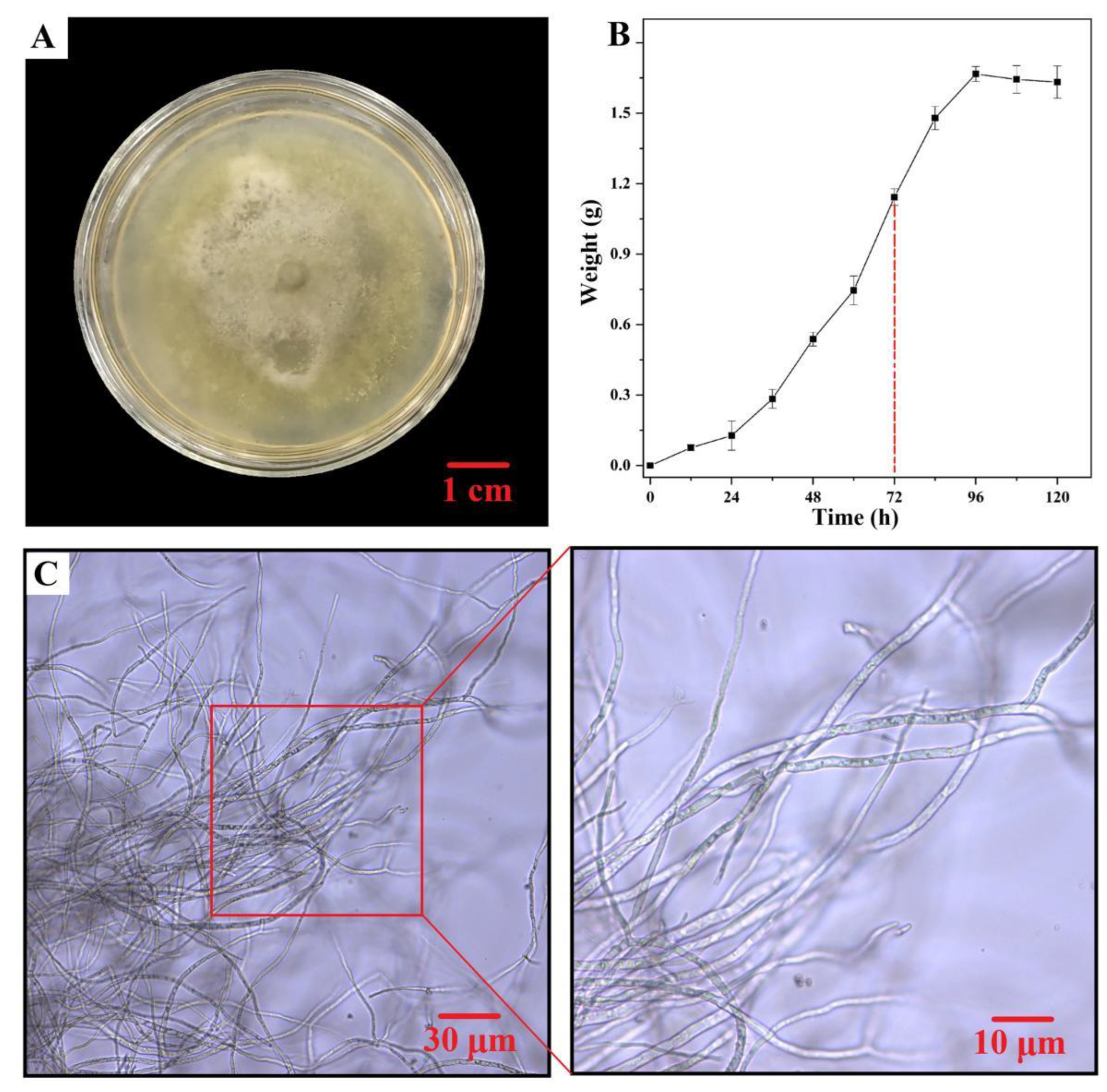
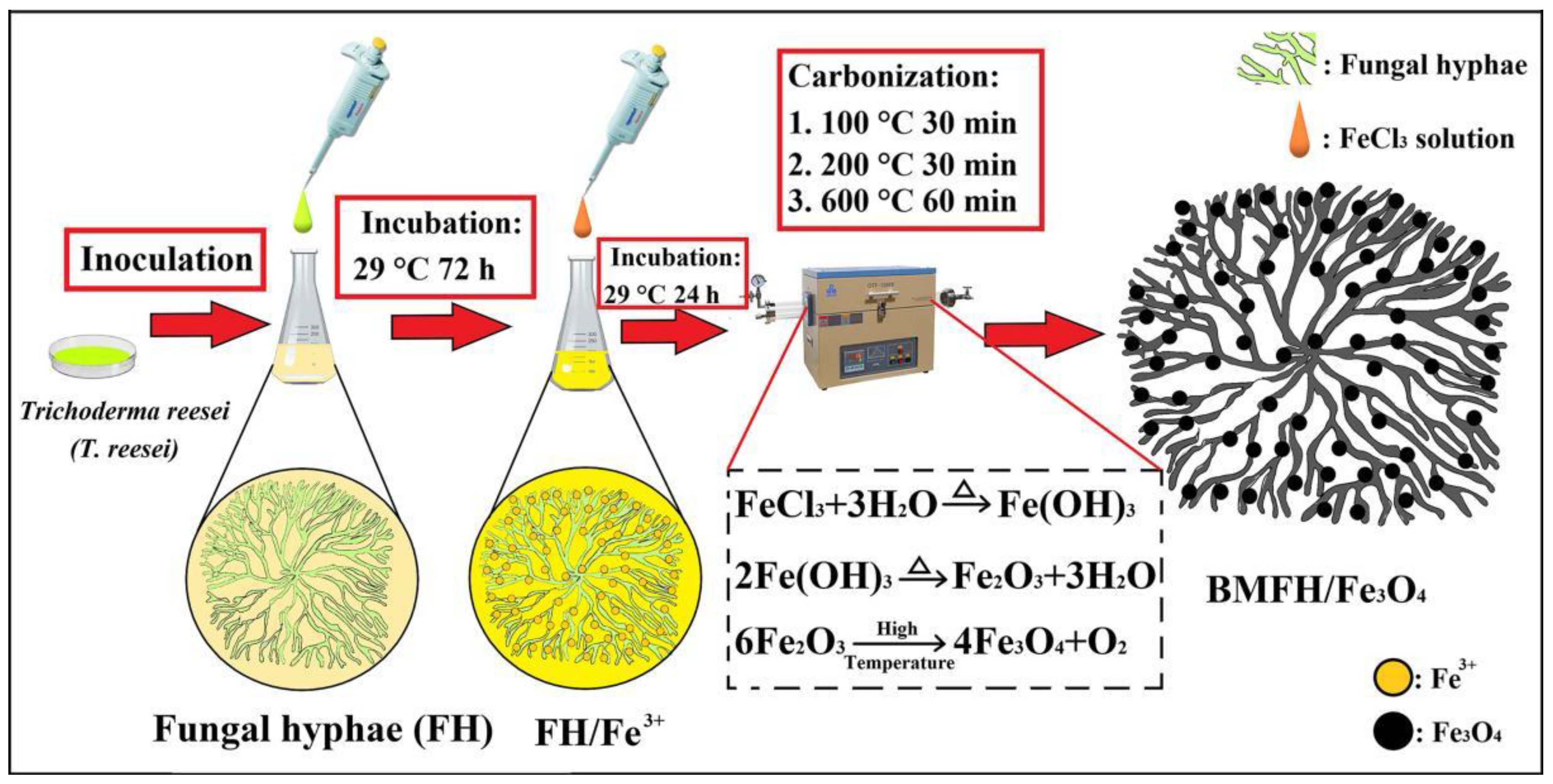
2.1. Preparation of BMFH/Fe3O4
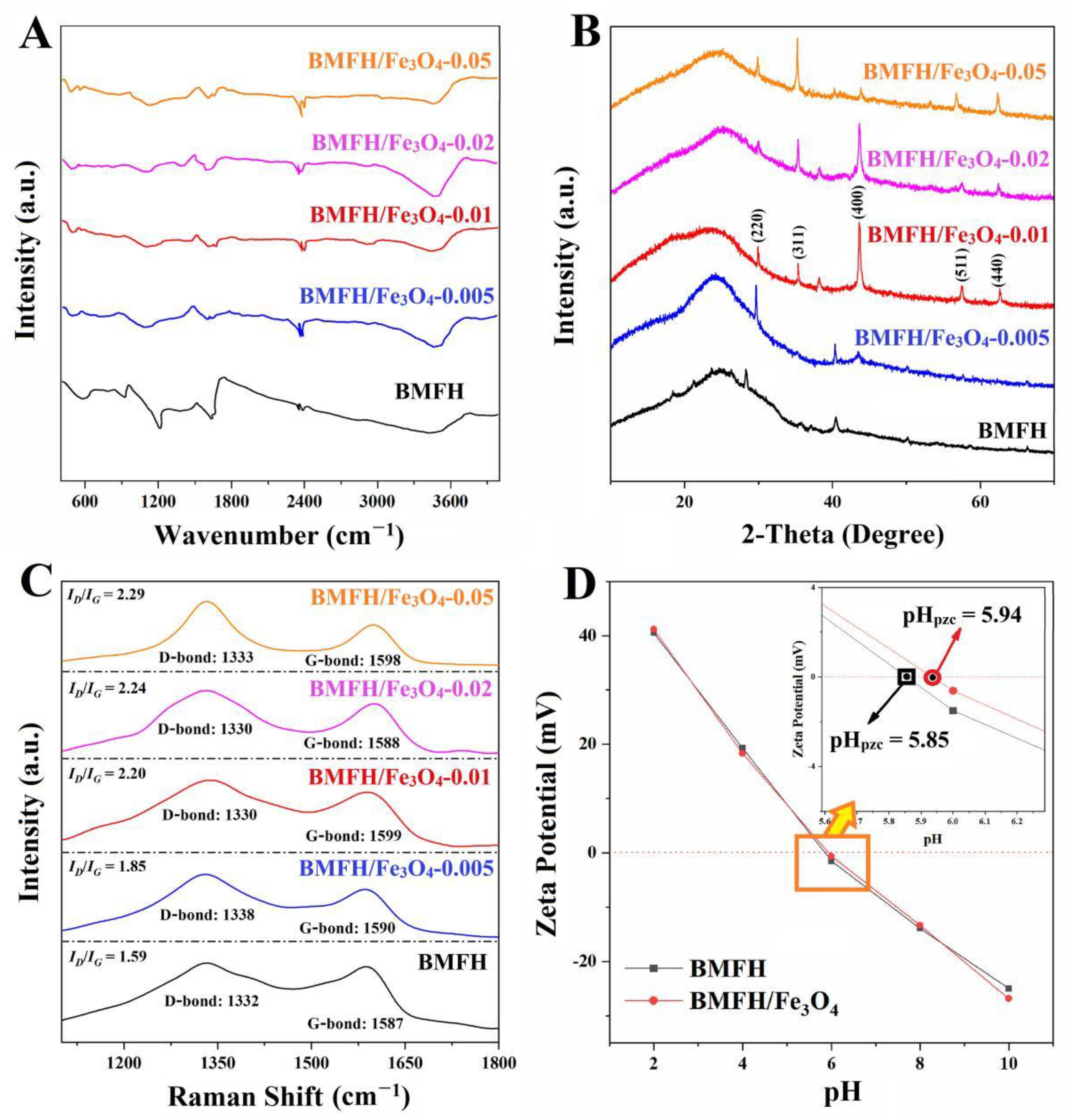
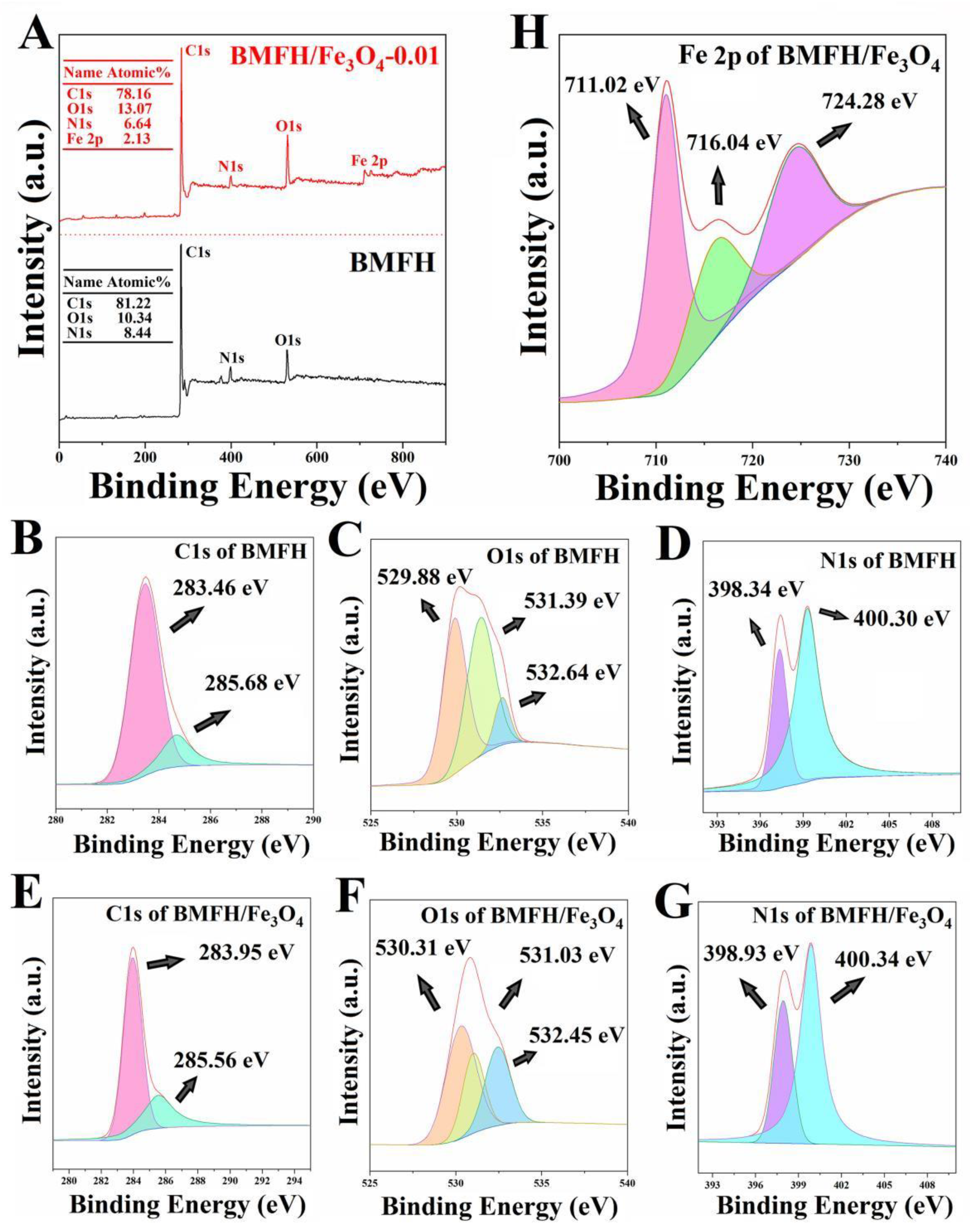
2.2. Characterization Results
2.3. Adsorption Performances
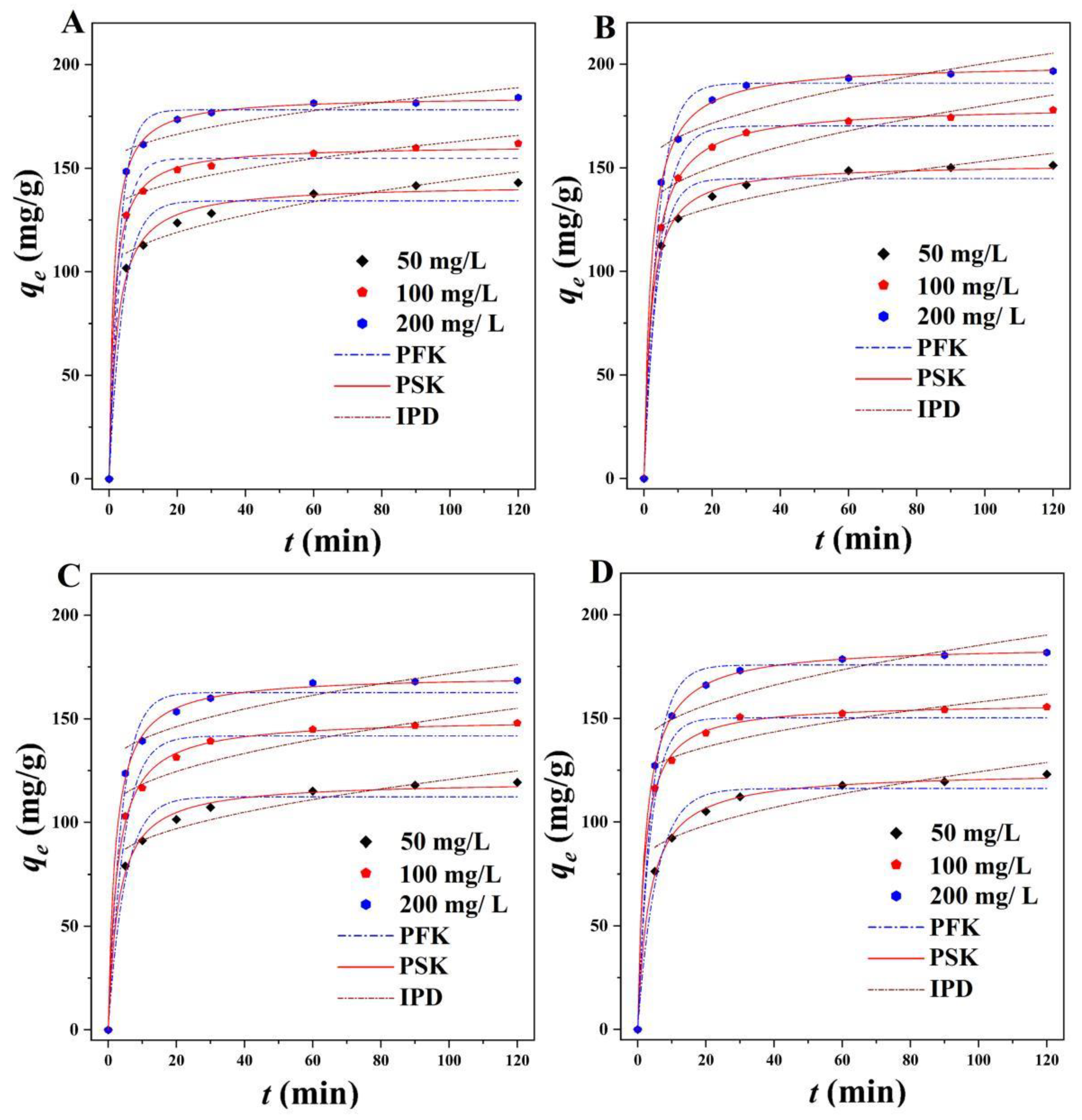
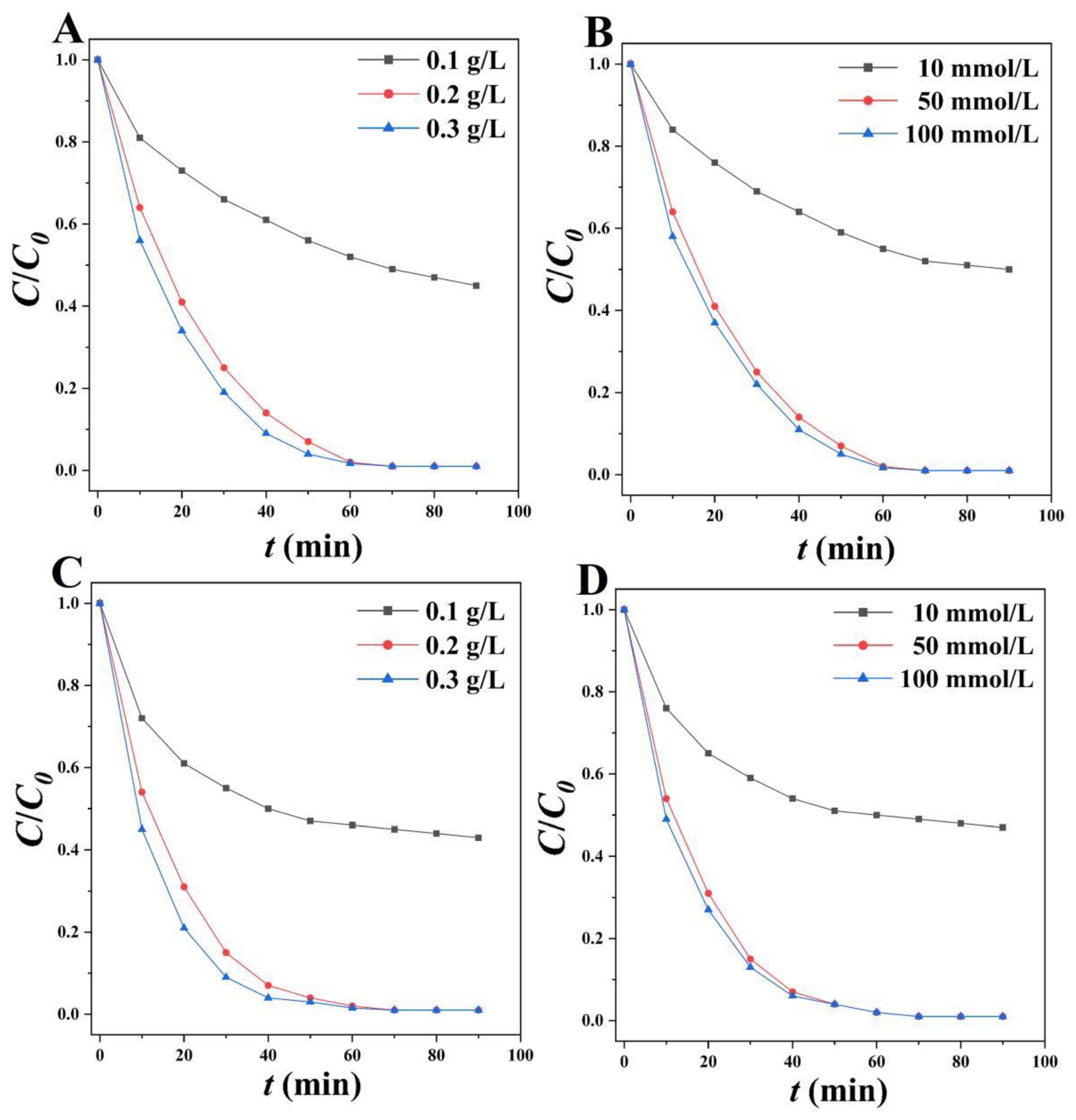
2.3.1. Adsorption Kinetic
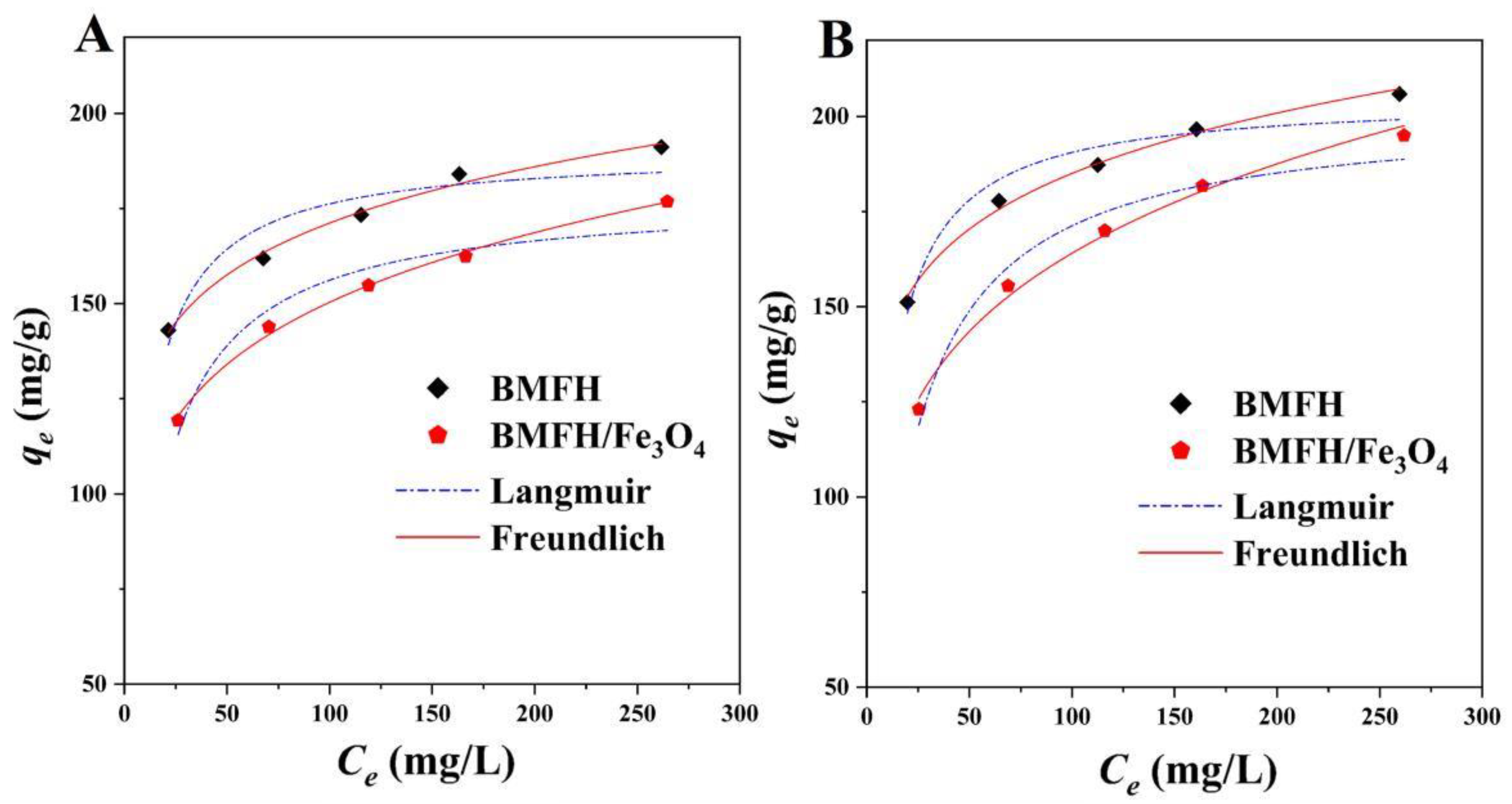
2.3.2. Adsorption Isotherm
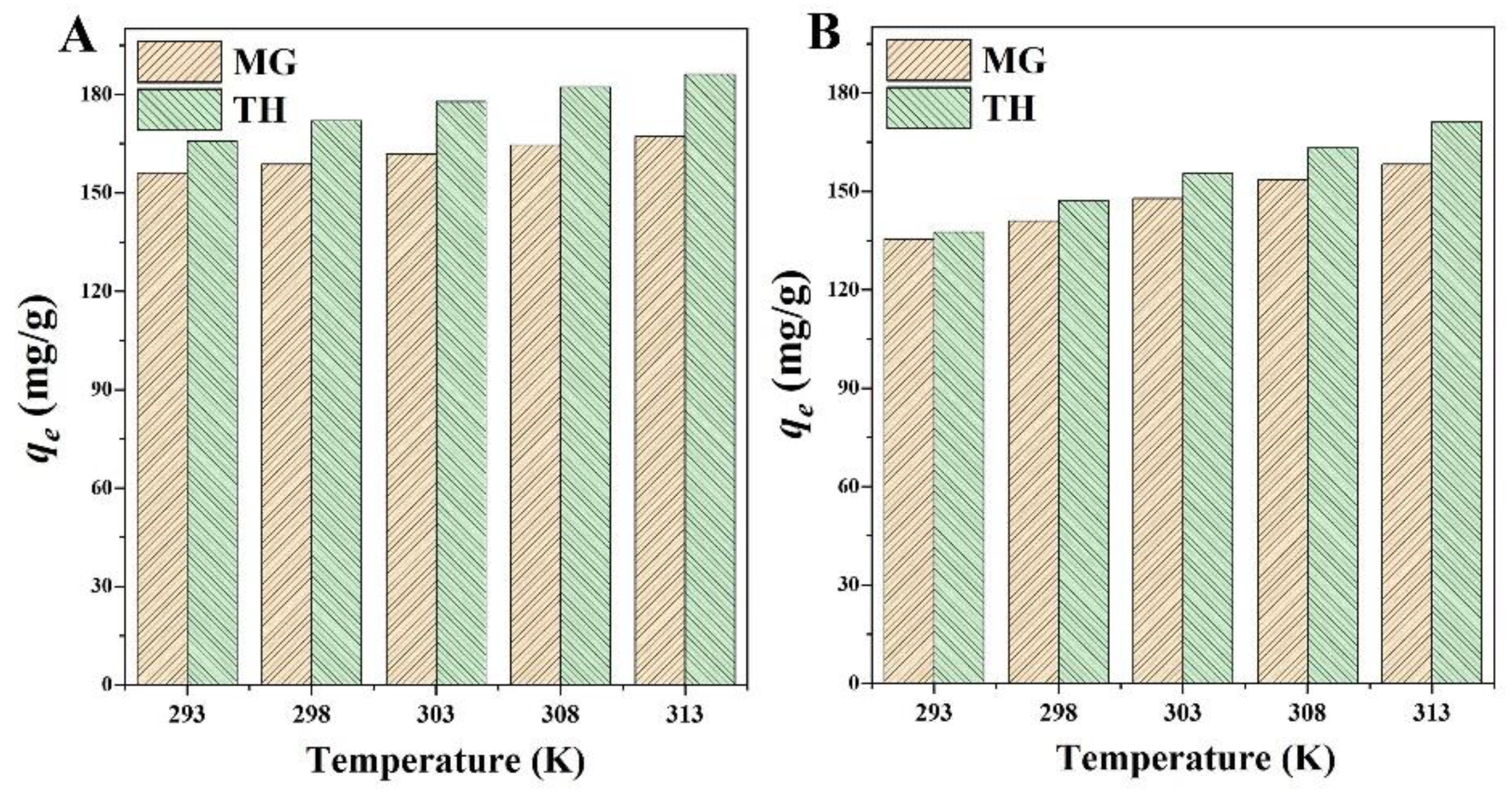
2.3.3. Adsorption Thermodynamic Results
2.4. Effect of pH
2.5. Fenton-like Catalysis Performance Results
2.5.1. Effect of BMFH/Fe3O4 and H2O2 on Catalytic Performance
2.5.2. Oxidative Radicals Quenching Experiment Results
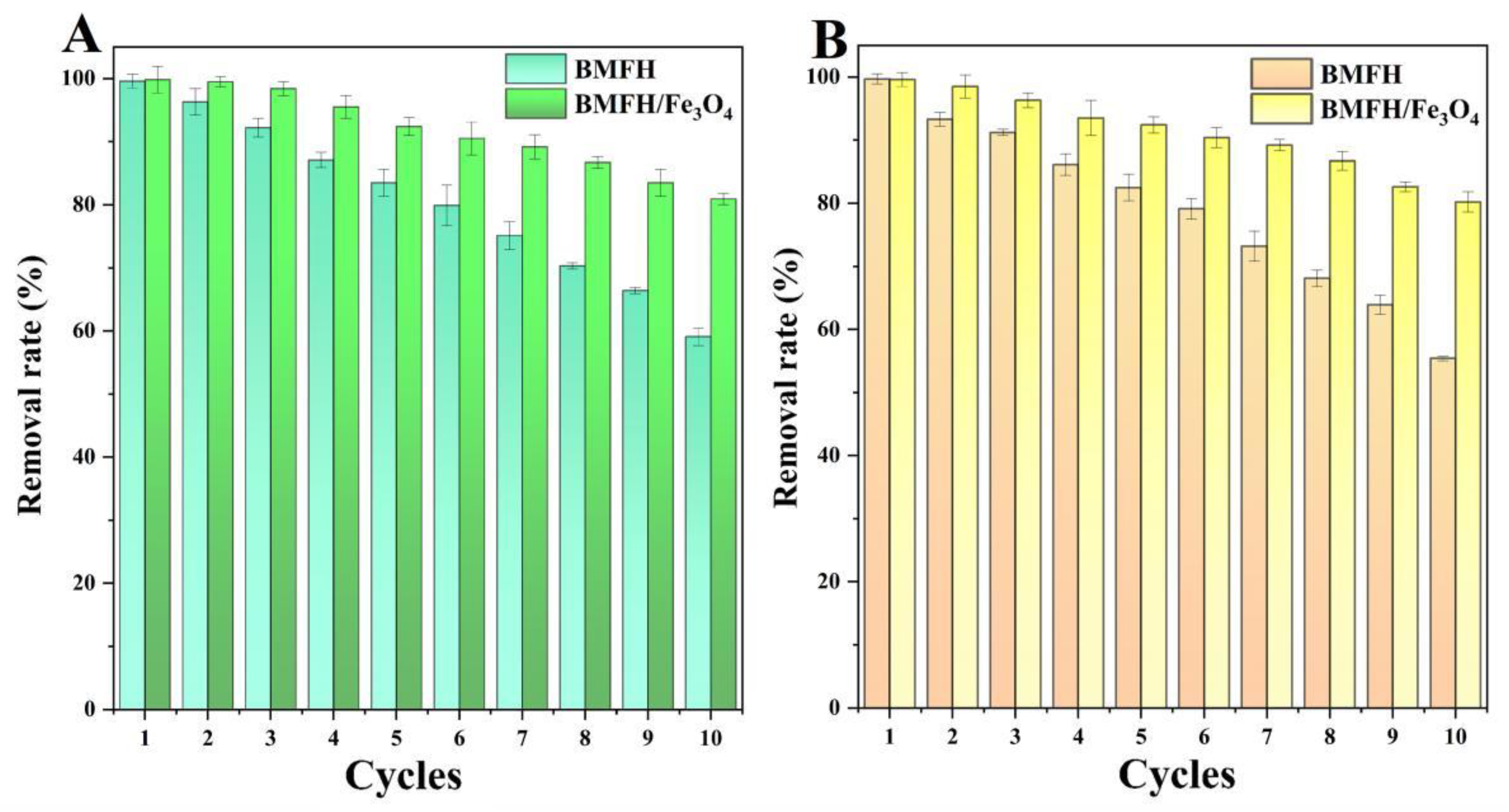
2.6. Cycling Stability and Performance Comparison of BMFH and BMFH/Fe3O4
2.7. Comparison with Other Biochars
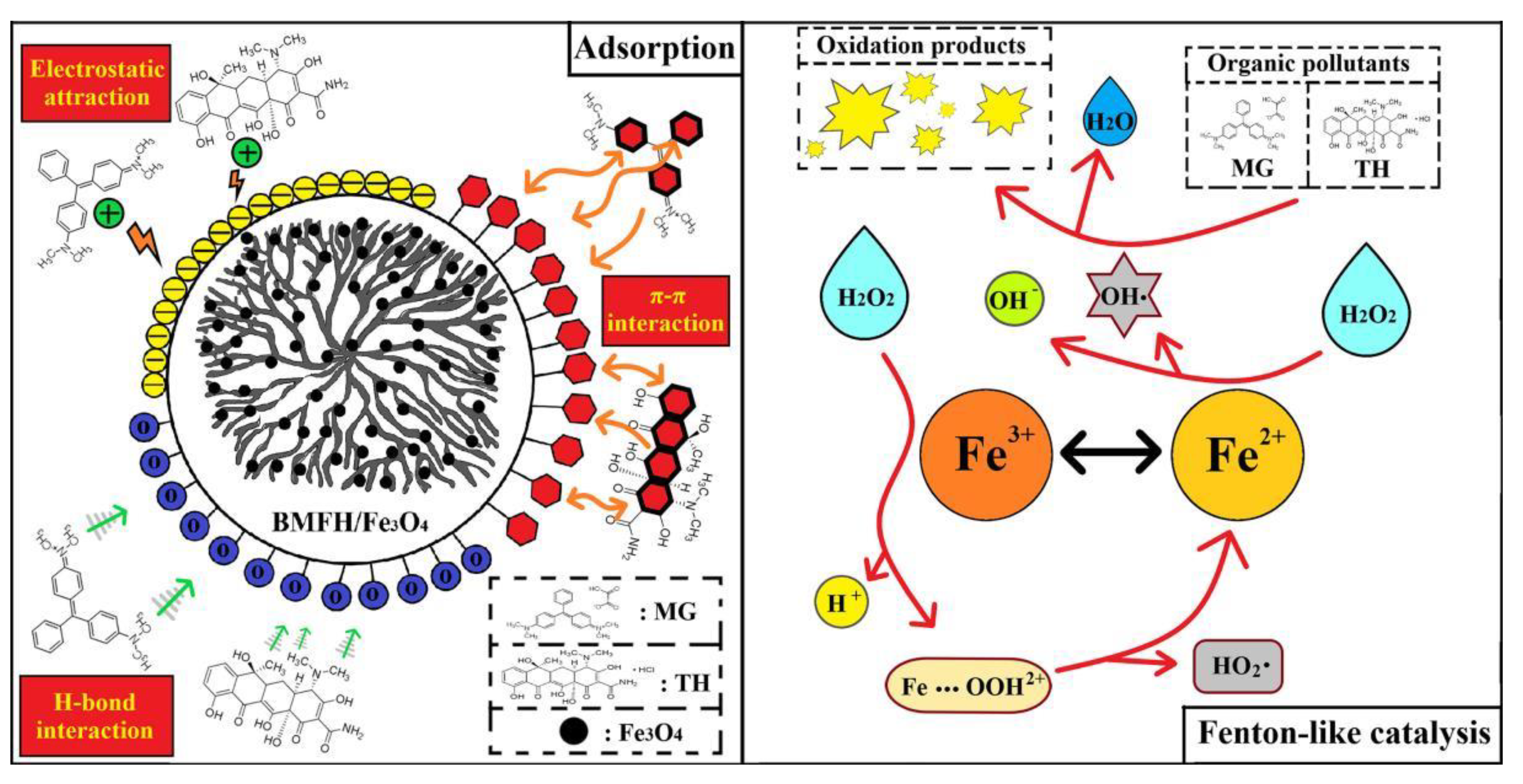
2.8. Possible Mechanisms
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of BMFH/Fe3O4
3.3. Adsorption Performances
3.3.1. Adsorption Kinetic Performances
3.3.2. Adsorption Isotherm Experiments
3.3.3. Adsorption Thermodynamic Experiments
3.3.4. The Effect of pH on Adsorption Capacities
3.4. Fenton-like Catalysis Performances
3.4.1. Catalytic Activity Test of BMFH/Fe3O4
3.4.2. Oxidative Radicals Quenching Experiments
3.5. Cycling Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agasti, N.; Gautam, V.; Priyanka; Manju; Pandey, N.; Genwa, M.; Meena, P.L.; Tandon, S.; Samantaray, R. Carbon nanotube based magnetic composites for decontamination of organic chemical pollutants in water: A review. Appl. Surf. Sci. Adv. 2022, 10, 100270. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Gunten, U.V.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.A.; Iltaf, J.; Zaheer, T.; Tariq, L.; Amir, M.B.; Fatima, R.; Asbat, A.; Kabeer, T.; Fahad, M.; Naeem, H.; et al. Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Sci. Total Environ. 2022, 850, 157961. [Google Scholar] [CrossRef]
- Ang, W.L.; McHugh, P.J.; Symes, M.D. Sonoelectrochemical processes for the degradation of persistent organic pollutants. Chem. Eng. J. 2022, 444, 136573. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Ruan, Y.; Kong, L.; Zhong, Y.; Diao, Z.; Shih, K.; Hou, L.; Wang, S.; Chen, D. Review on the synthesis and activity of iron-based catalyst in catalytic oxidation of refractory organic pollutants in wastewater. J. Clean. Prod. 2021, 321, 128924. [Google Scholar] [CrossRef]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X.; et al. Applications of functionalized magnetic biochar in environmental remediation: A review. J. Hazard. Mater. 2022, 434, 128841. [Google Scholar] [CrossRef]
- Adeola, A.O.; Abiodun, B.A.; Adenuga, D.O.; Nomngongo, P.N. Adsorptive and photocatalytic remediation of hazardous organic chemical pollutants in aqueous medium: A review. J. Contam. Hydrol. 2022, 248, 104019. [Google Scholar] [CrossRef]
- Khan, A.H.; Khan, N.A.; Zubair, M.; Shaida, M.A.; Manzar, M.S.; Abutaleb, A.; Naushad, M.; Iqbal, J. Sustainable green nanoadsorbents for remediation of pharmaceuticals from water and wastewater: A critical review. Environ. Res. 2022, 204, 112243. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; García-Payo, M.C.; Khayet, M.; Kharraz, J.E.; Chaouch, M. Application of direct contact membrane distillation for textile wastewater treatment and fouling study. Sep. Purif. Technol. 2019, 209, 815–825. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palacio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Gou, Z.; Hopla, G.A.; Yao, M.; Cui, B.; Su, Y.; Rinklebe, J.; Sun, C.; Chen, G.; Ma, N.L.; Sun, Y. Removal of dye pollution by an oxidase derived from mutagenesis of the Deuteromycete Myrothecium with high potential in industrial applications. Environ. Pollut. 2022, 310, 119726. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Al-Gheethi, A.; Iqbal, J. Engineered nanoparticles for removal of pollutants from wastewater: Current status and future prospects of nanotechnology for remediation strategies. J. Environ. Chem. Eng. 2021, 9, 106160. [Google Scholar] [CrossRef]
- Chen, S.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Disassembly of lignocellulose into cellulose, hemicellulose, and lignin for preparation of porous carbon materials with enhanced performances. J. Hazard. Mater. 2021, 408, 124956. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.; Chen, B.; Bu, Y.; Chen, Y.; Ma, J.; Rosario-Ortiz, F.L.; Zhu, R. Some issues limiting photo(cata)lysis application in water pollutant control: A critical review from chemistry perspectives. Water Res. 2020, 174, 115605. [Google Scholar] [CrossRef]
- Hou, C.; Jiang, X.; Chen, D.; Zhang, X.; Liu, X.; Mu, Y.; Shen, J. Ag-TiO2/biofilm/nitrate interface enhanced visible light-assisted biodegradation of tetracycline: The key role of nitrate as the electron accepter. Water Res. 2022, 215, 118212. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, R.; Tong, S.; Ma, C. Preparation of Ti/PTFE-F-PbO2 electrode with a long life from the sulfamic acid bath and its application in organic degradation. Chin. J. Chem. Eng. 2011, 19, 1033–1038. [Google Scholar] [CrossRef]
- Dihingia, H.; Tiwari, D. Impact and implications of nanocatalyst in the Fenton-like processes for remediation of aquatic environment contaminated with micro-pollutants: A critical review. J. Water Process Eng. 2022, 45, 102500. [Google Scholar] [CrossRef]
- Yang, R.; Peng, Q.; Yu, B.; Shen, Y.; Cong, H. Yolk-shell Fe3O4@MOF-5 nanocomposites as a heterogeneous Fenton-like catalyst for organic dye removal. Sep. Purif. Technol. 2021, 267, 118620. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Fenton-like degradation of sulfamethoxazole using Fe-based magnetic nanoparticles embedded into mesoporous carbon hybrid as an efficient catalyst. Chem. Eng. J. 2018, 351, 1085–1094. [Google Scholar] [CrossRef]
- Ke, P.; Zeng, D.; Wang, R.; Cui, J.; Li, X.; Fu, Y. Magnetic carbon microspheres as a reusable catalyst in heterogeneous Fenton system for the efficient degradation of phenol in wastewater. Colloids Surf. A 2022, 638, 128265. [Google Scholar] [CrossRef]
- Shin, J.; Bae, S.; Chon, K. Fenton oxidation of synthetic food dyes by Fe-embedded coffee biochar catalysts prepared at different pyrolysis temperatures: A mechanism study. Chem. Eng. J. 2021, 421, 129943. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, Y.; Qi, J.; Chen, H.; Chen, G.; Tang, S. Porous carbon materials based on Physalis alkekengi L. husk and its application for removal of malachite green. Environ. Technol. Innov. 2021, 21, 101343. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, B.; Chen, G.; Chen, H.; Tang, S. Combining biological and chemical methods to disassemble of cellulose from corn straw for the preparation of porous carbons with enhanced adsorption performance. Int. J. Biol. Macromol. 2022, 209, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, G.; Chen, H.; Sun, Y.; Yu, X.; Su, Y.; Tang, S. Preparation of porous carbon-based material from corn straw via mixed alkali and its application for removal of dye. Colloids Surf. A 2019, 568, 173–183. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, B.; Xia, Y.; Chen, H.; Chen, G.; Tang, S. Influence of mixed alkali on the preparation of edible fungus substrate porous carbon material and its application for the removal of dye. Colloids Surf. A 2021, 609, 125675. [Google Scholar] [CrossRef]
- Chen, X.; Yu, G.; Chen, Y.; Tang, S.; Su, Y. Cow dung-based biochar materials prepared via mixed base and its application in the removal of organic pollutants. Int. J. Mol. Sci. 2022, 23, 10094. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Deng, S.; Xie, D.; Shen, S.; Zhang, K.; Guo, W.; Wang, X.; Tu, J. Spore carbon from Aspergillus Oryzae for advanced electrochemical energy storage. Adv. Mater. 2018, 30, 1805165. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, Y.; Huang, X.; Tang, S.; Chen, H.; Su, Y.; Yu, X.; Chen, S.; Chen, G. Biological self-assembled hyphae/starch porous carbon composites for removal of organic pollutants from water. Chem. Eng. J. 2022, 450, 138264. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Porous carbon material derived from fungal hyphae and its application for the removal of dye. RSC Adv. 2019, 9, 25480. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, Y.; Qi, J.; Chen, H.; Chen, G.; Tang, S. Preparation of biomass carbon material based on Fomes fomentarius via alkali activation and its application for the removal of brilliant green in wastewater. Environ. Technol. Innov. 2021, 23, 101659. [Google Scholar] [CrossRef]
- Pant, S.; Ritika; Nag, P.; Ghati, A.; Chakraborty, D.; Maximiano, M.R.; Franco, O.L.; Mandal, A.K.; Kuila, A. Employment of the CRISPR/Cas9 system to improve cellulase production in Trichoderma reesei. Biotechnol. Adv. 2022, 60, 108022. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, X.; He, A.; Xia, J.; He, J.; Deng, Y.; Xu, N.; Qiu, Z.; Wang, X.; Zhao, P. One-pot fermentation for erythritol production from distillers grains by the co-cultivation of Yarrowia lipolytica and Trichoderma reesei. Bioresour. Technol. 2022, 351, 127053. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, N. A facile synthesis of nitrogen-doped porous carbons from lignocellulose and protein wastes for VOCs sorption. Environ. Res. 2020, 189, 109956. [Google Scholar] [CrossRef]
- Giri, B.S.; Sonwani, R.K.; Varjani, S.; Chaurasia, D.; Varadavenkatesan, T.; Chaturvedi, P.; Yadav, S.; Katiyar, V.; Singh, R.S.; Pandey, A. Highly efficient bio-adsorption of Malachite green using Chinese Fan-Palm Biochar (Livistona chinensis). Chemosphere 2022, 287, 132282. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Bibi, S.; Ali, N.; Ali, Z.; Said, A.; Wahab, Z.U.; Bilal, M.; Iqbal, H.M.N. Sorptive removal of malachite green dye by activated charcoal: Process optimization, kinetic, and thermodynamic evaluation. Case Stud. Chem. Environ. Eng. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sirajudheen, P.; Karthikeyan, P.; Meenakshi, S. Fabrication of sulfur-doped biochar derived from tapioca peel waste with superior adsorption performance for the removal of Malachite green and Rhodamine B dyes. Surf. Interfaces 2021, 23, 100920. [Google Scholar] [CrossRef]
- Leng, L.; Yuan, X.; Zeng, G.; Shao, J.; Chen, X.; Wu, Z.; Wang, H.; Peng, X. Surface characterization of rice husk bio-char produced by liquefaction and application for cationic dye (Malachite green) adsorption. Fuel 2015, 155, 77–85. [Google Scholar] [CrossRef]
- Motaghi, H.; Arabkhani, P.; Parvinnia, M.; Asfaram, A. Simultaneous adsorption of cobalt ions, azo dye, and imidacloprid pesticide on the magnetic chitosan/activated carbon@UiO-66 bio-nanocomposite: Optimization, mechanisms, regeneration, and application. Sep. Purif. Technol. 2022, 284, 120258. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Alrozi, R. Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 171, 510–516. [Google Scholar] [CrossRef]
- Altintig, E.; Onaran, M.; Sarı, A.; Altundag, H.; Tuzen, M. Preparation, characterization and evaluation of bio-based magnetic activated carbon for effective adsorption of malachite green from aqueous solution. Mater. Chem. Phys. 2018, 220, 313–321. [Google Scholar] [CrossRef]
- Sharma, G.; Sharmac, S.; Kumar, A.; Naushad, M.; Du, B.; Ahamad, T.; Ghfar, A.A.; Alqadami, A.A.; Stadler, F.J. Honeycomb structured activated carbon synthesized from Pinus roxburghii cone as effective bioadsorbent for toxic malachite green dye. J. Water Process Eng. 2019, 32, 100931. [Google Scholar] [CrossRef]
- Tsai, C.; Lin, P.; Hsieh, S.; Kirankumar, R.; Patel, A.K.; Singhania, R.; Dong, C.; Chen, C.; Hsieh, S. Engineered mesoporous biochar derived from rice husk for efficient removal of malachite green from wastewaters. Bioresour. Technol. 2022, 347, 126749. [Google Scholar] [CrossRef] [PubMed]
- Eltaweil, A.S.; Mohamed, H.A.; El-Monaem, E.M.A.; El-Subruiti, G.M. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol. 2020, 31, 1253–1263. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Katsou, E.; Jouhara, H. The removal of tetracycline from water using biochar produced from agricultural discarded material. Sci. Total Environ. 2021, 751, 141755. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wu, M.; Pang, Y.; Hao, Z.; Hu, M.; Qiu, R.; Chen, Z. Enhanced adsorption of tetracycline by an iron and manganese oxides loaded biochar: Kinetics, mechanism and column adsorption. Bioresour. Technol. 2021, 320, 124264. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.; Jiang, H.; Chen, J.; Li, W.; Yu, H. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Mu, Y.; He, W.; Ma, H. Enhanced adsorption of tetracycline by the modified tea-based biochar with the developed mesoporous and surface alkalinity. Bioresour. Technol. 2021, 342, 126001. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G.; Li, G. The characteristics of pharmaceutical sludge-derived biochar and its application for the adsorption of tetracycline. Sci. Total Environ. 2020, 747, 141492. [Google Scholar] [CrossRef]
- Dai, J.; Meng, X.; Zhang, Y.; Huang, Y. Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour. Technol. 2020, 311, 123455. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef] [PubMed]
- Khanday, W.A.; Hameed, B.H. Zeolite-hydroxyapatite-activated oil palm ash composite for antibiotic tetracycline adsorption. Fuel 2018, 215, 499–505. [Google Scholar] [CrossRef]
- Wang, J.; Lei, S.; Liang, L. Preparation of porous activated carbon from semi-coke by high temperature activation with KOH for the high-efficiency adsorption of aqueous tetracycline. Appl. Surf. Sci. 2020, 530, 147187. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G.; Li, G. Preparation of porous biochar based on pharmaceutical sludge activated by NaOH and its application in the adsorption of tetracycline. J. Colloid Interface Sci. 2021, 587, 271–278. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; He, J.; Liu, T.; Zhang, K.; Huang, X.; Kong, L.; Liu, J. EDTA-Fe(III) Fenton-like oxidation for the degradation of malachite green. J. Environ. Manag. 2018, 226, 256–263. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, S.; Wang, F.; Megharaj, M.; Naidu, R.; Chen, Z. Heterogeneous Fenton-like oxidation of malachite green by iron-based nanoparticles synthesized by tea extract as a catalyst. Sep. Purif. Technol. 2015, 154, 161–167. [Google Scholar] [CrossRef]
- Elhalil, A.; Tounsadi, H.; Elmoubarki, R.; Mahjoubi, F.Z.; Farnane, M.; Sadiq, M.; Abdennouri, M.; Qourzal, S.; Barka, N. Factorial experimental design for the optimization of catalytic degradation of malachite green dye in aqueous solution by Fenton process. Water Resour. Ind. 2016, 15, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Fu, X.; Yu, J.; Xu, Y.; Huang, J.; Li, Q.; Sun, D. Green synthesized iron nanoparticles as highly efficient fenton-like catalyst for degradation of dyes. Chemosphere 2020, 261, 127618. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Lei, J.; Ao, Z.; Zhou, Y. Fe3O4/graphene aerogels: A stable and efficient persulfate activator for the rapid degradation of malachite green. Chemosphere 2020, 251, 126402. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, J.; Yi, Q.; Li, D.; Liu, C.; Liu, Y. Construction of core-shell Fe3O4@GO-CoPc photo-Fenton catalyst for superior removal of tetracycline: The role of GO in promotion of H2O2 to OH conversion. J. Environ. Manag. 2022, 308, 114613. [Google Scholar] [CrossRef]
- Jafari, A.J.; Kakavandi, B.; Jaafarzadeh, N.; Kalantary, R.R.; Ahmadi, M.; Babaei, A.A. Fenton-like catalytic oxidation of tetracycline by AC@Fe3O4 as a heterogeneous persulfate activator: Adsorption and degradation studies. J. Ind. Eng. Chem. 2017, 45, 323–333. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, B.; Li, X.; Liu, B.; Wang, S.; Yu, P.; Xu, Y.; Jiang, G. High-efficiency removal of tetracycline by carbon-bridge-doped g-C3N4/Fe3O4 magnetic heterogeneous catalyst through photo-Fenton process. J. Hazard. Mater. 2021, 418, 126333. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Wang, C.; Wang, P.; Fu, H.; Zhao, C. Photocatalysis activation of peroxodisulfate over the supported Fe3O4 catalyst derived from MIL-88A(Fe) for efficient tetracycline hydrochloride degradation. Chem. Eng. J. 2021, 426, 131927. [Google Scholar] [CrossRef]
- Khodadadi, M.; Panahi, A.H.; Al-Musawi, T.J.; Ehrampoush, M.H.; Mahvi, A.H. The catalytic activity of FeNi3@SiO2 magnetic nanoparticles for the degradation of tetracycline in the heterogeneous Fenton-like treatment method. J. Water Process Eng. 2019, 32, 100943. [Google Scholar] [CrossRef]

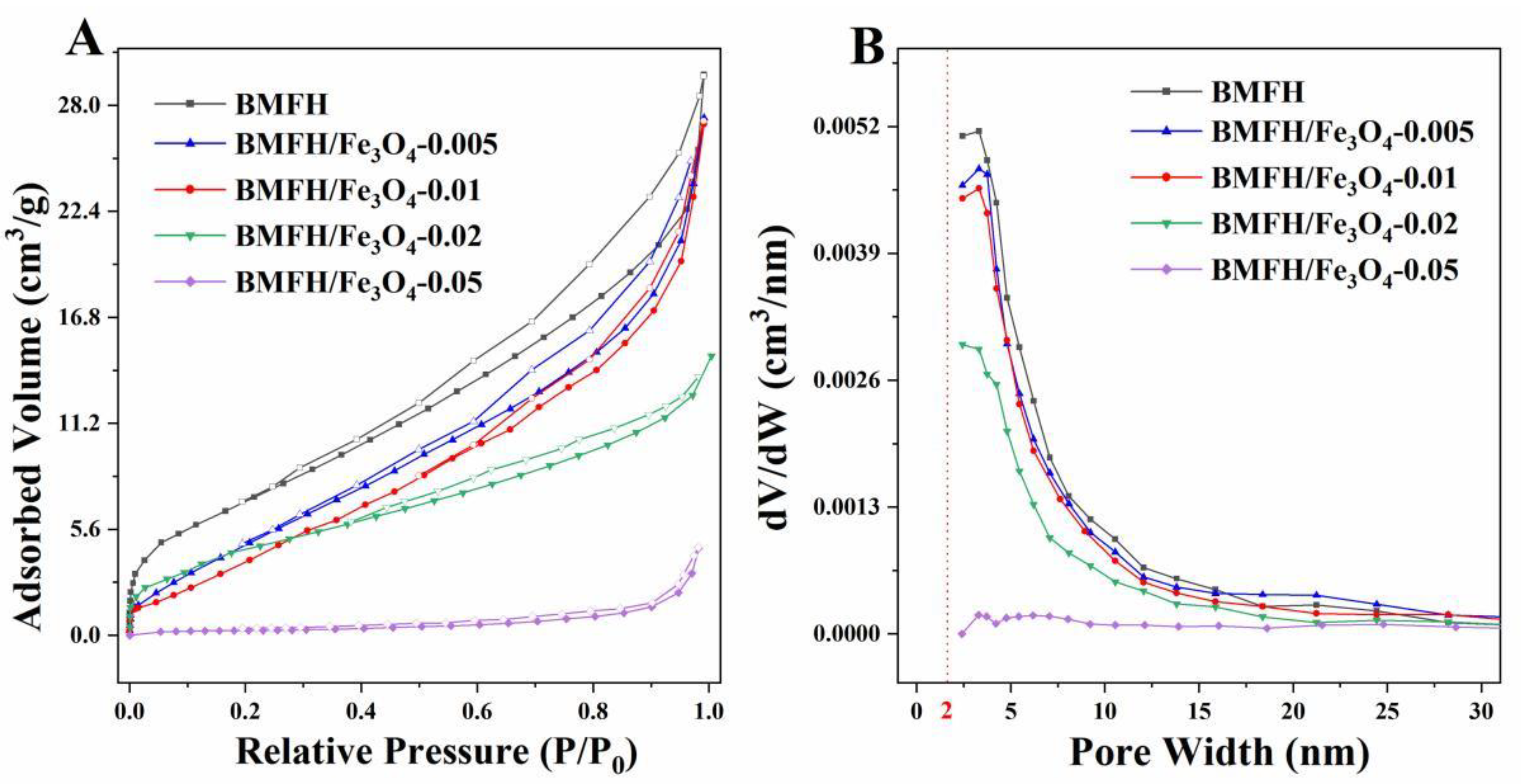


| Samples | SBET (m2/g) | Pm (nm) | Vtotal (cm3/g) |
|---|---|---|---|
| BMFH | 27.91 | 5.69 | 0.0369 |
| BMFH/Fe3O4-0.005 | 22.77 | 5.34 | 0.0236 |
| BMFH/Fe3O4-0.01 | 21.59 | 5.16 | 0.0214 |
| BMFH/Fe3O4-0.02 | 13.40 | 6.05 | 0.0202 |
| BMFH/Fe3O4-0.05 | 0.89 | 31.93 | 0.0071 |
| Adsorbates | Adsorbents | Models | Parameters | C0 (mg L−1) | ||
|---|---|---|---|---|---|---|
| 50 | 100 | 200 | ||||
| MG | BMFH | qe (mg/g) | 142.99 | 161.89 | 184.05 | |
| PFK | k1 (min−1) | 0.2438 | 0.3179 | 0.3319 | ||
| qe.cat (mg/g) | 134.21 | 154.69 | 178.15 | |||
| R2 | 0.9732 | 0.9888 | 0.9926 | |||
| PSK | k2 (g mg−1 min−1) | 0.0031 | 0.0042 | 0.0042 | ||
| qe.cat (mg/g) | 144.36 | 162.15 | 185.38 | |||
| R2 | 0.9949 | 0.9988 | 0.9996 | |||
| IPD | k3 (mg g−1 min−0.5) | 4.4963 | 3.4926 | 3.4629 | ||
| B | 98.96 | 127.57 | 150.89 | |||
| R2 | 0.9050 | 0.8452 | 0.7582 | |||
| BMFH/Fe3O4 | qe (mg/g) | 119.33 | 147.90 | 168.44 | ||
| PFK | k1 (min−1) | 0.2039 | 0.2208 | 0.2526 | ||
| qe.cat (mg/g) | 112.33 | 141.29 | 162.65 | |||
| R2 | 0.9737 | 0.9819 | 0.9862 | |||
| PSK | k2 (g mg−1 min−1) | 0.0029 | 0.0027 | 0.0027 | ||
| qe.cat (mg/g) | 119.99 | 150.15 | 171.13 | |||
| R2 | 0.9966 | 0.9984 | 0.9991 | |||
| IPD | k3 (mg g−1 min−0.5) | 4.2913 | 4.6966 | 4.6213 | ||
| B | 77.76 | 103.57 | 125.51 | |||
| R2 | 0.8741 | 0.8037 | 0.7824 | |||
| TH | BMFH | qe (mg/g) | 151.20 | 177.81 | 196.65 | |
| PFK | k1 (min−1) | 0.2674 | 0.2241 | 0.2476 | ||
| qe.cat (mg/g) | 144.76 | 170.19 | 190.80 | |||
| R2 | 0.9847 | 0.9899 | 0.9909 | |||
| PSK | k2 (g mg−1 min−1) | 0.0034 | 0.0023 | 0.0024 | ||
| qe.cat (mg/g) | 152.12 | 179.98 | 200.38 | |||
| R2 | 0.9986 | 0.9998 | 0.9996 | |||
| IPD | k3 (mg g−1 min−0.5) | 4.0254 | 5.2583 | 5.3479 | ||
| B | 112.90 | 126.09 | 148.21 | |||
| R2 | 0.8271 | 0.7566 | 0.7178 | |||
| BMFH/Fe3O4 | qe (mg/g) | 123.03 | 155.47 | 181.75 | ||
| PFK | k1 (min−1) | 0.1807 | 0.2642 | 0.2336 | ||
| qe.cat (mg/g) | 116.14 | 150.24 | 175.74 | |||
| R2 | 0.9821 | 0.9883 | 0.9912 | |||
| PSK | k2 (g mg−1 min−1) | 0.0024 | 0.0033 | 0.0023 | ||
| qe.cat (mg/g) | 124.42 | 157.48 | 185.32 | |||
| R2 | 0.9991 | 0.9990 | 0.9999 | |||
| IPD | k3 (mg g−1 min−0.5) | 4.6731 | 3.9191 | 5.2245 | ||
| B | 77.47 | 118.65 | 132.95 | |||
| R2 | 0.8163 | 0.7471 | 0.7341 | |||
| Adsorbates | Adsorbents | Types | Parameters | |
|---|---|---|---|---|
| MG | BMFH | Langmuir | qm (mg/g) | 190.01 |
| KL (L/mg) | 0.1279 | |||
| R2 | 0.8905 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 98.73 | ||
| nF | 8.3675 | |||
| R2 | 0.9928 | |||
| BMFH/Fe3O4 | Langmuir | qm (mg/g) | 178.28 | |
| KL (L/mg) | 0.0707 | |||
| R2 | 0.9380 | |||
| Freundlich | KF (mg g−1(L mg-−1)1/n) | 70.12 | ||
| nF | 6.0368 | |||
| R2 | 0.9965 | |||
| TH | BMFH | Langmuir | qm (mg/g) | 204.94 |
| KL (L/mg) | 0.1324 | |||
| R2 | 0.9378 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 107.11 | ||
| nF | 8.4253 | |||
| R2 | 0.9945 | |||
| BMFH/Fe3O4 | Langmuir | qm (mg/g) | 201.47 | |
| KL (L/mg) | 0.0567 | |||
| R2 | 0.9659 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 67.44 | ||
| nF | 5.1808 | |||
| R2 | 0.9915 | |||
| Adsorbents | Adsorbates | T (K) | ∆G (kJ/mol) | ∆H (kJ/mol) | ∆S (J mol−1 K−1) |
|---|---|---|---|---|---|
| BMFH | MG | 293 | −2.00 | 3.88 | 20.06 |
| 303 | −2.19 | ||||
| 313 | −2.39 | ||||
| TH | 293 | −2.21 | 6.81 | 30.80 | |
| 303 | −2.56 | ||||
| 313 | −2.83 | ||||
| BMFH/Fe3O4 | MG | 293 | −1.51 | 8.43 | 33.92 |
| 303 | −1.87 | ||||
| 313 | −2.18 | ||||
| TH | 293 | −1.56 | 12.07 | 46.53 | |
| 303 | −2.05 | ||||
| 313 | −2.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Lin, J.; Su, Y.; Tang, S. One-Step Carbonization Synthesis of Magnetic Biochar with 3D Network Structure and Its Application in Organic Pollutant Control. Int. J. Mol. Sci. 2022, 23, 12579. https://doi.org/10.3390/ijms232012579
Chen X, Lin J, Su Y, Tang S. One-Step Carbonization Synthesis of Magnetic Biochar with 3D Network Structure and Its Application in Organic Pollutant Control. International Journal of Molecular Sciences. 2022; 23(20):12579. https://doi.org/10.3390/ijms232012579
Chicago/Turabian StyleChen, Xiaoxin, Jiacheng Lin, Yingjie Su, and Shanshan Tang. 2022. "One-Step Carbonization Synthesis of Magnetic Biochar with 3D Network Structure and Its Application in Organic Pollutant Control" International Journal of Molecular Sciences 23, no. 20: 12579. https://doi.org/10.3390/ijms232012579





