Importance of Micromilieu for Pathophysiologic Mineralocorticoid Receptor Activity—When the Mineralocorticoid Receptor Resides in the Wrong Neighborhood
Abstract
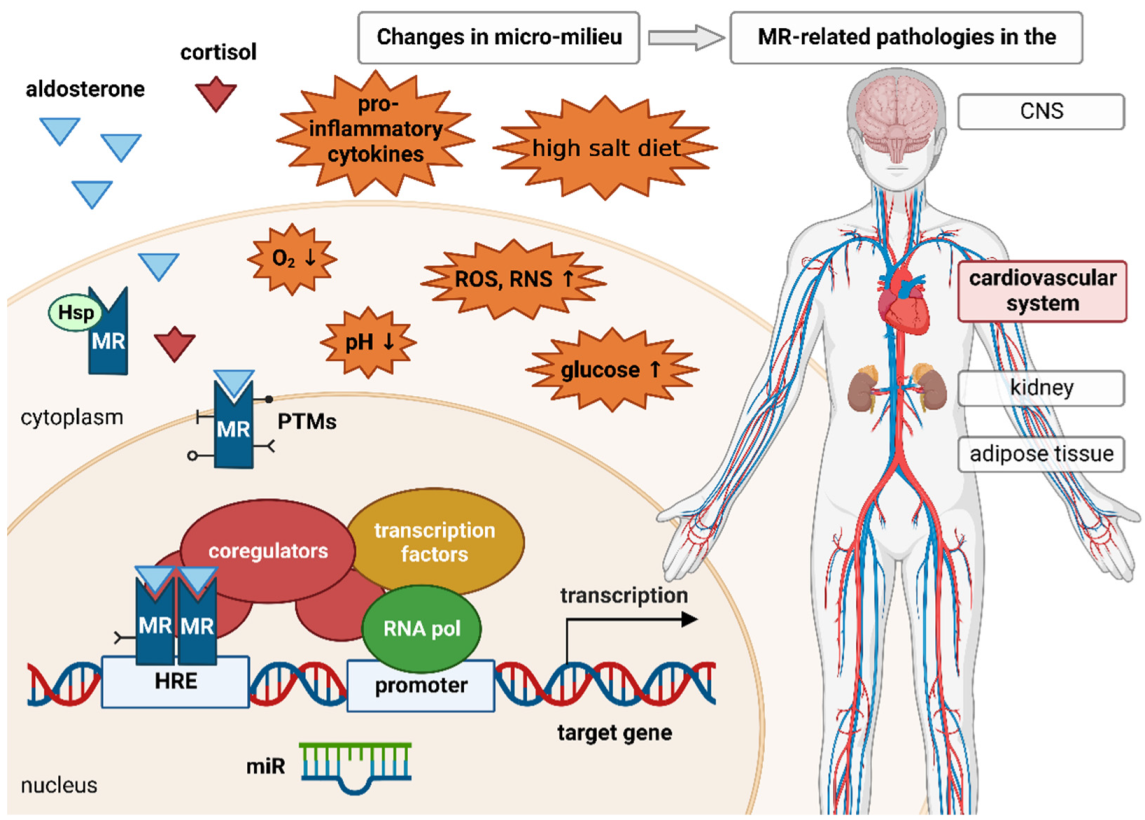
:1. Physiologic and Pathophysiologic Role of the MR
2. Over-Active MR in the Absence of Elevated Aldosterone Levels
2.1. High Salt Diet
2.2. Hyperglycemia
2.3. Inflammation
2.4. Oxidative Stress
2.5. Hypoxia, Ischemia and Reperfusions
3. MR Signaling and Micromilieu
3.1. Posttranslational Modifications
3.2. Coregulators
3.3. Non-Coding RNAs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nahum, L.H. The renin angiotensin-aldosterone system (RAAS) in normal man. Conn. Med. 1965, 29, 710–711. [Google Scholar] [PubMed]
- Pearce, D.; Bhargava, A.; Cole, T.J. Aldosterone: Its receptor, target genes, and actions. Vitam. Horm. 2003, 66, 29–76. [Google Scholar] [PubMed]
- Kanatsou, S.; Joels, M.; Krugers, H. Brain Mineralocorticoid Receptors and Resilience to Stress. Vitam. Horm. 2019, 109, 341–359. [Google Scholar] [PubMed]
- Marzolla, V.; Armani, A.; Feraco, A.; De Martino, M.U.; Fabbri, A.; Rosano, G.; Caprio, M. Mineralocorticoid receptor in adipocytes and macrophages: A promising target to fight metabolic syndrome. Steroids 2014, 91, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Clyne, C.D. Mineralocorticoid receptor actions in cardiovascular development and disease. Essays Biochem. 2021, 65, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; McMurray, J.J.V.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms. N. Engl. J. Med. 2010, 364, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sadaba, J.R.; Zannad, F.; Rossignol, P.; Lopez-Andres, N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail 2015, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Z.; Morgan, J.; Tesch, G.H.; Rickard, A.J.; Chrissobolis, S.; Drummond, G.R.; Fuller, P.J.; Young, M.J. Cardiac Tissue Injury and Remodeling Is Dependent Upon MR Regulation of Activation Pathways in Cardiac Tissue Macrophages. Endocrinology 2016, 157, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J.; Lother, A. Mineralocorticoid receptor activation and antagonism in cardiovascular disease: Cellular and molecular mechanisms. Kidney Int. Suppl. (2011) 2022, 12, 19–26. [Google Scholar] [CrossRef]
- Brilla, C.G.; Weber, K.T. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J. Lab Clin. Med. 1992, 120, 893–901. [Google Scholar]
- Wang, Q.; Clement, S.; Gabbiani, G.; Horisberger, J.D.; Burnier, M.; Rossier, B.C.; Hummler, E. Chronic hyperaldosteronism in a transgenic mouse model fails to induce cardiac remodeling and fibrosis under a normal-salt diet. Am. J. Physiol. Ren. Physiol. 2004, 286, F1178–F1184. [Google Scholar] [CrossRef]
- Nagata, K.; Obata, K.; Xu, J.; Ichihara, S.; Noda, A.; Kimata, H.; Kato, T.; Izawa, H.; Murohara, T.; Yokota, M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension 2006, 47, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sawano, T.; Sen, A.; Hossain, A.; Jahan, N.; Kobara, H.; Masaki, T.; Kosaka, S.; Kitada, K.; Nakano, D.; et al. Cardioprotective Effects of a Nonsteroidal Mineralocorticoid Receptor Blocker, Esaxerenone, in Dahl Salt-Sensitive Hypertensive Rats. Int. J. Mol. Sci. 2021, 22, 2069. [Google Scholar] [CrossRef]
- Kawarazaki, W.; Nagase, M.; Yoshida, S.; Takeuchi, M.; Ishizawa, K.; Ayuzawa, N.; Ueda, K.; Fujita, T. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J. Am. Soc. Nephrol. 2012, 23, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Shibata, S.; Mu, S.; Kawarazaki, H.; Muraoka, K.; Ishizawa, K.; Yoshida, S.; Kawarazaki, W.; Takeuchi, M.; Ayuzawa, N.; Miyoshi, J.; et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J. Clin. Investig. 2011, 121, 3233–3243. [Google Scholar] [CrossRef]
- Silva, G.B.; Garvin, J.L. Rac1 mediates NaCl-induced superoxide generation in the thick ascending limb. Am. J. Physiol. Ren. Physiol. 2010, 298, F421–F425. [Google Scholar] [CrossRef] [Green Version]
- Treesaranuwattana, T.; Wong, K.Y.H.; Brooks, D.L.; Tay, C.S.; Williams, G.H.; Williams, J.S.; Pojoga, L.H. Lysine-Specific Demethylase-1 Deficiency Increases Agonist Signaling Via the Mineralocorticoid Receptor. Hypertension 2020, 75, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Shibata, H.; Kurihara, I.; Yokota, K.; Mitsuishi, Y.; Ohashi, K.; Murai-Takeda, A.; Jo, R.; Ohyama, T.; Sakamoto, M.; et al. High Glucose Stimulates Mineralocorticoid Receptor Transcriptional Activity Through the Protein Kinase C beta Signaling. Int. Heart J. 2017, 58, 794–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, J.F.; Uzui, H.; Guo, H.Y.; Ueda, T.; Lee, J.D. Effects of eplerenone on the activation of matrix metalloproteinase-2 stimulated by high glucose and interleukin-1beta in human cardiac fibroblasts. Genet Mol. Res. 2014, 13, 4845–4855. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Wang, J.Y.; Ko, J.Y.; Surendran, K.; Huang, Y.T.; Kuo, Y.H.; Wang, F.S. Superoxide destabilization of beta-catenin augments apoptosis of high-glucose-stressed mesangial cells. Endocrinology 2008, 149, 2934–2942. [Google Scholar] [CrossRef] [Green Version]
- Shen, E.; Li, Y.; Li, Y.; Shan, L.; Zhu, H.; Feng, Q.; Arnold, J.M.; Peng, T. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes 2009, 58, 2386–2395. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Reddy, M.A.; Sun, G.; Lanting, L.; Wang, M.; Kato, M.; Natarajan, R. Involvement of p300/CBP and epigenetic histone acetylation in TGF-beta1-mediated gene transcription in mesangial cells. Am. J. Physiol.-Ren. Physiol. 2012, 304, F601–F613. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Yao, F.; Lang, Y.; Feng, X. Elevated Circulating LINC-P21 Serves as a Diagnostic Biomarker of Type 2 Diabetes Mellitus and Regulates Pancreatic beta-cell Function by Sponging miR-766-3p to Upregulate NR3C2. Exp. Clin. Endocrinol. Diabetes 2022, 130, 156–164. [Google Scholar]
- O’Keefe, J.H.; Abuissa, H.; Pitt, B. Eplerenone improves prognosis in postmyocardial infarction diabetic patients with heart failure: Results from EPHESUS. Diabetes Obes. Metab. 2008, 10, 492–497. [Google Scholar] [CrossRef]
- Ruhs, S.; Stratz, N.; Quarch, K.; Masch, A.; Schutkowski, M.; Gekle, M.; Grossmann, C. Modulation of transcriptional mineralocorticoid receptor activity by casein kinase 2. Sci. Rep. 2017, 7, 15340. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Geng, J.; Gao, S.X.; Yue, W.W.; Liu, Q. Eplerenone Modulates Interleukin-33/sST2 Signaling and IL-1beta in Left Ventricular Systolic Dysfunction After Acute Myocardial Infarction. J. Interferon Cytokine Res. 2018, 38, 137–144. [Google Scholar] [CrossRef]
- Papaharalambus, C.; Sajjad, W.; Syed, A.; Zhang, C.; Bergo, M.O.; Alexander, R.W.; Ahmad, M. Tumor necrosis factor alpha stimulation of Rac1 activity. Role of isoprenylcysteine carboxylmethyltransferase. J. Biol. Chem. 2005, 280, 18790–18796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.; Dong, R.; Zhang, Y.; Yang, Y.; Chen, Z.; Tang, Y.; Fu, M.; Xu, X.; Tu, L. Age−related changes in mineralocorticoid receptors in rat hearts. Mol. Med. Rep. 2020, 22, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.M.; Mishra, V.; Bhatnagar, S.; Tajrishi, M.M.; Ogura, Y.; Yan, Z.; Burkly, L.C.; Zheng, T.S.; Kumar, A. Regulatory circuitry of TWEAK-Fn14 system and PGC-1alpha in skeletal muscle atrophy program. FASEB J. 2014, 28, 1398–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Sweeney, T.R.; Shigenaga, J.K.; Chui, L.G.; Moser, A.; Grunfeld, C.; Feingold, K.R. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism 2007, 56, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Andres, O.; Suarez-Alvarez, B.; Sanchez-Ramos, C.; Monsalve, M.; Sanchez-Nino, M.D.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A.; Sanz, A.B. The inflammatory cytokine TWEAK decreases PGC-1alpha expression and mitochondrial function in acute kidney injury. Kidney Int. 2016, 89, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Leite, R.S.; Brown, A.G.; Strauss, J.F., III. Tumor necrosis factor-alpha suppresses the expression of steroid receptor coactivator-1 and -2: A possible mechanism contributing to changes in steroid hormone responsiveness. FASEB J. 2004, 18, 1418–1420. [Google Scholar] [CrossRef]
- Ruhs, S.; Straetz, N.; Schloer, K.; Meinel, S.; Mildenberger, S.; Rabe, S.; Gekle, M.; Grossmann, C. Modulation of transcriptional mineralocorticoid receptor activity by nitrosative stress. Free Radic. Biol. Med. 2012, 53, 1088–1100. [Google Scholar] [CrossRef]
- Dikshit, P.; Jana, N.R. The co-chaperone CHIP is induced in various stresses and confers protection to cells. Biochem. Biophys. Res. Commun. 2007, 357, 761–765. [Google Scholar] [CrossRef]
- Stankovic-Valentin, N.; Drzewicka, K.; Koenig, C.; Schiebel, E.; Melchior, F. Redox regulation of SUMO enzymes is required for ATM activity and survival in oxidative stress. EMBO J. 2016, 35, 1312–1329. [Google Scholar] [CrossRef] [Green Version]
- Nagase, M.; Ayuzawa, N.; Kawarazaki, W.; Ishizawa, K.; Ueda, K.; Yoshida, S.; Fujita, T. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: Role of small GTPase Rac1. Hypertension 2012, 59, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Omori, Y.; Mano, T.; Ohtani, T.; Sakata, Y.; Takeda, Y.; Tamaki, S.; Tsukamoto, Y.; Miwa, T.; Yamamoto, K.; Komuro, I. Glucocorticoids Induce Cardiac Fibrosis via Mineralocorticoid Receptor in Oxidative Stress: Contribution of Elongation Factor Eleven-Nineteen Lysine-Rich Leukemia (ELL). Yonago Acta Med. 2014, 57, 109–116. [Google Scholar] [PubMed]
- Kim, Y.; Nam, H.J.; Lee, J.; Park, D.Y.; Kim, C.; Yu, Y.S.; Kim, D.; Park, S.W.; Bhin, J.; Hwang, D.; et al. Methylation-dependent regulation of HIF-1alpha stability restricts retinal and tumour angiogenesis. Nat. Commun. 2016, 7, 10347. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Xiong, Y.; Lei, Y.; Huang, Q.; Liu, H.; Zhao, X.; Chen, Z.; Chen, H.; Liu, X.; Wang, L.; et al. Lysine-specific demethylase 1 aggravated oxidative stress and ferroptosis induced by renal ischemia and reperfusion injury through activation of TLR4/NOX4 pathway in mice. J. Cell. Mol. Med. 2022, 26, 4254–4267. [Google Scholar] [CrossRef]
- Wang, P.; Fan, F.; Li, X.; Sun, X.; Ma, L.; Wu, J.; Shen, C.; Zhu, H.; Dong, Z.; Wang, C.; et al. Riboflavin attenuates myocardial injury via LSD1-mediated crosstalk between phospholipid metabolism and histone methylation in mice with experimental myocardial infarction. J. Mol. Cell. Cardiol. 2018, 115, 115–129. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhang, Q.H.; Ye, H.; Zhang, Y.; Luo, Y.M.; Ji, X.M.; Su, Y.Y. Distribution of lysine-specific demethylase 1 in the brain of rat and its response in transient global cerebral ischemia. Neurosci. Res. 2010, 68, 66–72. [Google Scholar] [CrossRef]
- Liu, L.; Ai, J.; Xiao, W.; Liu, J.; Wang, Y.; Xin, D.; He, Z.; Guo, Y.; Wang, Z. ELL is an HIF-1alpha partner that regulates and responds to hypoxia response in PC3 cells. Prostate 2010, 70, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Hu, F. Long noncoding RNA SOX2OT silencing alleviates cerebral ischemia-reperfusion injury via miR-135a-5p-mediated NR3C2 inhibition. Brain Res. Bull. 2021, 173, 193–202. [Google Scholar] [CrossRef]
- Cai, J.; Shangguan, S.; Li, G.; Cai, Y.; Chen, Y.; Ma, G.; Miao, Z.; Liu, L.; Deng, Y. Knockdown of lncRNA Gm11974 protect against cerebral ischemic reperfusion through miR-766-3p/NR3C2 axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3847–3853. [Google Scholar] [CrossRef] [Green Version]
- Bulluck, H.; Fröhlich, G.M.; Nicholas, J.M.; Mohdnazri, S.; Gamma, R.; Davies, J.; Sirker, A.; Mathur, A.; Blackman, D.; Garg, P.; et al. Mineralocorticoid receptor antagonist pre-treatment and early post-treatment to minimize reperfusion injury after ST-elevation myocardial infarction: The MINIMIZE STEMI trial. Am. Heart J. 2019, 211, 60–67. [Google Scholar] [CrossRef]
- Hayashi, M.; Tsutamoto, T.; Wada, A.; Tsutsui, T.; Ishii, C.; Ohno, K.; Fujii, M.; Taniguchi, A.; Hamatani, T.; Nozato, Y.; et al. Immediate Administration of Mineralocorticoid Receptor Antagonist Spironolactone Prevents Post-Infarct Left Ventricular Remodeling Associated With Suppression of a Marker of Myocardial Collagen Synthesis in Patients With First Anterior Acute Myocardial Infarction. Circulation 2003, 107, 2559–2565. [Google Scholar]
- Oakley, R.H.; Cruz-Topete, D.; He, B.; Foley, J.F.; Myers, P.H.; Xu, X.; Gomez-Sanchez, C.E.; Chambon, P.; Willis, M.S.; Cidlowski, J.A. Cardiomyocyte glucocorticoid and mineralocorticoid receptors directly and antagonistically regulate heart disease in mice. Sci. Signal. 2019, 12, eaau9685. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Aldosterone, Mineralocorticoid Receptor Activation, and Cardiovascular Remodeling. Circulation 2011, 124, e466–e468. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Newfell, B.G.; la Sala, A.; Baur, W.; Fabbri, A.; Rosano, G.; Mendelsohn, M.E.; Jaffe, I.Z. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ. Res. 2008, 102, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Lother, A.; Fürst, D.; Bergemann, S.; Gilsbach, R.; Grahammer, F.; Huber, T.B.; Hilgendorf, I.; Bode, C.; Moser, M.; Hein, L. Deoxycorticosterone Acetate/Salt-Induced Cardiac But Not Renal Injury Is Mediated By Endothelial Mineralocorticoid Receptors Independently From Blood Pressure. Hypertension 2016, 67, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Iwashima, F.; Yoshimoto, T.; Minami, I.; Sakurada, M.; Hirono, Y.; Hirata, Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology 2008, 149, 1009–1014. [Google Scholar] [CrossRef]
- Jani, B.; Rajkumar, C. Ageing and vascular ageing. Postgrad Med. J. 2006, 82, 357. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; McCurley, A.T.; DuPont, J.J.; Aronovitz, M.; Moss, M.E.; Stillman, I.E.; Karumanchi, S.A.; Christou, D.D.; Jaffe, I.Z. Smooth Muscle Cell Mineralocorticoid Receptor as a Mediator of Cardiovascular Stiffness With Aging. Hypertension 2018, 71, 609–621. [Google Scholar] [CrossRef]
- Vargas, R.A.; Millan, J.M.; Bonilla, E. Renin angiotensin system: Basic and clinical aspects—A general perspective. Endocrinol. Diabetes Y Nutr. 2022, 69, 52–62. [Google Scholar] [CrossRef]
- Mascolo, A.; Scavone, C.; Rafaniello, C.; Ferrajolo, C.; Racagni, G.; Berrino, L.; Paolisso, G.; Rossi, F.; Capuano, A. Renin-Angiotensin System and Coronavirus Disease 2019: A Narrative Review. Front. Cardiovasc. Med. 2020, 7, 143. [Google Scholar] [CrossRef]
- Lefranc, C.; Friederich-Persson, M.; Foufelle, F.; Nguyen Dinh Cat, A.l.; Jaisser, F. Adipocyte-Mineralocorticoid Receptor Alters Mitochondrial Quality Control Leading to Mitochondrial Dysfunction and Senescence of Visceral Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 2881. [Google Scholar] [CrossRef]
- Marzolla, V.; Armani, A.; Mammi, C.; Moss, M.E.; Pagliarini, V.; Pontecorvo, L.; Antelmi, A.; Fabbri, A.; Rosano, G.; Jaffe, I.Z.; et al. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. Int. J. Cardiol. 2017, 232, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.Y.; Kohno, M.; Hitomi, H.; Kitada, K.; Fujisawa, Y.; Yatabe, J.; Yatabe, M.; Felder, R.A.; Ohsaki, H.; Rafiq, K.; et al. Aldosterone/Mineralocorticoid Receptor Stimulation Induces Cellular Senescence in the Kidney. Endocrinology 2011, 152, 680–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krug, A.W.; Allenhofer, L.; Monticone, R.; Spinetti, G.; Gekle, M.; Wang, M.; Lakatta, E.G. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension 2010, 55, 1476–1483. [Google Scholar] [PubMed]
- Stroedecke, K.; Meinel, S.; Markwardt, F.; Kloeckner, U.; Straetz, N.; Quarch, K.; Schreier, B.; Kopf, M.; Gekle, M.; Grossmann, C. The mineralocorticoid receptor leads to increased expression of EGFR and T-type calcium channels that support HL-1 cell hypertrophy. Sci. Rep. 2021, 11, 13229. [Google Scholar] [CrossRef]
- Menard, J. The 45-year story of the development of an anti-aldosterone more specific than spironolactone. Mol. Cell Endocrinol. 2004, 217, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lainscak, M.; Pelliccia, F.; Rosano, G.; Vitale, C.; Schiariti, M.; Greco, C.; Speziale, G.; Gaudio, C. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int. J. Cardiol. 2015, 200, 25–29. [Google Scholar] [CrossRef]
- Pei, H.; Wang, W.; Zhao, D.; Wang, L.; Su, G.H.; Zhao, Z. The use of a novel non-steroidal mineralocorticoid receptor antagonist finerenone for the treatment of chronic heart failure: A systematic review and meta-analysis. Medicine 2018, 97, e0254. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, E.P. Third-generation Mineralocorticoid Receptor Antagonists: Why Do We Need a Fourth? J Cardiovasc Pharm. 2016, 57, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Funder, J.W. RALES, EPHESUS and redox. J. Steroid Biochem. Mol. Biol. 2005, 93, 121–125. [Google Scholar] [CrossRef]
- Funder, J.W. Reconsidering the roles of the mineralocorticoid receptor. Hypertension 2009, 53, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Reichek, N.; Willenbrock, R.; Zannad, F.; Phillips, R.A.; Roniker, B.; Kleiman, J.; Krause, S.; Burns, D.; Williams, G.H. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: The 4E-left ventricular hypertrophy study. Circulation 2003, 108, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.H. Age-related changes in the renin-aldosterone system. Physiological effects and clinical implications. Drugs Aging 1993, 3, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, P.; De Myttenaere-Bursztein, S.; Maxwell, M.H.; de Lima, J. Effect on aging on plasma renin and aldosterone in normal man. Kidney Int. 1975, 8, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.; Holmes, M.; Seckl, J. 11beta-hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef] [Green Version]
- Campino, C.; Martinez-Aguayo, A.; Baudrand, R.; Carvajal, C.A.; Aglony, M.; Garcia, H.; Padilla, O.; Kalergis, A.M.; Fardella, C.E. Age-related changes in 11beta-hydroxysteroid dehydrogenase type 2 activity in normotensive subjects. Am. J. Hypertens. 2013, 26, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Chen, Y.; Li, X.; Xu, D. New insights into the roles of glucocorticoid signaling dysregulation in pathological cardiac hypertrophy. Heart Fail. Rev. 2021, 27, 1431–1441. [Google Scholar] [CrossRef]
- Luft, F.C. 11beta-Hydroxysteroid Dehydrogenase-2 and Salt-Sensitive Hypertension. Circulation 2016, 133, 1335–1337. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, S.; Gravez, B.; Tarjus, A.; Pelloux, V.; Ouvrard-Pascaud, A.; Delcayre, C.; Samuel, J.; Launay, J.M.; Sierra-Ramos, C.; de la Rosa, D.A.; et al. Aldosterone-Specific Activation of Cardiomyocyte Mineralocorticoid Receptor In Vivo. Hypertension 2013, 61, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Rossier, M.F.; Python, M.; Maturana, A.D. Contribution of mineralocorticoid and glucocorticoid receptors to the chronotropic and hypertrophic actions of aldosterone in neonatal rat ventricular myocytes. Endocrinology 2010, 151, 2777–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.T.; Chiang, F.T.; Tseng, C.D.; Hwang, J.J.; Kuo, K.T.; Wu, C.K.; Yu, C.C.; Wang, Y.C.; Lai, L.P.; Lin, J.L. Increased Expression of Mineralocorticoid Receptor in Human Atrial Fibrillation and a Cellular Model of Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 55, 758–770. [Google Scholar] [CrossRef] [Green Version]
- Maeoka, Y.; Su, X.T.; Wang, W.H.; Duan, X.P.; Sharma, A.; Li, N.; Staub, O.; McCormick, J.A.; Ellison, D.H. Mineralocorticoid Receptor Antagonists Cause Natriuresis in the Absence of Aldosterone. Hypertension 2022, 79, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Kataoka, T.; Yamamoto, Y.; Asano, G.; Fukamoto, A.; Hotta, Y.; Maeda, Y.; Takahashi, M.; Kanayama, H.o.; Kimura, K. High Salt Intake Impairs Erectile Function in Salt-Sensitive Rats Through Mineralocorticoid Receptor Pathway Beyond Its Effect on Blood Pressure. J. Sex. Med. 2020, 17, 1280–1287. [Google Scholar] [CrossRef]
- Cabrera, D.; Rao, I.; Raasch, F.; Solis, N.; Pizarro, M.; Freire, M.; Sáenz De Urturi, D.; Ramírez, C.A.; Triantafilo, N.; León, J.; et al. Mineralocorticoid receptor modulation by dietary sodium influences NAFLD development in mice. Ann. Hepatol. 2021, 24, 100357. [Google Scholar] [CrossRef] [PubMed]
- Parksook, W.W.; Heydarpour, M.; Gholami, S.K.; Luther, J.M.; Hopkins, P.N.; Pojoga, L.H.; Williams, J.S. Salt Sensitivity of Blood Pressure and Aldosterone: Interaction Between the Lysine-specific Demethylase 1 Gene, Sex, and Age. J. Clin. Endocrinol. Metab. 2022, 107, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T. Mineralocorticoid Receptors, Salt-Sensitive Hypertension, and Metabolic Syndrome. Hypertension 2010, 55, 813–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funder, J.W. Aldosterone and Mineralocorticoid Receptors-Physiology and Pathophysiology. Int. J. Mol. Sci. 2017, 18, 1032. [Google Scholar] [CrossRef] [Green Version]
- Martus, W.; Kim, D.; Garvin, J.L.; Beierwaltes, W.H. Commercial rodent diets contain more sodium than rats need. Am. J. Physiol. -Ren. Physiol. 2005, 288, F428–F431. [Google Scholar] [CrossRef] [Green Version]
- Bonny, O.; Rossier, B.C. Disturbances of Na/K Balance: Pseudohypoaldosteronism Revisited. J. Am. Soc. Nephrol. 2002, 13, 2399. [Google Scholar] [CrossRef] [Green Version]
- Martens, P.; Ferreira, J.P.; Vincent, J.; Abreu, P.; Busselen, M.; Mullens, W.; Tang, W.W.H.; Bohm, M.; Pitt, B.; Zannad, F.; et al. Serum sodium and eplerenone use in patients with a myocardial infarction and left ventricular dysfunction or heart failure: Insights from the EPHESUS trial. Clin. Res. Cardiol. 2022, 111, 380–392. [Google Scholar] [CrossRef]
- Girndt, M. Electrolyte disorders. Internist 2011, 52, 963–974. [Google Scholar] [CrossRef]
- Olde Engberink, R.H.G.; Selvarajah, V.; Vogt, L. Clinical impact of tissue sodium storage. Pediatr. Nephrol. 2020, 35, 1373–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulyok, E.; Farkas, B.; Nagy, B.; Varnagy, A.; Kovacs, K. Tissue Sodium Accumulation: Pathophysiology and Clinical Implications. Tissue Sodium Accumul. Pathophysiol. Clin. Implic. 2022, 11, 750. [Google Scholar] [CrossRef]
- Nakajima, T.; Murata, M.; Nitta, S.; Shitara, T.; Kazama, H.; Satoh, Y.; Takizawa, M.; Mori, A.; Kobayashi, Y.; Adachi, H. Sodium Restriction Counseling Reduces Cardiac Events in Patients With Heart Failure. Circ. J. 2021, 85, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Gekle, M.; Grossmann, C. Actions of aldosterone in the cardiovascular system: The good, the bad, and the ugly? Pflug. Arch. 2009, 458, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Gratze, P.; Boschmann, M.; Dechend, R.; Qadri, F.; Malchow, J.; Graeske, S.; Engeli, S.; Janke, J.; Springer, J.; Contrepas, A.; et al. Energy Metabolism in Human Renin-Gene Transgenic Rats. Hypertension 2009, 53, 516–523. [Google Scholar] [CrossRef] [Green Version]
- de Kloet, A.D.; Krause, E.G.; Kim, D.H.; Sakai, R.R.; Seeley, R.J.; Woods, S.C. The Effect of Angiotensin-Converting Enzyme Inhibition Using Captopril on Energy Balance and Glucose Homeostasis. Endocrinology 2009, 150, 4114–4123. [Google Scholar] [CrossRef] [Green Version]
- Potere, N.; Del Buono, M.G.; Vecchie, A.; Porreca, E.; Abbate, A.; Dentali, F.; Bonaventura, A. Diabetes mellitus and heart failure: An update on pathophysiology and therapy. Minerva Cardiol. Angiol. 2022, 70, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Durango, N.; Vecchiola, A.; Gonzalez-Gomez, L.M.; Simon, F.; Riedel, C.A.; Fardella, C.E.; Kalergis, A.M. Modulation of Immunity and Inflammation by the Mineralocorticoid Receptor and Aldosterone. Biomed. Res. Int. 2015, 2015, 652738. [Google Scholar] [CrossRef] [Green Version]
- Funder, J.W. Mineralocorticoid receptor activation and oxidative stress. Hypertension 2007, 50, 840–841. [Google Scholar] [CrossRef] [Green Version]
- Eskurza, I.; Myerburgh, L.A.; Kahn, Z.D.; Seals, D.R. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J. Physiol. 2005, 568, 1057–1065. [Google Scholar] [CrossRef]
- Adler, A.; Messina, E.; Sherman, B.; Wang, Z.; Huang, H.; Linke, A.; Hintze, T.H. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am. J. Physiol.-Heart Circ. Physiol. 2003, 285, H1015–H1022. [Google Scholar] [CrossRef]
- Keidar, S.; Kaplan, M.; Pavlotzky, E.; Coleman, R.; Hayek, T.; Hamoud, S.; Aviram, M. Aldosterone Administration to Mice Stimulates Macrophage NADPH Oxidase and Increases Atherosclerosis Development. Circulation 2004, 109, 2213–2220. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Li, J.; Zhai, Y.; Li, Q.; Xie, D.; Zhang, J.; Xiao, Y.; Gao, X. Spironolactone suppresses aldosterone-induced Kv1.5 expression by attenuating mineralocorticoid receptor-Nox1/2/4-mediated ROS generation in neonatal rat atrial myocytes. Biochem. Biophys. Res. Commun. 2019, 520, 379–384. [Google Scholar] [CrossRef]
- Matsushima, S.; Sadoshima, J. Yin and Yang of NADPH Oxidases in Myocardial Ischemia-Reperfusion. Antioxidants 2022, 11, 1069. [Google Scholar] [CrossRef]
- Dragasevic, N.; Jakovljevic, V.; Zivkovic, V.; Draginic, N.; Andjic, M.; Bolevich, S.; Jovic, S. The role of aldosterone inhibitors in cardiac ischemia-reperfusion injury. Can. J. Physiol. Pharm. 2021, 99, 18–29. [Google Scholar] [CrossRef]
- Fraccarollo, D.; Berger, S.; Galuppo, P.; Kneitz, S.; Hein, L.; Schutz, G.; Frantz, S.; Ertl, G.; Bauersachs, J. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 2011, 123, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Bienvenu, L.A.; Reichelt, M.E.; Morgan, J.; Fletcher, E.K.; Bell, J.R.; Rickard, A.J.; Delbridge, L.M.; Young, M.J. Cardiomyocyte Mineralocorticoid Receptor Activation Impairs Acute Cardiac Functional Recovery After Ischemic Insult. Hypertension 2015, 66, 970–977. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; Tissier, R.; Ghaleh, B.; Drogies, T.; Felix, S.B.; Krieg, T. Cardioprotective effects of mineralocorticoid receptor antagonists at reperfusion. Eur. Heart J. 2010, 31, 1655–1662. [Google Scholar] [CrossRef]
- Pascual-Le Tallec, L.; Kirsh, O.; Lecomte, M.C.; Viengchareun, S.; Zennaro, M.C.; Dejean, A.; Lombes, M. Protein Inhibitor of Activated Signal Transducer and Activator of Transcription 1 Interacts with the N-Terminal Domain of Mineralocorticoid Receptor and Represses Its Transcriptional Activity: Implication of Small Ubiquitin-Related Modifier 1 Modification. Mol. Endocrinol. 2003, 17, 2529–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, B.E.; Holaska, J.M.; Rastinejad, F.; Paschal, B.M. DNA binding domains in diverse nuclear receptors function as nuclear export signals. Curr. Biol. 2001, 11, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Le Tallec, L.; Lombes, M. The mineralocorticoid receptor: A journey exploring its diversity and specificity of action. Mol. Endocrinol. 2005, 19, 2211–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Diaz, I.; Giraldez, T.; Arnau, M.ü.R.; Smits, V.A.J.; Jaisser, F.; Farman, N.; Alvarez de la Rosa, D. The Mineralocorticoid Receptor Is a Constitutive Nuclear Factor in Cardiomyocytes due to Hyperactive Nuclear Localization Signals. Endocrinology 2010, 151, 3888–3899. [Google Scholar] [CrossRef] [PubMed]
- Vienchareun, S.; Le Menuet, D.; Martinerie, L.; Munier, M.; Pascual-Le Tallec, L.; Lombes, M. The mineralocorticoid receptor: Insights into its molecular and (patho)physiological biology. Nucl. Recept. Signal. 2007, 30, e012. [Google Scholar]
- Li, Y.; Suino, K.; Daugherty, J.; Xu, H.E. Structural and Biochemical Mechanisms for the Specificity of Hormone Binding and Coactivator Assembly by Mineralocorticoid Receptor. Mol. Cell 2005, 19, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Lombes, M.; Binart, N.; Delahaye, F.; Baulieu, E.E.; Rafestin-Oblin, M.E. Differential intracellular localization of human mineralocorticosteroid receptor on binding of agonists and antagonists. Biochem. J. 1994, 302, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Rafestin-Oblin, M.E.; Couette, B.; Radanyi, C.; Lombes, M.; Baulieu, E.E. Mineralocorticosteroid receptor of the chick intestine. Oligomeric structure and transformation. J. Biol. Chem. 1989, 264, 9304–9309. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Erlejman, A.G.; Monte, M.; Gomez-Sanchez, C.; Piwien-Pilipuk, G. The hsp90-FKBP52 Complex Links the Mineralocorticoid Receptor to Motor Proteins and Persists Bound to the Receptor in Early Nuclear Events. Mol. Cell Biol. 2010, 30, 1285–1298. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, T.; Ohara-Nemoto, Y.; Sato, N.; Ota, M. Dual roles of 90-kDa heat shock protein in the function of the mineralocorticoid receptor. J. Biochem. 1993, 113, 769–775. [Google Scholar]
- Grossmann, C.; Ruhs, S.; Langenbruch, L.; Mildenberger, S.; Straetz, N.; Schumann, K.; Gekle, M. Nuclear Shuttling Precedes Dimerization in Mineralocorticoid Receptor Signaling. Chem. Biol. 2012, 19, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Nishi, M.; Tanaka, M.; Matsuda, K.i.; Sunaguchi, M.; Kawata, M. Visualization of Glucocorticoid Receptor and Mineralocorticoid Receptor Interactions in Living Cells with GFP-Based Fluorescence Resonance Energy Transfer. J. Neurosci. 2004, 24, 4918–4927. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, C.; Krug, A.W.; Freudinger, R.; Mildenberger, S.; Voelker, K.; Gekle, M. Aldosterone-induced EGFR expression: Interaction between the human mineralocorticoid receptor and the human EGFR promoter. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1790–E1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obradovic, D.; Tirard, M.; Nemethy, Z.; Hirsch, O.; Gronemeyer, H.; Almeida, O.F. DAXX, FLASH, and FAF-1 modulate mineralocorticoid and glucocorticoid receptor-mediated transcription in hippocampal cells--toward a basis for the opposite actions elicited by two nuclear receptors? Mol. Pharm. 2004, 65, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Ruhs, S.; Nolze, A.; Huebschmann, R.; Grossmann, C. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Nongenomic effects via the mineralocorticoid receptor. J. Endocrinol. 2017, 234, T107–T124. [Google Scholar] [CrossRef] [Green Version]
- Funder, J.W. The nongenomic actions of aldosterone. Endocr. Rev. 2005, 26, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, C.; Gekle, M. Non-classical actions of the mineralocorticoid receptor: Misuse of EGF receptors? Mol. Cell. Endocrinol. 2007, 277, 6–12. [Google Scholar] [CrossRef]
- Gadasheva, Y.; Nolze, A.; Grossmann, C. Posttranslational Modifications of the Mineralocorticoid Receptor and Cardiovascular Aging. Front Mol Biosci 2021, 28, 667990. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, N.; Blind, R.; Garabedian, M.J. Stabilization of the Unliganded Glucocorticoid Receptor by TSG101. J. Biol. Chem. 2005, 280, 11120–11126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokota, K.; Shibata, H.; Kobayashi, S.; Suda, N.; Murai, A.; Kurihara, I.; Saito, I.; Saruta, T. Proteasome−Mediated Mineralocorticoid Receptor Degradation Attenuates Transcriptional Response to Aldosterone. Endocr. Res. 2004, 30, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Faresse, N.; Ruffieux-Daidie, D.; Salamin, M.; Gomez-Sanchez, C.E.; Staub, O. Mineralocorticoid receptor degradation is promoted by Hsp90 inhibition and the ubiquitin-protein ligase CHIP. Am. J. Physiol. Ren. Physiol. 2010, 299, F1462–F1472. [Google Scholar] [CrossRef] [Green Version]
- Yokota, K.; Shibata, H.; Kurihara, I.; Kobayashi, S.; Suda, N.; Murai-Takeda, A.; Saito, I.; Kitagawa, H.; Kato, S.; Saruta, T.; et al. Coactivation of the N-terminal Transactivation of Mineralocorticoid Receptor by Ubc9. J. Biol. Chem. 2007, 282, 1998–2010. [Google Scholar] [CrossRef] [Green Version]
- Tirard, M.; Almeida, O.F.X.; Hutzler, P.; Melchior, F.; Michaelidis, T.M. Sumoylation and proteasomal activity determine the transactivation properties of the mineralocorticoid receptor. Mol. Cell. Endocrinol. 2007, 268, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Faresse, N.; Vitagliano, J.J.; Staub, O. Differential ubiquitylation of the mineralocorticoid receptor is regulated by phosphorylation. FASEB J. 2012, 26, 4373–4382. [Google Scholar] [CrossRef]
- Nagarajan, S.; Vohra, T.; Loffing, J.; Faresse, N. Protein Phosphatase 1 alpha enhances renal aldosterone signaling via mineralocorticoid receptor stabilization. Mol. Cell. Endocrinol. 2017, 450, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Galigniana, M.D. Native rat kidney mineralocorticoid receptor is a phosphoprotein whose transformation to a DNA-binding form is induced by phosphatases. Biochem. J. 1998, 333, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Shibata, S.; Rinehart, J.; Zhang, J.; Moeckel, G.; Castaneda-Bueno, M.; Stiegler, A.L.; Boggon, T.J.; Gamba, G.; Lifton, R.P. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013, 18, 660–671. [Google Scholar] [CrossRef]
- Faresse, N. Post-translational modifications of the mineralocorticoid receptor: How to dress the receptor according to the circumstances? J. Steroid Biochem. Mol. Biol. 2014, 143, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Ishizawa, K.; Wang, Q.; Xu, N.; Fujita, T.; Uchida, S.; Lifton, R.P. ULK1 Phosphorylates and Regulates Mineralocorticoid Receptor. Cell Rep. 2018, 24, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Ayuzawa, N.; Nagase, M.; Ueda, K.; Nishimoto, M.; Kawarazaki, W.; Marumo, T.; Aiba, A.; Sakurai, T.; Shindo, T.; Fujita, T. Rac1-Mediated Activation of Mineralocorticoid Receptor in Pressure Overload-Induced Cardiac Injury. Hypertension 2016, 67, 99–106. [Google Scholar] [CrossRef]
- Shibata, S.; Nagase, M.; Yoshida, S.; Kawarazaki, W.; Kurihara, H.; Tanaka, H.; Miyoshi, J.; Takai, Y.; Fujita, T. Modification of mineralocorticoid receptor function by Rac1 GTPase: Implication in proteinuric kidney disease. Nat. Med. 2008, 14, 1370–1376. [Google Scholar] [CrossRef]
- Nagase, M.; Fujita, T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat. Rev. Nephrol. 2013, 9, 86–98. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Cho, H.; Kim, S.; Iwasaki, Y.; Kim, I.K. Histone Deacetylase Inhibition Attenuates Transcriptional Activity of Mineralocorticoid Receptor Through Its Acetylation and Prevents Development of Hypertension. Circ. Res. 2013, 112, 1004–1012. [Google Scholar] [CrossRef] [Green Version]
- Fuller, P.J.; Yang, J.; Young, M.J. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Coregulators as mediators of mineralocorticoid receptor signalling diversity. J. Endocrinol. 2017, 234, T23–T34. [Google Scholar] [CrossRef] [Green Version]
- Fuse, H.; Kitagawa, H.; Kato, S. Characterization of transactivational property and coactivator mediation of rat mineralocorticoid receptor activation function-1 (AF-1). Mol. Endocrinol. 2000, 14, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Seok, Y.M.; Song, M.J.; Lee, H.A.; Kurz, T.; Kim, I. Histone deacetylase inhibition attenuates cardiac hypertrophy and fibrosis through acetylation of mineralocorticoid receptor in spontaneously hypertensive rats. Mol. Pharm. 2015, 87, 782–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghe, W.V.; De Bosscher, K.; Boone, E.; Plaisance, S.p.; Haegeman, G. The Nuclear Factor-κB Engages CBP/p300 and Histone Acetyltransferase Activity for Transcriptional Activation of the Interleukin-6 Gene Promoter*. J. Biol. Chem. 1999, 274, 32091–32098. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation and aging. Free Radic. Res. 2006, 40, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Galigniana, M.D.; Piwien-Pilipuk, G. Comparative inhibition by hard and soft metal ions of steroid-binding capacity of renal mineralocorticoid receptor cross-linked to the 90-kDa heat-shock protein heterocomplex. Biochem. J. 1999, 341, 585–592. [Google Scholar] [CrossRef]
- Piwien-Pilipuk, G.; Ayala, A.; Machado, A.; Galigniana, M.D. Impairment of Mineralocorticoid Receptor (MR)-dependent Biological Response by Oxidative Stress and Aging: Correlation with Post-translational Modification of MR and Decreased ADP-ribosylatatable Level of Elongation Factor 2 in Kidney Cellls. J. Biol. Chem. 2002, 277, 11896–11903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piwien-Pilipuk, G.; Galigniana, M.D. Oxidative stress induced by L-buthionine-(S,R)-sulfoximine, a selective inhibitor of glutathione metabolism, abrogates mouse kidney mineralocorticoid receptor function. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2000, 1495, 263–280. [Google Scholar] [CrossRef] [Green Version]
- Auboeuf, D.; Batsché, E.; Dutertre, M.; Muchardt, C.; O’Malley, B.W. Coregulators: Transducing signal from transcription to alternative splicing. Trends Endocrinol. Metab. 2007, 18, 122–129. [Google Scholar] [CrossRef]
- O’Malley, B.W. Origins of the Field of Molecular Endocrinology: A Personal Perspective. Mol. Endocrinol. 2016, 30, 1015–1018. [Google Scholar] [PubMed] [Green Version]
- McInerney, E.M.; Rose, D.W.; Flynn, S.E.; Westin, S.; Mullen, T.M.; Krones, A.; Inostroza, J.; Torchia, J.; Nolte, R.T.; Assa-Munt, N.; et al. Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation. Genes Dev. 1998, 12, 3357–3368. [Google Scholar] [CrossRef] [Green Version]
- Rogerson, F.M.; Yao, Y.Z.; Young, M.J.; Fuller, P.J. Identification and characterization of a ligand-selective mineralocorticoid receptor coactivator. FASEB J. 2014, 28, 4200–4210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Fuller, P.J.; Morgan, J.; Shibata, H.; Clyne, C.D.; Young, M.J. GEMIN4 functions as a coregulator of the mineralocorticoid receptor. J. Mol. Endocrinol. 2015, 54, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fuller, P.J.; Morgan, J.; Shibata, H.; McDonnell, D.P.; Clyne, C.D.; Young, M.J. Use of phage display to identify novel mineralocorticoid receptor-interacting proteins. Mol. Endocrinol. 2014, 28, 1571–1584. [Google Scholar] [CrossRef]
- Kohata, N.; Kurihara, I.; Yokota, K.; Kobayashi, S.; Murai-Takeda, A.; Mitsuishi, Y.; Nakamura, T.; Morisaki, M.; Kozuma, T.; Torimitsu, T.; et al. Lysine-specific demethylase 1 as a corepressor of mineralocorticoid receptor. Hypertens. Res. 2022, 45, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Malagraba, G.; Yarmohammadi, M.; Javed, A.; Barcelo, C.; Rubio-Tomas, T. The Role of LSD1 and LSD2 in Cancers of the Gastrointestinal System: An Update. Biomolecules 2022, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Le Tallec, L.; Simone, F.; Viengchareun, S.; Meduri, G.; Thirman, M.J.; Lombes, M. The elongation factor ELL (eleven-nineteen lysine-rich leukemia) is a selective coregulator for steroid receptor functions. Mol. Endocrinol. 2005, 19, 1158–1169. [Google Scholar] [CrossRef] [Green Version]
- Hultman, M.L.; Krasnoperova, N.V.; Li, S.; Du, S.; Xia, C.; Dietz, J.D.; Lala, D.S.; Welsch, D.J.; Hu, X. The Ligand-Dependent Interaction of Mineralocorticoid Receptor with Coactivator and Corepressor Peptides Suggests Multiple Activation Mechanisms. Mol. Endocrinol. 2005, 19, 1460–1473. [Google Scholar] [CrossRef] [Green Version]
- Knutti, D.; Kaul, A.; Kralli, A. A Tissue-Specific Coactivator of Steroid Receptors, Identified in a Functional Genetic Screen. Mol. Cell Biol. 2000, 20, 2411–2422. [Google Scholar] [CrossRef] [Green Version]
- Borniquel, S.; Valle, I.; Cadenas, S.; Lamas, S.; Monsalve, M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J. 2006, 20, 1889–1891. [Google Scholar] [CrossRef] [PubMed]
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-Nino, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1alpha and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Syed, S.N.; Brune, B. Exosomal and Non-Exosomal MicroRNAs: New Kids on the Block for Cancer Therapy. Int. J. Mol. Sci. 2022, 9, 4493. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Sengar, R.S. Biogenesis and mechanisms of microRNA-mediated gene regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef]
- Kawamata, T.; Tomari, Y. Making RISC. Trends Biochem. Sci. 2010, 35, 368–376. [Google Scholar] [CrossRef]
- Vilimova, M.; Pfeffer, S. Post-transcriptional regulation of polycistronic microRNAs. WIREs RNA, 2022; e1749, epub ahead of print. [Google Scholar] [CrossRef]
- de Lucia, C.; Komici, K.; Borghetti, G.; Femminella, G.D.; Bencivenga, L.; Cannavo, A.; Corbi, G.; Ferrara, N.; Houser, S.R.; Koch, W.J.; et al. microRNA in Cardiovascular Aging and Age-Related Cardiovascular Diseases. Front. Med. 2017, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, M.B. Non-coding RNAs and the mineralocorticoid receptor in the kidney. Mol. Cell. Endocrinol. 2021, 521, 111115. [Google Scholar] [CrossRef]
- Rezaei, M.; Andrieu, T.; Neuenschwander, S.; Bruggmann, R.; Mordasini, D.; Frey, F.J.; Vogt, B.; Frey, B.M. Regulation of 11beta-hydroxysteroid dehydrogenase type 2 by microRNA. Hypertension 2014, 64, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Ozbaki-Yagan, N.; Liu, X.; Bodnar, A.J.; Ho, J.; Butterworth, M.B. Aldosterone-induced microRNAs act as feedback regulators of mineralocorticoid receptor signaling in kidney epithelia. FASEB J. 2020, 34, 11714–11728. [Google Scholar] [CrossRef]
- Sober, S.; Laan, M.; Annilo, T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem. Biophys. Res. Commun. 2010, 391, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Mannironi, C.; Camon, J.; De Vito, F.; Biundo, A.; De Stefano, M.E.; Persiconi, I.; Bozzoni, I.; Fragapane, P.; Mele, A.; Presutti, C. Acute stress alters amygdala microRNA miR-135a and miR-124 expression: Inferences for corticosteroid dependent stress response. PLoS ONE 2013, 8, e73385. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, C.; Li, J.; Che, J.; Yang, X.; Xian, Y.; Li, X.; Cao, C. Long non-coding RNA MALAT1 promotes cardiac remodeling in hypertensive rats by inhibiting the transcription of MyoD. Aging 2019, 11, 8792–8809. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Hu, P.; Wu, J. Long Non-Coding RNA Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) Promotes Hypertension by Modulating the Hsa-miR-124-3p/Nuclear Receptor Subfamily 3, Group C, Member 2 (NR3C2) and Hsa-miR-135a-5p/NR3C2 Axis. Aging 2020, 26, e920478. [Google Scholar] [CrossRef]
- Nong, R.; Qin, C.; Lin, Q.; Lu, Y.; Li, J. Down-regulated HDAC1 and up-regulated microRNA-124-5p recover myocardial damage of septic mice. Bioengineered 2022, 13, 7168–7180. [Google Scholar] [CrossRef]
- Yang, C.; Ma, X.; Guan, G.; Liu, H.; Yang, Y.; Niu, Q.; Wu, Z.; Jiang, Y.; Bian, C.; Zang, Y.; et al. MicroRNA-766 promotes cancer progression by targeting NR3C2 in hepatocellular carcinoma. FASEB J. 2019, 33, 1456–1467. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kawasaki, M.; Hirai, T.; Yoshida, Y.; Tsushima, H.; Fujishiro, M.; Ikeda, K.; Morimoto, S.; Takamori, K.; Sekigawa, I. MicroRNA-766-3p Contributes to Anti-Inflammatory Responses through the Indirect Inhibition of NF-kappaB Signaling. Int. J. Mol. Sci. 2019, 20, 809. [Google Scholar] [CrossRef] [Green Version]
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Roxe, T.; Muller-Ardogan, M.; et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Wang, P.; Zhao, C.; Yin, G.; Meng, X.; Li, L.; Cai, S.; Meng, B. MicroRNA-155-5p Targets NR3C2 to Promote Malignant Progression of Clear Cell Renal Cell Carcinoma. Kidney Blood Press Res. 2022, 47, 354–362. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Qin, Z.; Liu, N.; Zhang, Z.; Lu, Y.; Xu, Y.; Zhang, J.; Tang, J. Diagnostic and Predictive Values of Circulating Extracellular Vesicle-Carried microRNAs in Ischemic Heart Disease Patients With Type 2 Diabetes Mellitus. Front. Cardiovasc. Med 2022, 9, 813310. [Google Scholar] [CrossRef]
- Marques, F.Z.; Vizi, D.; Khammy, O.; Mariani, J.A.; Kaye, D.M. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur. J. Heart Fail. 2016, 18, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Foinquinos, A.; Jung, M.; Janssen-Peters, H.; Biss, S.; Bauersachs, J.; Gupta, S.K.; Thum, T. MiRNA-181a is a novel regulator of aldosterone-mineralocorticoid receptor-mediated cardiac remodelling. Eur. J. Heart Fail. 2020, 22, 1366–1377. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, J.; Zhang, F.; Lei, Q.; Zhang, T.; Li, K.; Guo, J.; Hong, Y.; Bu, G.; Lv, X.; et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Leroy, V.; De Seigneux, S.; Agassiz, V.; Hasler, U.; Rafestin-Oblin, M.E.; Vinciguerra, M.; Martin, P.Y.; Feraille, E. Aldosterone activates NF-kappaB in the collecting duct. J. Am. Soc. Nephrol. 2009, 20, 131–144. [Google Scholar] [CrossRef]
- Li, A.L.; Lv, J.B.; Gao, L. MiR-181a mediates Ang II-induced myocardial hypertrophy by mediating autophagy. Eur. Rev. Med. Pharm. Sci. 2017, 21, 5462–5470. [Google Scholar]
- Guo, J.Y.; Wang, Y.K.; Lv, B.; Jin, H. miR-454 performs tumor-promoting effects in oral squamous cell carcinoma via reducing NR3C2. J. Oral. Pathol. Med. 2020, 49, 286–293. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, W.; Bai, X.; Wang, X.; Wang, Y.; Yin, Y. microRNA-454-mediated NEDD4-2/TrkA/cAMP axis in heart failure: Mechanisms and cardioprotective implications. J. Cell Mol. Med. 2021, 25, 5082–5098. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- Palanisamy, S.; Funes Hernandez, M.; Chang, T.I.; Mahaffey, K.W. Cardiovascular and Renal Outcomes with Finerenone, a Selective Mineralocorticoid Receptor Antagonist. Cardiol. Ther. 2022, 3, 337–354. [Google Scholar] [CrossRef]
- Milliez, P.; Girerd, X.; Plouin, P.F.; Blacher, J.; Safar, M.E.; Mourad, J.J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Liu, P.Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Cao, T.; Ding, W.; Liang, L.; Fan, G.C.; Qu, L.; Peng, T. Pharmacological inhibition of Rac1 attenuates myocardial abnormalities in tail-suspended mice. J. Cardiovasc. Transl. Res. 2022; epub ahead of print. [Google Scholar] [CrossRef]
- Furuzono, S.; Meguro, M.; Miyauchi, S.; Inoue, S.; Homma, T.; Yamada, K.; Tagawa, Y.i.; Nara, F.; Nagayama, T. A novel aldosterone synthase inhibitor ameliorates mortality in pressure-overload mice with heart failure. Eur. J. Pharmacol. 2017, 795, 58–65. [Google Scholar] [CrossRef]
- Cardama, G.A.; Alonso, D.F.; Gonzalez, N.; Maggio, J.; Gomez, D.E.; Rolfo, C.; Menna, P.L. Relevance of small GTPase Rac1 pathway in drug and radio-resistance mechanisms: Opportunities in cancer therapeutics. Crit. Rev. Oncol./Hematol. 2018, 124, 29–36. [Google Scholar] [CrossRef]
- Strum, S.W.; Gyenis, L.; Litchfield, D.W. CSNK2 in cancer: Pathophysiology and translational applications. Br. J. Cancer 2022, 7, 994–1003. [Google Scholar] [CrossRef]
- Sayed, A.; Abdelfattah, O.M.; Munir, M.; Shazly, O.; Awad, A.K.; Ghaith, H.S.; Moustafa, K.; Gerew, M.; Guha, A.; Barac, A.; et al. Long-term effectiveness of empiric cardio-protection in patients receiving cardiotoxic chemotherapies: A systematic review & bayesian network meta-analysis. Eur. J. Cancer 2022, 169, 82–92. [Google Scholar]
| Micromilieu Change | Effect on MR | Reference |
|---|---|---|
| high salt diet | Pre-clinical findings | |
| Elevated aldosterone levels cause cardiac fibrosis only on high/normal salt diet but not when deprived of sodium in rats and mice. | [14,15] | |
| MR antagonists mitigate high-salt diet-induced cardiac remodeling in Dahl salt-sensitive rats. | [16,17] | |
| The GTPase Rac1 is able to induce nuclear translocation of the MR and MR-dependent gene transcription in the absence of aldosterone in salt-induced kidney injury. | [18,19] | |
| Rac1 mediates NaCl-induced superoxide generation. | [20] | |
| Dysregulation of the MR corepressor LSD1 leads to salt-sensitivity and hypertension in rodents and humans. | [21] | |
| hyperglycemia | Pre-clinical findings | |
| High glucose levels upregulate MR expression and MR transcriptional activity in cell culture experiments. | [22,23] | |
| High glucose levels activate kinase PKC-beta, promoting MR expression and transcriptional activity and reducing ubiquitination in mice. | [22] | |
| High glucose induces Rac1 activation in mesangial cell culture. | [24] | |
| Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. | [25] | |
| TGF-beta1 induces expression of pathological genes involved in diabetic nephropathy via CBP (CREB-binding protein)/p300, a histone acetyltransferase and coactivator of the MR, in rat renal mesangial cells in high glucose conditions and in glomeruli from diabetic mice. | [26] | |
| LINC-P21, a long non-coding RNA known to be upregulated in patients with type 2 diabetes mellitus, upregulates MR expression in pancreatic islet cells. | [27] | |
| Clinical findings | ||
| The use of MR antagonist eplerenone leads to a significant reduction in instances of hospitalization and mortality in diabetic patients with post-acute myocardial infarction heart failure compared to non-diabetic patients. | [28] | |
| inflammation | Pre-clinical findings | |
| Costimulation of MR with cytokines and aldosterone induces the expression of inflammation-associated MR target genes. | [29] | |
| The expression of MR and CK2, a kinase phosphorylating the MR, thereby increasing its transcriptional activity, is enhanced in the presence of pro-inflammatory cytokines. | [29] | |
| IL-1beta influences MR signaling amplifying cardiac fibrosis in mice. | [30] | |
| TNF-alpha stimulates Rac1 activity. | [31] | |
| TNF-alpha, TWEAK, and interleukin 1 reduce the expression of the MR coactivator PGC-1alpha, which is associated with mitochondrial dysfunction and increased ROS accumulation. | [32,33,34,35] | |
| TNF-alpha and interleukin 1 affect the expression of MR coactivators SRC-1 and SRC-2. | [34,36] | |
| oxidative stress | Pre-clinical findings | |
| RNS accumulation in pathologically altered cardiovascular tissues can activate the MR by promoting its nuclear translocation. | [37] | |
| Upregulation of ubiquitin-protein-ligase CHIP marks the MR for proteasomal degradation under oxidative stress in HeLa cells. | [38] | |
| SUMO E1 and E2 enzymes that also mark the MR for sumoylation and degradation are affected by redox changes caused by ROS. | [39] | |
| Oxidative stress causes MR activation in rat cardiomyocytes via Rac1. | [40] | |
| Under oxidative stress, glucocorticoids can promote cardiac fibrosis through MR via upregulated expression of its coactivator ELL and binding of ELL to the MR in rat cardiac fibroblasts. | [41] | |
| hypoxia, ischemia and reperfusion | Pre-clinical findings | |
| Dysregulation of the MR corepressor LSD1 plays a role in myocardial, cerebral, and renal ischemia reperfusion injury by stabilizing HIF-1alpha. | [42,43,44,45] | |
| MR coactivator ELL and HIF-1alpha modulate each other’s function during hypoxia response in cancer cells. | [46] | |
| In neuronal cells, SOX2OT and Gm11974, long non-coding RNAs that upregulate MR expression, are upregulated upon ischemia/reperfusion. | [47,48] | |
| Clinical findings | ||
| Post-MI inhibition with eplerenone reduces mortality and morbidity. | [7] | |
| Peri- and post-reperfusion MR inhibition can improve the ejection fraction and the left ventricular end diastolic and end systolic volume. | [49] | |
| Administration of spinolactone after MI can significantly reduce fibrosis markers. | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griesler, B.; Schuelke, C.; Uhlig, C.; Gadasheva, Y.; Grossmann, C. Importance of Micromilieu for Pathophysiologic Mineralocorticoid Receptor Activity—When the Mineralocorticoid Receptor Resides in the Wrong Neighborhood. Int. J. Mol. Sci. 2022, 23, 12592. https://doi.org/10.3390/ijms232012592
Griesler B, Schuelke C, Uhlig C, Gadasheva Y, Grossmann C. Importance of Micromilieu for Pathophysiologic Mineralocorticoid Receptor Activity—When the Mineralocorticoid Receptor Resides in the Wrong Neighborhood. International Journal of Molecular Sciences. 2022; 23(20):12592. https://doi.org/10.3390/ijms232012592
Chicago/Turabian StyleGriesler, Bruno, Christin Schuelke, Christian Uhlig, Yekaterina Gadasheva, and Claudia Grossmann. 2022. "Importance of Micromilieu for Pathophysiologic Mineralocorticoid Receptor Activity—When the Mineralocorticoid Receptor Resides in the Wrong Neighborhood" International Journal of Molecular Sciences 23, no. 20: 12592. https://doi.org/10.3390/ijms232012592
APA StyleGriesler, B., Schuelke, C., Uhlig, C., Gadasheva, Y., & Grossmann, C. (2022). Importance of Micromilieu for Pathophysiologic Mineralocorticoid Receptor Activity—When the Mineralocorticoid Receptor Resides in the Wrong Neighborhood. International Journal of Molecular Sciences, 23(20), 12592. https://doi.org/10.3390/ijms232012592






