High-Molecular-Weight Dextran-Type Exopolysaccharide Produced by the Novel Apilactobacillus waqarii Improves Metabolic Syndrome: In Vitro and In Vivo Analyses
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Process Parameters for Exopolysaccharide Production by Bacteria
2.2. Monosaccharide Composition of the Exopolysaccharide
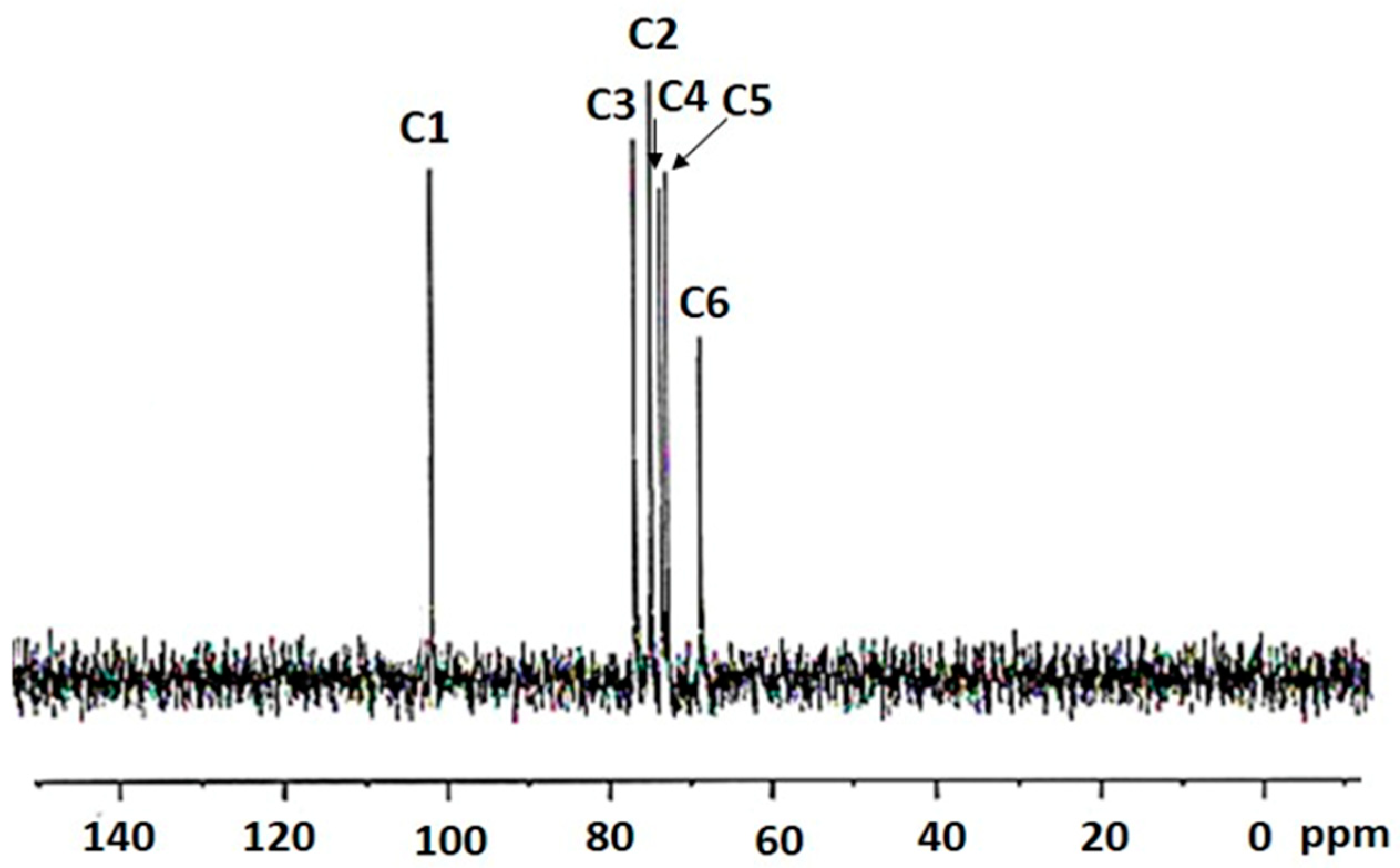
2.3. 13C NMR Spectroscopy of the Bacterial-Derived Glucan
2.4. Apilactobacillus waqarii Dextran-Type Glucan Linkage Analysis
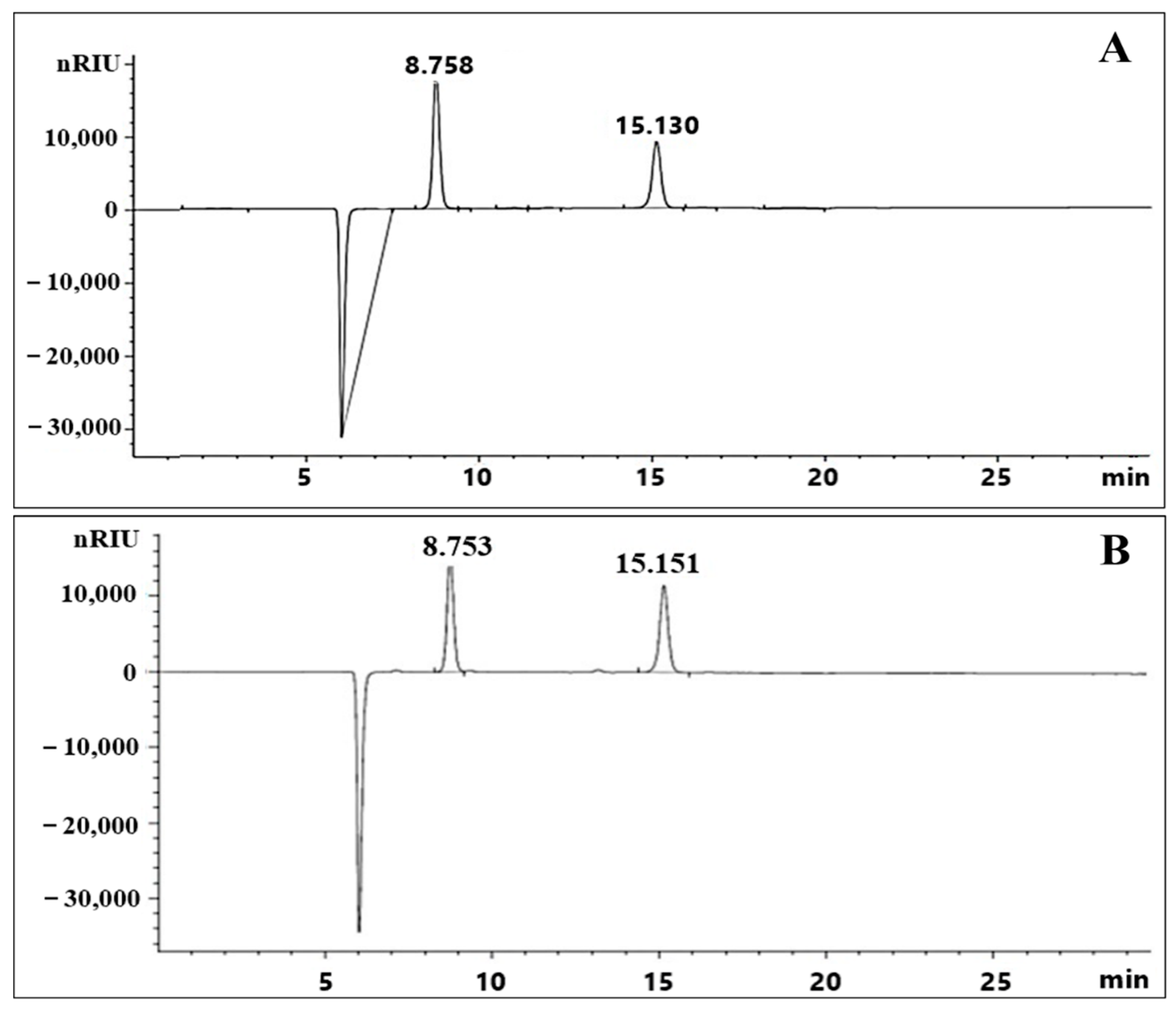
2.5. Exopolysaccharide Molecular Weight Distribution
2.6. Cell Lines Experiments
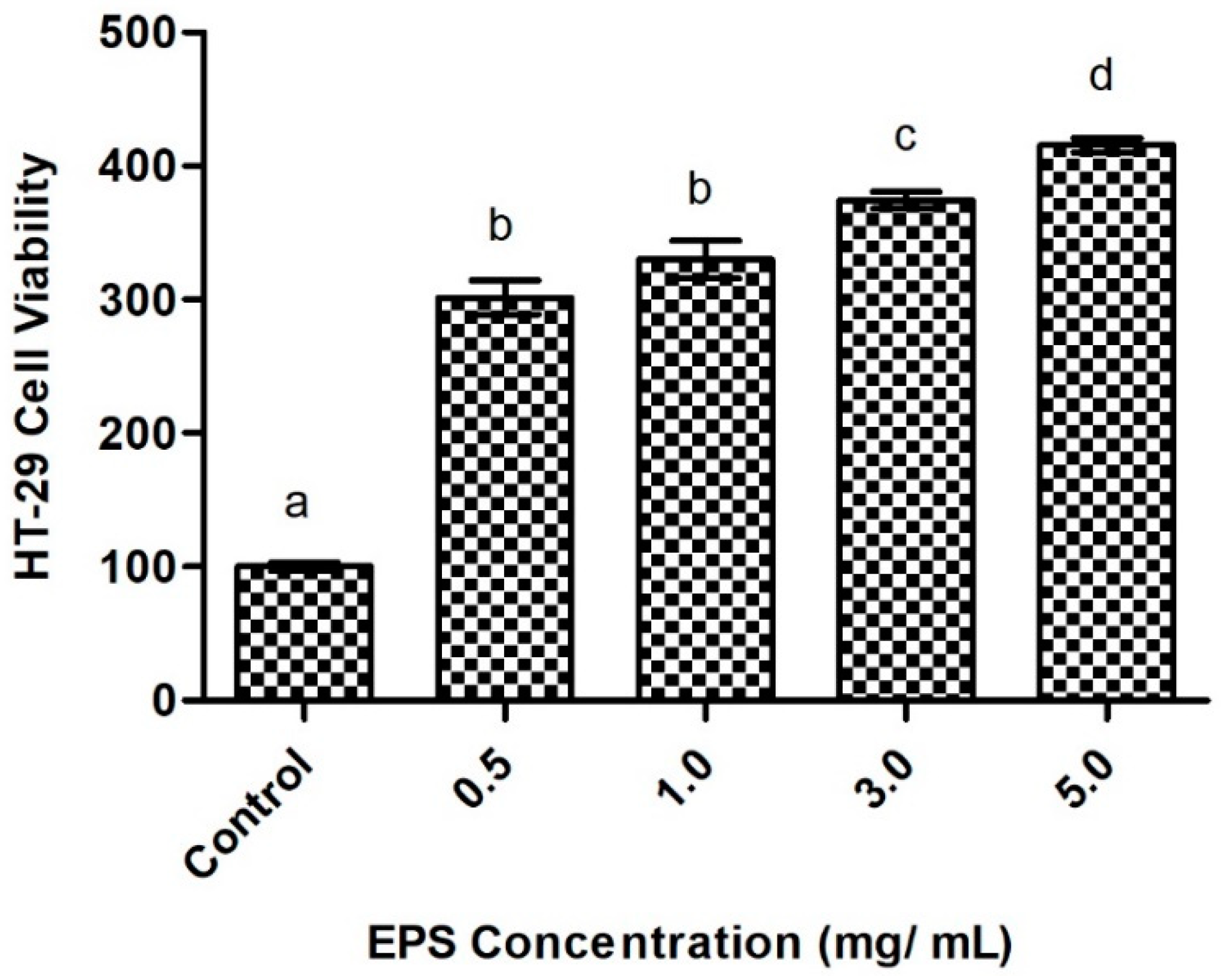
2.6.1. Intestinal Cell Viability in Response to Exopolysaccharide Exposure
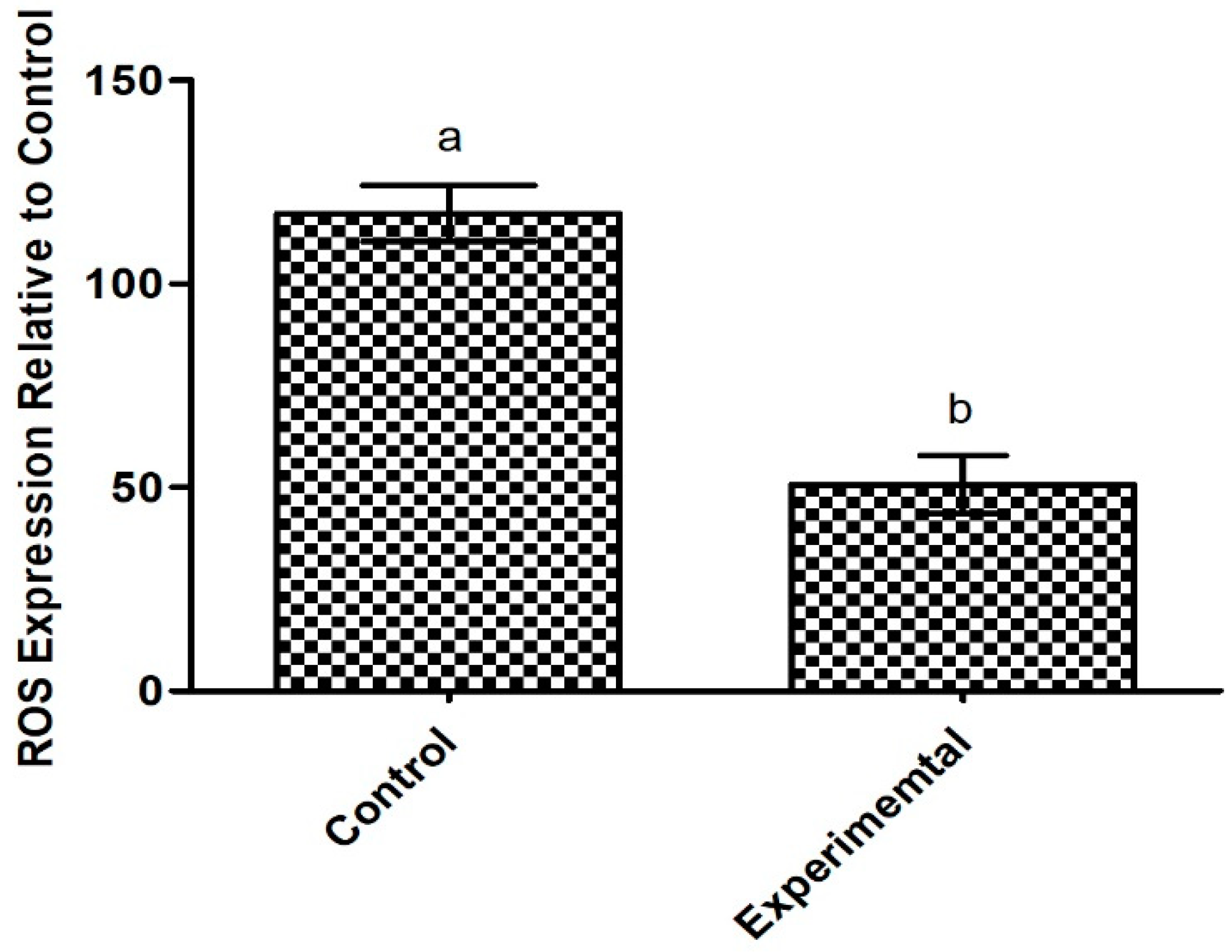
2.6.2. Antioxidant Potential of the Exopolysaccharide Produced by Apilactobacillus waqarii
2.7. Investigation of Metabolic Syndrome Pre-Clinical Efficacy in Experimental Animals
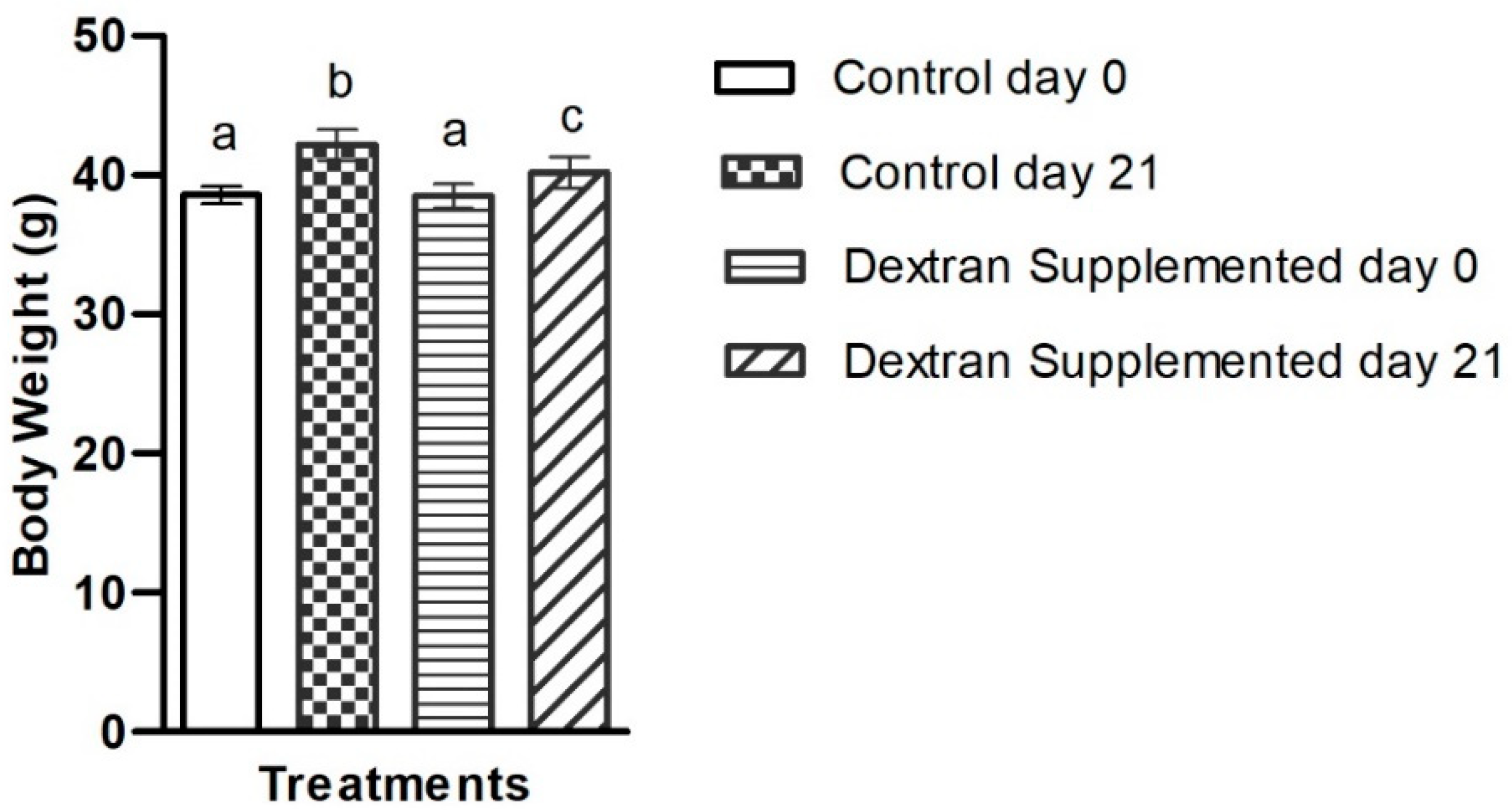
2.7.1. Determination of the Exopolysaccharide’s Impact on Body Weight
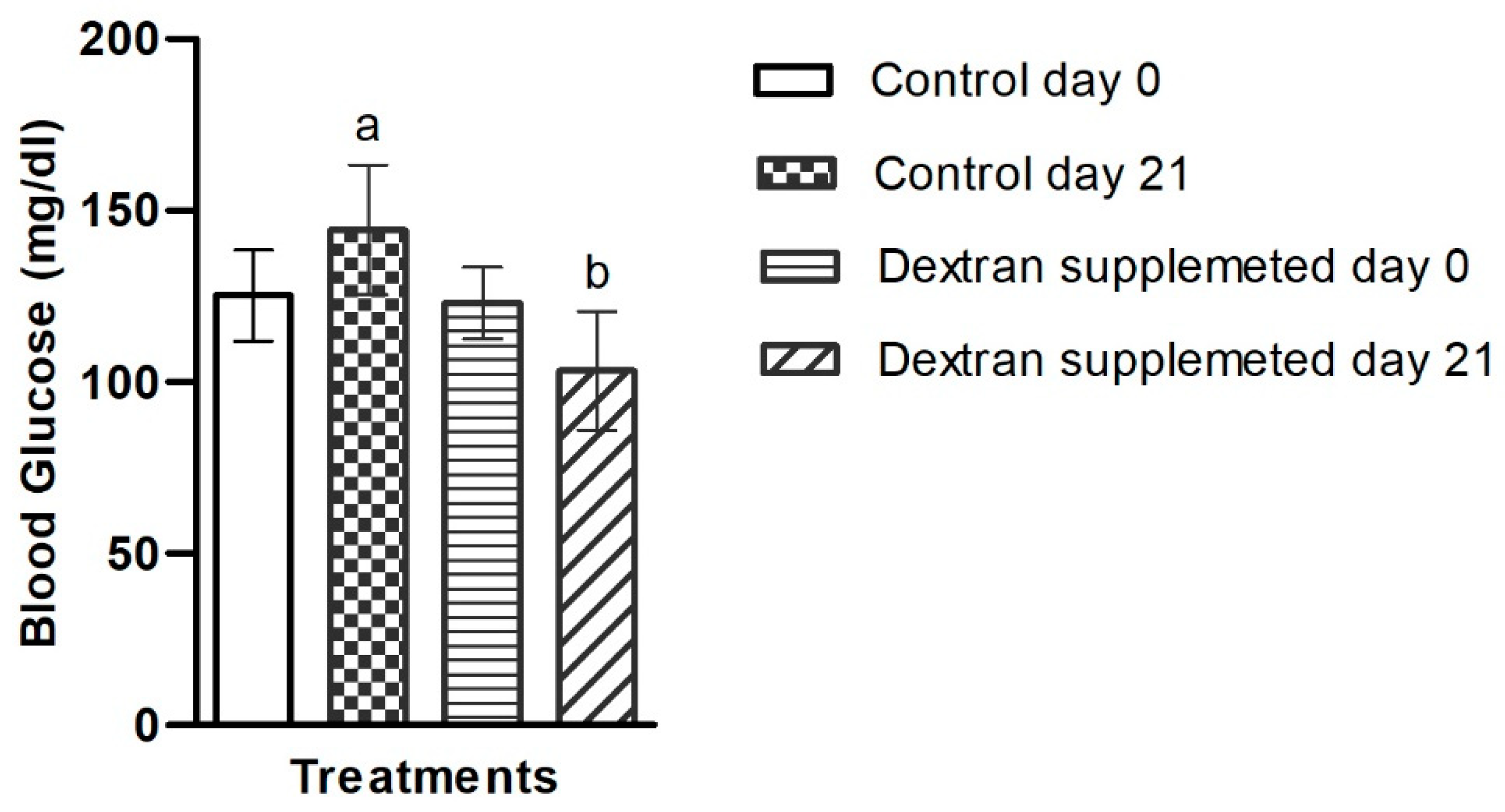
2.7.2. Investigation of Blood Glucose Level in Mice Treated with Bacterial Exopolysaccharide
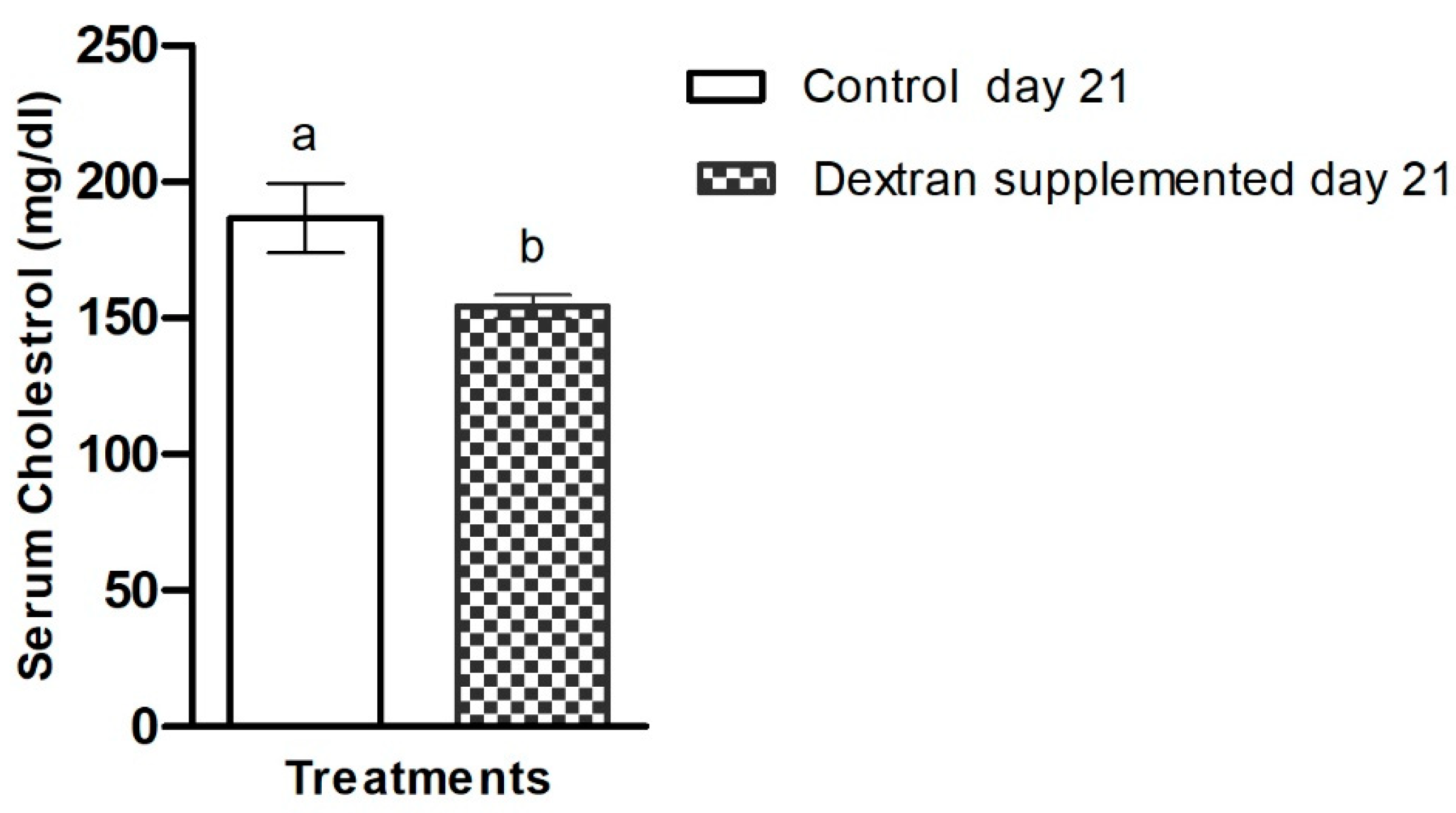
2.7.3. Investigation of Serum Cholesterol in Mice
3. Material and Methods
3.1. Screening and Identification of the Bacterial Isolate
3.2. Bacterial Optimization of Culturing Parameters
3.3. Extraction and Purification of EPS
3.4. Characterizations of EPS
3.4.1. Determination of Sugar Composition and Moisture
3.4.2. 13C NMR Spectroscopy
3.4.3. Methylation Analysis
3.4.4. Molecular Weight Determination
3.5. Cell Line Experiments
3.5.1. Cell Viability and Cytotoxicity
3.5.2. Intracellular Reactive Oxygen Species (ROS) Assay
3.6. Experiments on Mice Models
3.6.1. Formulation of Mice Feed
3.6.2. Animals and Diet Design
3.6.3. Determination of Body Weight
3.6.4. Blood Glucose Analysis
3.6.5. Serum Cholesterol Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Brown, W.V. Metabolic syndrome and risk of stroke. Clin. Cornerstone 2004, 6, S30–S34. [Google Scholar] [CrossRef]
- Shin, J.A.; Lee, J.H.; Lim, S.Y.; Ha, H.S.; Kwon, H.S.; Park, Y.M.; Lee, W.C.; Kang, M.I.; Yim, H.W.; Yoon, K.H.; et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J. Diabetes Investig. 2013, 4, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Chang, H.-T.; Tseng, Y.-H.; Chen, H.-S.; Chiang, S.-C.; Chen, T.-J.; Hwang, S.-J. Changes in metabolic syndrome affect the health-related quality of life of community-dwelling adults. Sci. Rep. 2021, 11, 20267. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Cordero, P.; Li, J.; Oben, J.A. Bariatric surgery as a treatment for metabolic syndrome. J. R. Coll. Phys. Edinb. 2017, 47, 364–368. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2019, 20, 128. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Lillich, F.F.; Imig, J.D.; Proschak, E. Multi-Target Approaches in Metabolic Syndrome. Front. Pharmacol. 2021, 11, 554961. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Cruz, A.G.; Cadena, R.S.; Walter, E.H.; Mortazavian, A.M.; Granato, D.; Faria, J.A.; Bolini, H.M. Sensory analysis: Relevance for prebiotic, probiotic, and synbiotic product development. Compr. Rev. Food Sci. Food Saf. 2010, 9, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Langella, P. Emerging health concepts in the probiotics field: Streamlining the definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.T.; Teixeira, M.M.; Martins, F.d.S. The role of probiotics and prebiotics in inducing gut immunity. Front. Immunol. 2013, 4, 445. [Google Scholar] [CrossRef]
- De Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Prebiotics: Trends in food, health and technological applications. Trends Food Sci. Technol. 2019, 93, 23–35. [Google Scholar] [CrossRef]
- Llavata, B.; García-Pérez, J.V.; Simal, S.; Cárcel, J.A. Innovative pre-treatments to enhance food drying: A current review. Curr. Opin. Food Sci. 2020, 35, 20–26. [Google Scholar] [CrossRef]
- Mohanty, D.; Misra, S.; Mohapatra, S.; Sahu, P.S. Prebiotics and synbiotics: Recent concepts in nutrition. Food Biosci. 2018, 26, 152–160. [Google Scholar] [CrossRef]
- Xie, X.; He, Y.; Li, H.; Yu, D.; Na, L.; Sun, T.; Zhang, D.; Shi, X.; Xia, Y.; Jiang, T. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition 2019, 61, 132–142. [Google Scholar] [CrossRef]
- Althubiani, A.S.; Al-Ghamdi, S.B.; Qais, F.A.; Khan, M.S.; Ahmad, I.; Malak, H.A. Plant-Derived Prebiotics and Its Health Benefits. In New Look to Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 63–88. [Google Scholar]
- Colantonio, A.G.; Werner, S.L.; Brown, M. The effects of prebiotics and substances with prebiotic properties on metabolic and inflammatory biomarkers in individuals with type 2 diabetes mellitus: A systematic review. J. Acad. Nutr. Diet. 2020, 120, 587–607.e2. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Meta-analysis of the effect of β-glucan intake on blood cholesterol and glucose levels. Nutrition 2011, 27, 1008–1016. [Google Scholar] [CrossRef]
- Wang, P.X.; Deng, X.R.; Zhang, C.H.; Yuan, H.J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef] [PubMed]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef] [PubMed]
- Tingirikari, J.M.R.; Kothari, D.; Goyal, A. Superior prebiotic and physicochemical properties of novel dextran from Weissella cibaria JAG8 for potential food applications. Food Funct. 2014, 5, 2324–2330. [Google Scholar] [CrossRef]
- Vettori, M.H.P.B.; Franchetti, S.M.M.; Contiero, J. Structural characterization of a new dextran with a low degree of branching produced by Leuconostoc mesenteroides FT045B dextransucrase. Carbohydr. Polym. 2012, 88, 1440–1444. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Q.; Guo, Y.; Han, Y.; Xiao, H.; Zhou, Z. Isolation and characterization of dextran produced by Leuconostoc citreum NM105 from manchurian sauerkraut. Carbohydr. Polym. 2015, 133, 365–372. [Google Scholar] [CrossRef]
- Paulo, E.M.; Boffo, E.F.; Branco, A.; Valente, Â.M.; Melo, I.S.; Ferreira, A.G.; Roque, M.R.; Assis, S.A.D. Production, extraction and characterization of exopolysaccharides produced by the native Leuconostoc pseudomesenteroides R2 strain. An. Da Acad. Bras. De Ciências 2012, 84, 495–508. [Google Scholar] [CrossRef]
- Ma’unatin, A.; Harijono, H.; Zubaidah, E.; Rifa’i, M.J.I.J.O.M. The isolation of exopolysaccharide-producing lactic acid bacteria from lontar (Borassus flabellifer L.) sap. Iran. J. Microbiol. 2020, 12, 437. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, F.; Zhou, Q.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Isolation, purification and characterization of exopolysaccharide produced by Leuconostoc pseudomesenteroides YF32 from soybean paste. Int. J. Biol. Macromol. 2018, 114, 529–535. [Google Scholar] [CrossRef]
- Sarwat, F.; Qader, S.A.U.; Aman, A.; Ahmed, N. Production & characterization of a unique dextran from an indigenous Leuconostoc mesenteroides CMG713. Int. J. Biol. Sci. 2008, 4, 379. [Google Scholar]
- Ahmed, R.Z.; Siddiqui, K.; Arman, M.; Ahmed, N. Characterization of high molecular weight dextran produced by Weissella cibaria CMGDEX3. Carbohydr. Polym. 2012, 90, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Bashari, M.; Lagnika, C.; Ocen, D.; Chen, H.; Wang, J.; Xu, X.; Jin, Z. Separation and characterization of dextran extracted from deteriorated sugarcane. Int. J. Biol. Macromol. 2013, 59, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Valafar, H.; Valafar, F. Identification of/sup 1/H-NMR spectra of N-linked oligosaccharides using artificial neural networks. In Proceedings of the First Joint BMES/EMBS Conference. 1999 IEEE Engineering in Medicine and Biology 21st Annual Conference and the 1999 Annual Fall Meeting of the Biomedical Engineering Society, Atlanta, GA, USA, 13–16 October 1999. [Google Scholar]
- Dols, M.; Remaud-Simeon, M.; Willemot, R.-M.; Vignon, M.; Monsan, P.F. Characterization of dextransucrases from Leuconostoc mesenteroides NRRL B-1299. Appl. Biochem. Biotechnol. 1997, 62, 47–59. [Google Scholar] [CrossRef]
- Han, J.; Hang, F.; Guo, B.; Liu, Z.; You, C.; Wu, Z. Dextran synthesized by Leuconostoc mesenteroides BD1710 in tomato juice supplemented with sucrose. Carbohydr. Polym. 2014, 112, 556–562. [Google Scholar] [CrossRef]
- Ye, G.; Li, G.; Wang, C.; Ling, B.; Yang, R.; Huang, S. Extraction and characterization of dextran from Leuconostoc pseudomesenteroides YB-2 isolated from mango juice. Carbohydr. Polym. 2019, 207, 218–223. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, F.; Zhou, Q.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Isolation, purification, and characterization of exopolysaccharide produced by Leuconostoc Citreum N21 from dried milk cake. Trans. Tianjin Univ. 2019, 25, 161–168. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef]
- Yuan, L.; Chu, B.; Chen, S.; Li, Y.; Liu, N.; Zhu, Y.; Zhou, D. Exopolysaccharides from Bifidobacterium animalis Ameliorate Escherichia coli-Induced IPEC-J2 Cell Damage via Inhibiting Apoptosis and Restoring Autophagy. Microorganisms 2021, 9, 2363. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Ren, F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules 2019, 24, 513. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, W.; Hong, T.; Xiong, T.; Gong, D.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus plantarum NCU116 Regulate Intestinal Barrier Function via STAT3 Signaling Pathway. J. Agric. Food Chem. 2018, 66, 9719–9727. [Google Scholar] [CrossRef]
- Sommer, K.; Wiendl, M.; Müller, T.M.; Heidbreder, K.; Voskens, C.; Neurath, M.F.; Zundler, S. Intestinal Mucosal Wound Healing and Barrier Integrity in IBD-Crosstalk and Trafficking of Cellular Players. Front. Med. 2021, 8, 643973. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.M.; Opdenaker, L.M.; Flynn, D.; Sims-Mourtada, J. Wound healing and cancer stem cells: Inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis 2015, 8, CGM-S11286. [Google Scholar] [CrossRef]
- Sun, M.; Liu, W.; Song, Y.; Tuo, Y.; Mu, G.; Ma, F. The Effects of Lactobacillus plantarum-12 Crude Exopolysaccharides on the Cell Proliferation and Apoptosis of Human Colon Cancer (HT-29) Cells. Probiotics Antimicrob. Proteins 2021, 13, 413–421. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Yi, H.; Han, X.; Zhang, Y.; Xin, L. Exopolysaccharides produced by Lactobacillus strains suppress HT-29 cell growth via induction of G0/G1 cell cycle arrest and apoptosis. Oncol. Lett. 2018, 16, 3577–3586. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Sengül, N.; Isik, S.; Aslım, B.; Ucar, G.; Demirbağ, A. The Effect of Exopolysaccharide-Producing Probiotic Strains on Gut Oxidative Damage in Experimental Colitis. Dig. Dis. Sci. 2011, 56, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Kavitake, D.; Veerabhadrappa, B.; Sudharshan, S.J.; Kandasamy, S.; Devi, P.B.; Dyavaiah, M.; Shetty, P.H. Oxidative stress alleviating potential of galactan exopolysaccharide from Weissella confusa KR780676 in yeast model system. Sci. Rep. 2022, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Ma, L.; Qiao, Y.; Shi, B. A novel exopolysaccharide produced by Lactobacillus coryniformis NA-3 exhibits antioxidant and biofilm-inhibiting properties in vitro. Food Nutr. Res. 2020, 64, 3744. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.; Ishola, R.; Oyewunmi, T. Characterization, antioxidant and immunomodulatory potential on exopolysaccharide produced by wild type and mutant Weissella confusa strains. Biotechnol. Rep. 2018, 19, e00271. [Google Scholar] [CrossRef]
- Bilbis, L.S.; Muhammad, S.A.; Saidu, Y. The Potentials of Antioxidant Micronutrients in the Management of Metabolic Syndrome. J. Antioxid. Act. 2016, 1, 1–21. [Google Scholar] [CrossRef][Green Version]
- Litvinova, L.; Atochin, D.N.; Fattakhov, N.; Vasilenko, M.; Zatolokin, P.; Kirienkova, E. Nitric oxide and mitochondria in metabolic syndrome. Front. Physiol. 2015, 6, 20. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. The Two Faces of Reactive Oxygen Species in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Uetaki, M.; Tabata, S.; Nakasuka, F.; Soga, T.; Tomita, M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci. Rep. 2015, 5, 13896. [Google Scholar] [CrossRef]
- Phang, C.W.; Karsani, S.A.; Abd Malek, S.N. Induction of Apoptosis and Cell Cycle Arrest by Flavokawain C on HT-29 Human Colon Adenocarcinoma via Enhancement of Reactive Oxygen Species Generation, Upregulation of p21, p27, and GADD153, and Inactivation of Inhibitor of Apoptosis Proteins. Pharmacogn. Mag. 2017, 13, S321–S328. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, Z.; Jin, Q.; Chen, G.; Guo, M. Cell Cycle Arrest and Apoptosis in HT-29 Cells Induced by Dichloromethane Fraction From Toddalia asiatica (L.) Lam. Front. Pharmacol. 2018, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kale, M.; Kim, D.-H.; Kim, H.-S.; Chon, J.-W.; Seo, K.-H.; Lee, H.G.; Yokoyama, W.; Kim, H.J.J.O.A.; Chemistry, F. Antiobesity effect of exopolysaccharides isolated from kefir grains. J. Agric. Food Chem. 2017, 65, 10011–10019. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016633371. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.W. Prevention of Obesity and Metabolic Syndrome in Children. Front. Endocrinol. 2019, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Gepstein, V.; Weiss, R. Obesity as the Main Risk Factor for Metabolic Syndrome in Children. Front. Endocrinol. 2019, 10, 568. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; MacCallum, P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part, I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J. Cardiometab. Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef]
- Cao, Y.; Zou, S.; Xu, H.; Li, M.; Tong, Z.; Xu, M.; Xu, X. Hypoglycemic activity of the Baker’s yeast β-glucan in obese/type 2 diabetic mice and the underlying mechanism. Mol. Nutr. Food Res. 2016, 60, 2678–2690. [Google Scholar] [CrossRef]
- Hu, J.; Pang, W.; Bai, S.; Zheng, Z.; Wu, X. Hypoglycemic effect of polysaccharides with different molecular weight of Pseudostellaria heterophylla. BMC Complement. Altern. Med. 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Titty, F.K. Glycaemic control, dyslipidaemia and metabolic syndrome among recently diagnosed diabetes mellitus patients in Tamale Teaching Hospital, Ghana. West Afr. J. Med. 2010, 29, 8–11. [Google Scholar] [CrossRef]
- Mårtensson, O.; Biörklund, M.; Lambo, A.M.; Dueñas-Chasco, M.; Irastorza, A.; Holst, O.; Norin, E.; Welling, G.; Öste, R.; Önning, G. Fermented, ropy, oat-based products reduce cholesterol levels and stimulate the bifidobacteria flora in humans. Nutr. Res. 2005, 25, 429–442. [Google Scholar] [CrossRef]
- Favier, M.-L.; Moundras, C.; Demigné, C.; Rémésy, C. Fermentable carbohydrates exert a more potent cholesterol-lowering effect than cholestyramine. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1995, 1258, 115–121. [Google Scholar] [CrossRef]
- Ahmad, W.; Khaliq, S.; Akhtar, N.; El Arab, J.; Akhtar, K.; Prakash, S.; Anwar, M.A.; Munawar, N.J.M. Whole Genome Sequence Analysis of a Novel Apilactobacillus Species from Giant Honeybee (Apis dorsata) Gut Reveals Occurrence of Genetic Elements Coding Prebiotic and Probiotic Traits. Microorganisms 2022, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Nasir, A.; Sattar, F.; Ashfaq, I.; Chen, M.-H.; Hayat, A.; Zhao, S.; Khaliq, S.; Ghauri, M.A.; Anwar, M.A.J.F.M. Production of bimodal molecular weight levan by a Lactobacillus reuteri isolate from fish gut. Folia Microbiol. 2022, 67, 21–31. [Google Scholar] [CrossRef]
- Tahir, M.; Majeed, M.I.; Nawaz, H.; Ali, S.; Rashid, N.; Kashif, M.; Ashfaq, I.; Ahmad, W.; Ghauri, K.; Sattar, F.J.S.A.P.A.M.; et al. Raman spectroscopy for the analysis of different exo-polysaccharides produced by bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 237, 118408. [Google Scholar] [CrossRef] [PubMed]
- Foyle, T.; Jennings, L.; Mulcahy, P. Compositional analysis of lignocellulosic materials: Evaluation of methods used for sugar analysis of waste paper and straw. Bioresour. Technol. 2007, 98, 3026–3036. [Google Scholar] [CrossRef]
- Walford, S.N. Sugarcane Bagasse: How Easy Is It to Mea-sure Its Constituents? In Proceedings of the South African Sugar Technologists Association, Durban, South Africa, 29–31 July 2008; pp. 266–273. [Google Scholar]
- Nasir, A.; Sattar, F.; Ashfaq, I.; Lindemann, S.R.; Chen, M.-H.; Van den Ende, W.; Öner, E.T.; Kirtel, O.; Khaliq, S.; Ghauri, M.A.J.L. Production and characterization of a high molecular weight levan and fructooligosaccharides from a rhizospheric isolate of Bacillus aryabhattai. LWT 2020, 123, 109093. [Google Scholar] [CrossRef]
- Yao, T.; Chen, M.-H.; Lindemann, S.R. Carbohydrate complexity structures stable diversity in gut-derived microbial consortia under high dilution pressure. bioRxiv 2020. [Google Scholar] [CrossRef]
- Care:, J.W.G.O.V. Guidelines for the veterinary care of laboratory animals: Report of the FELASA/ECLAM/ESLAV Joint Working Group on Veterinary Care. Lab. Anim. 2008, 42, 1–11. [Google Scholar]
- Burnett, L.C.; Lunn, G.; Coico, R. Biosafety: Guidelines for working with pathogenic and infectious microorganisms. Curr. Protoc. Microbiol. 2009, 13, 1A-1. [Google Scholar] [CrossRef]


| Retention Time | Methylated Sugar | Primary MS Fragments (m/z) | Linkage Type | Molar Ratio (%) |
|---|---|---|---|---|
| 8.657 | 1,5-Di-O-acetyl-2,3,4,6-tetra-O-methyl-D-glucitol | 87, 102, 118, 129, 145, 161/162, 205 | Terminal Glcp | 21.5 ± 0.5 |
| 10.635 | 1,5,6-Tri-O-acetyl-2,3,4-tri-O-methyl-D-glucitol | 87, 99, 102, 118, 129, 162, 189, 233 | 1→6-linked Glcp | 66.74 ± 0.2 |
| 12.607 | 1,3,5,6-Tetra-O-acetyl-2,4-di-O-methyl-D-glucitol | 87, 101, 118, 139, 160, 174, 189, 234, 305 | 1→3,6-linked Glcp | 11.76 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, W.; Boyajian, J.L.; Abosalha, A.; Nasir, A.; Ashfaq, I.; Islam, P.; Schaly, S.; Thareja, R.; Hayat, A.; Rehman, M.u.; et al. High-Molecular-Weight Dextran-Type Exopolysaccharide Produced by the Novel Apilactobacillus waqarii Improves Metabolic Syndrome: In Vitro and In Vivo Analyses. Int. J. Mol. Sci. 2022, 23, 12692. https://doi.org/10.3390/ijms232012692
Ahmad W, Boyajian JL, Abosalha A, Nasir A, Ashfaq I, Islam P, Schaly S, Thareja R, Hayat A, Rehman Mu, et al. High-Molecular-Weight Dextran-Type Exopolysaccharide Produced by the Novel Apilactobacillus waqarii Improves Metabolic Syndrome: In Vitro and In Vivo Analyses. International Journal of Molecular Sciences. 2022; 23(20):12692. https://doi.org/10.3390/ijms232012692
Chicago/Turabian StyleAhmad, Waqar, Jacqueline L. Boyajian, Ahmed Abosalha, Anam Nasir, Iram Ashfaq, Paromita Islam, Sabrina Schaly, Rahul Thareja, Azam Hayat, Mujaddad ur Rehman, and et al. 2022. "High-Molecular-Weight Dextran-Type Exopolysaccharide Produced by the Novel Apilactobacillus waqarii Improves Metabolic Syndrome: In Vitro and In Vivo Analyses" International Journal of Molecular Sciences 23, no. 20: 12692. https://doi.org/10.3390/ijms232012692
APA StyleAhmad, W., Boyajian, J. L., Abosalha, A., Nasir, A., Ashfaq, I., Islam, P., Schaly, S., Thareja, R., Hayat, A., Rehman, M. u., Anwar, M. A., & Prakash, S. (2022). High-Molecular-Weight Dextran-Type Exopolysaccharide Produced by the Novel Apilactobacillus waqarii Improves Metabolic Syndrome: In Vitro and In Vivo Analyses. International Journal of Molecular Sciences, 23(20), 12692. https://doi.org/10.3390/ijms232012692










