Thermophilic Inorganic Pyrophosphatase Ton1914 from Thermococcus onnurineus NA1 Removes the Inhibitory Effect of Pyrophosphate
Abstract
:1. Introduction
2. Results
2.1. Molecular Properties of Ton1914
2.2. Catalytic Properties of Ton1914
2.3. pH, Thermal and Metal Stability of Ton1914
2.4. Substrate Specificity of Ton1914
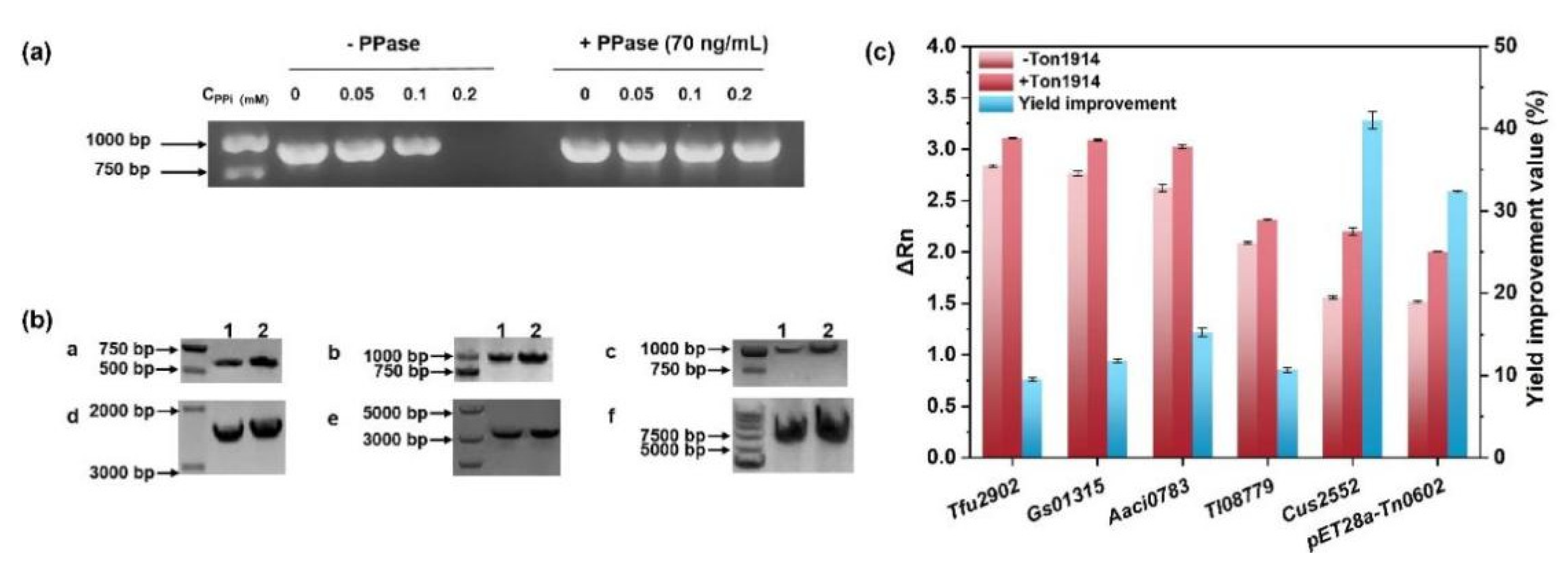
2.5. Ton1914 for Improving PCR Efficiency
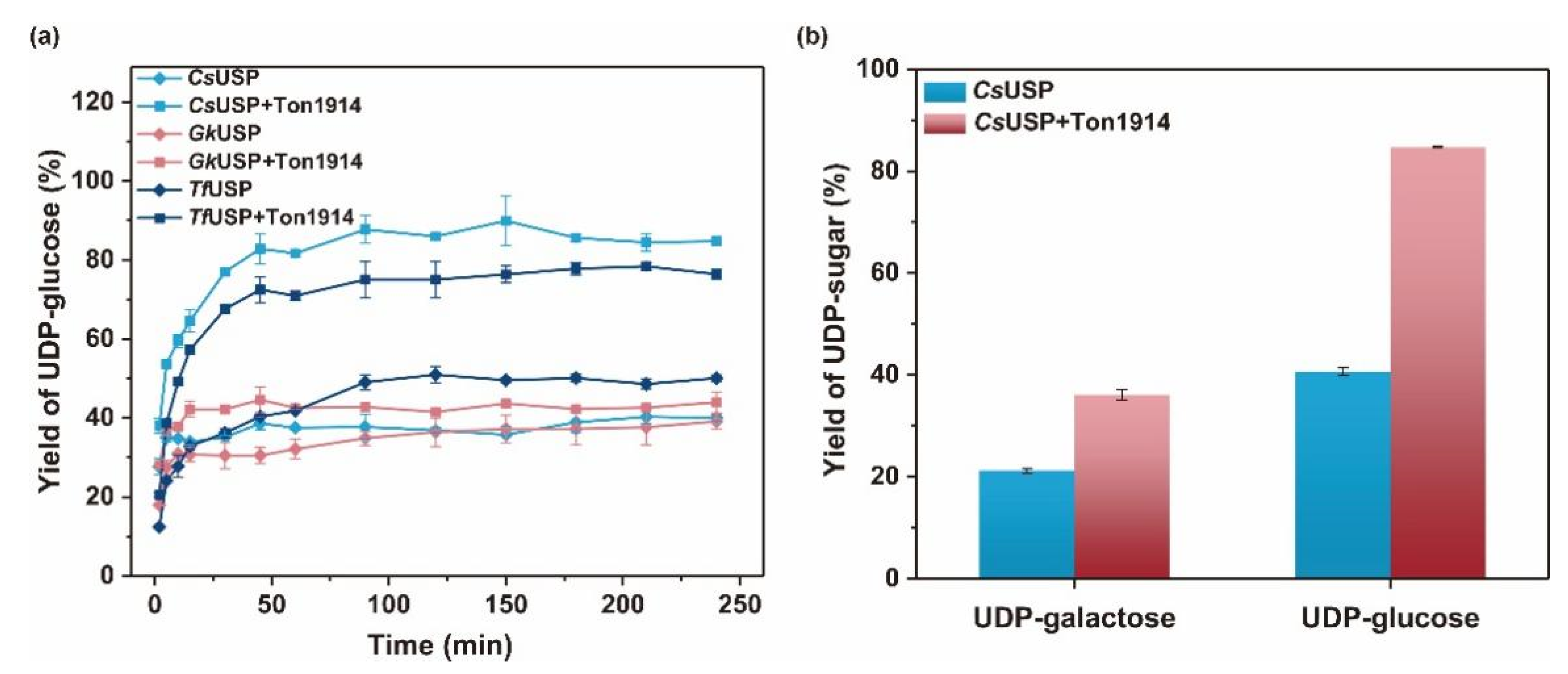
2.6. Use of Ton1914 to Improve UDP-Sugar Synthesis Yield
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, C.X.; Zeng, J.; Hao, H.N.; Xu, Y.X.Y.; Liu, F.; Liu, R.D.; Long, S.R.; Wang, Z.Q.; Cui, J. Biological properties and roles of a Trichinella spiralis inorganic pyrophosphatase in molting and developmental process of intestinal larval stages. Veter. Res. 2021, 52, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Xie, G.; Gao, R. Cloning, purification, and characterization of inorganic pyrophosphatase from the hyperthermophilic archaea Pyrococcus horikoshii. Protein Expr. Purif. 2014, 99, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.B.; Friesen, J.D.; Walker, J.A.; Peters, S.J.; Weitzel, C.S.; Friesen, J.A. Characterization of an archaeal inorganic pyrophosphatase from Sulfolobus islandicus using a [31P]-NMR-based assay. Biochem. Biophys. Res. Commun. 2021, 585, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Romanov, R.S.; Mariasina, S.S.; Efimov, S.V.; Klochkov, V.V.; Rodina, E.V.; Polshakov, V.I. Backbone resonance assignment and dynamics of 110 kDa hexameric inorganic pyrophosphatase from Mycobacterium tuberculosis. Biomol. NMR Assign. 2020, 14, 281–287. [Google Scholar] [CrossRef]
- Anashkin, V.A.; Salminen, A.; Orlov, V.N.; Lahti, R.; Baykov, A.A. The tetrameric structure of nucleotide-regulated pyrophosphatase and its modulation by deletion mutagenesis and ligand binding. Arch. Biochem. Biophys. 2020, 692, 108537. [Google Scholar] [CrossRef]
- Yan, X.; Dong, Q.; Zheng, M.; Yang, Z. Characterization of a family I-liked alkaline-resistant inorganic pyrophosphatase from the hyperthermophilic archaeon Pyrococcus furiosus for rapid determination of sugar nucleotidyltransferase at high temperature. J. Mol. Catal. B Enzym. 2013, 98, 15–20. [Google Scholar] [CrossRef]
- Muthana, M.M.; Qu, J.; Li, Y.; Zhang, L.; Yu, H.; Ding, L.; Malekan, H.; Chen, X. Efficient one-pot multienzyme synthesis of UDP-sugars using a promiscuous UDP-sugar pyrophosphorylase from Bifidobacterium longum (BLUSP). Chem. Commun. 2012, 48, 2728–2730. [Google Scholar] [CrossRef]
- Muthana, M.M.; Qu, J.; Xue, M.; Klyuchnik, T.; Siu, A.; Li, Y.; Zhang, L.; Yu, H.; Li, L.; Wang, P.G.; et al. Improved one-pot multienzyme (OPME) systems for synthesizing UDP-uronic acids and glucuronides. Chem. Commun. 2015, 51, 4595–4598. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Lee, B.; Park, K.S.; Chong, Y.; Yoon, M.Y.; Jeon, S.J.; Kim, D.E. Facilitation of polymerase chain reaction with thermostable inorganic pyrophosphatase from hyperthermophilic archaeon Pyrococcus horikoshii. Appl. Microbiol. Biotechnol. 2010, 85, 807–812. [Google Scholar] [CrossRef]
- Ralser, M.; Querfurth, R.; Warnatz, H.-J.; Lehrach, H.; Yaspo, M.-L.; Krobitsch, S. An efficient and economic enhancer mix for PCR. Biochem. Biophys. Res. Commun. 2006, 347, 747–751. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ryu, Y.G.; Lee, H.S.; Cho, Y.; Kwon, S.T.; Lee, J.H.; Kang, S.G. Characterization of a dITPase from the hyperthermophilic archaeon Thermococcus onnurineus NA1 and its application in PCR amplification. Appl. Microbiol. Biotechnol. 2008, 79, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Lee, H.S.; Kim, Y.J.; Kang, S.G.; Kim, S.J.; Lee, J.H. Characterization of a dUTPase from the hyperthermophilic archaeon Thermococcus onnurineus NA1 and its application in polymerase chain reaction amplification. Mar. Biotechnol. 2007, 9, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Kafafy, R.; Salleh, H.M.; Faris, W.F. Enhancing the efficiency of polymerase chain reaction using graphene nanoflakes. Nanotechnology 2012, 23, 455106. [Google Scholar] [PubMed]
- Cho, S.S.; Yu, M.; Kim, S.H.; Kwon, S.-T. Enhanced PCR efficiency of high-fidelity DNA polymerase from Thermococcus waiotapuensis. Enzym. Microb. Technol. 2014, 63, 39–45. [Google Scholar] [CrossRef]
- Tong, W.; Cao, X.; Wen, S.; Guo, R.; Shen, M.; Wang, J.; Shi, X. Enhancing the specificity and efficiency of polymerase chain reaction using polyethyleneimine-based derivatives and hybrid nanocomposites. Int. J. Nanomed. 2012, 7, 1069. [Google Scholar]
- Sakhabutdinova, A.R.; Chemeris, A.V.; Garafutdinov, R.R. Enhancement of PCR efficiency using mono- and disaccharides. Anal. Biochem. 2020, 606, 113858. [Google Scholar] [CrossRef]
- Aravind, L.; Koonin, E.V. Phosphoesterase domains associated with DNA polymerases of diverse origins. Nucleic Acids Res. 1998, 26, 3746–3752. [Google Scholar] [CrossRef] [Green Version]
- Lapenta, F.; Montón Silva, A.; Brandimarti, R.; Lanzi, M.; Gratani, F.L.; Vellosillo Gonzalez, P.; Perticarari, S.; Hochkoeppler, A. Escherichia coli DnaE polymerase couples pyrophosphatase activity to DNA replication. PLoS ONE 2016, 11, e0152915. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.; Zismann, T.; Lunau, N.; Warnecke, S.; Wendicke, S.; Meier, C. A convenient synthesis of nucleoside diphosphate glycopyranoses and other polyphosphorylated bioconjugates. Eur. J. Cell Biol. 2010, 89, 63–75. [Google Scholar] [CrossRef]
- Ma, X.; Stöckigt, J. High yielding one-pot enzyme-catalyzed synthesis of UDP-glucose in gram scales. Carbohydr. Res. 2001, 333, 159–163. [Google Scholar] [CrossRef]
- Tersteeg, S.; Mrozowich, T.; Henrickson, A.; Demeler, B.; Patel, T.R. Purification and Characterization of Inorganic Pyrophosphatase for in vitro RNA Transcription. Biochem. Cell Biol. 2022, 100, 18. [Google Scholar] [CrossRef] [PubMed]
- Oliva, G.; Romero, I.; Ayala, G.; Barrios-Jacobo, I.; Celis, H. Characterization of the inorganic pyrophosphatase from the pathogenic bacterium Helicobacter pylori. Arch. Microbiol. 2000, 174, 104–110. [Google Scholar] [CrossRef]
- Jeon, S.-J.; Ishikawa, K. Characterization of the Family I inorganic pyrophosphatase from Pyrococcus horikoshii OT3. Archaea 2005, 1, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Baykov, A.A.; Anashkin, V.A.; Salminen, A.; Lahti, R. Inorganic pyrophosphatases of Family II—Two decades after their discovery. FEBS Lett. 2017, 591, 3225–3234. [Google Scholar] [CrossRef] [Green Version]
- Gómez-García, M.R.; Ruiz-Pérez, L.M.; González-Pacanowska, D.; Serrano, A. A novel calcium-dependent soluble inorganic pyrophosphatase from the trypanosomatid Leishmania major. FEBS Lett. 2004, 560, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Van Alebeek, G.J.W.M.; Keltjens, J.T.; van der Drift, C. Purification and characterization of inorganic pyrophosphatase from Methanobacterium thernoautotrophicum (strain Δ H). Biochim. Biophys. Acta 1994, 1206, 231–239. [Google Scholar] [CrossRef]
- Verhoeven, J.A.; Schenck, K.M.; Meyer, R.R.; Trela, J.M. Purification and characterization of an inorganic pyrophosphatase from the extreme thermophile Thermus aquaticus. J. Bacteriol. 1986, 168, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Hoe, H.S.; Kim, H.K.; Kwon, S.-T. Expression in Escherichia coli of the Thermostable Inorganic Pyrophosphatase from the Aquifex aeolicus and Purification and Characterization of the Recombinant Enzyme. Protein Expr. Purif. 2001, 23, 242–248. [Google Scholar] [CrossRef]
- Hansen, T.; Urbanke, C.; Leppänen, V.M.; Goldman, A.; Brandenburg, K.; Schäfer, G. The Extreme Thermostable Pyrophosphatase from Sulfolobus acidocaldarius: Enzymatic and Comparative Biophysical Characterization. Arch. Biochem. Biophys. 1999, 363, 135–147. [Google Scholar] [CrossRef]
- Lee, H.S.; Cho, Y.; Kim, Y.-J.; Lho, T.-O.; Cha, S.-S.; Lee, J.-H.; Kang, S.G. A novel inorganic pyrophosphatase in Thermococcus onnurineus NA1. FEMS Microbiol. Lett. 2009, 300, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Kim, Y.J.; Lee, J.-H.; Kang, S.G. Identification and Characterization of Inorganic Pyrophosphatase and PAP Phosphatase from Thermococcus onnurineus NA1. J. Bacteriol. 2009, 191, 3415–3419. [Google Scholar] [CrossRef] [PubMed]
- Rodina, E.V.; Valueva, A.V.; Yakovlev, R.Y.; Vorobyeva, N.N.; Kulakova, I.I.; Lisichkin, G.V.; Leonidov, N.B. Immobilization of inorganic pyrophosphatase on nanodiamond particles retaining its high enzymatic activity. Biointerphases 2015, 10, 041005. [Google Scholar] [CrossRef] [PubMed]
- Valueva, A.V.; Romanov, R.S.; Vorobyeva, N.N.; Kurilova, S.A.; Rodina, E.V. Synthesis of Inorganic Pyrophosphatase–Nanodiamond Conjugates Resistant to Calcium and Fluoride. ACS Omega 2020, 5, 6641–6650. [Google Scholar] [CrossRef] [Green Version]
- Valueva, A.V.; Romanov, R.S.; Mariasina, S.S.; Eliseev, M.S.; Rodina, E.V. Inorganic Pyrophosphatase–Nanodiamond Conjugates Hydrolyze Pyrophosphate in Human Synovial Fluid. ACS Omega 2020, 5, 8579–8586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Guo, Y.; Zhao, M.; Lin, C.; Lin, Z.; Luo, F.; Chen, G. Fluorescence biosensor for inorganic pyrophosphatase activity. Anal. Bioanal. Chem. 2016, 409, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, Z.; Zhou, L.; Zheng, O.; Wu, X.; Guo, L.; Qiu, B.; Lin, Z.; Chen, G. Fluorometric Method for Inorganic Pyrophosphatase Activity Detection and Inhibitor Screening Based on Click Chemistry. Anal. Chem. 2014, 87, 816–820. [Google Scholar] [CrossRef]
- Chen, C.; Ruan, G.; Sun, Y.; Wang, L.; Zhang, C.; Liu, J. In situ Cu2+-triggered wavelength-tunable fluorescent sensor for highly sensitive sensing inorganic pyrophosphatase activity and its logic gate application. Sens. Actuators B Chem. 2021, 346, 130439. [Google Scholar] [CrossRef]
- Deng, H.-H.; Shi, X.-Q.; Peng, H.-P.; Zhuang, Q.-Q.; Yang, Y.; Liu, A.-L.; Xia, X.-H.; Chen, W. Gold Nanoparticle-Based Photoluminescent Nanoswitch Controlled by Host–Guest Recognition and Enzymatic Hydrolysis for Arginase Activity Assay. ACS Appl. Mater. Interfaces 2018, 10, 5358–5364. [Google Scholar] [CrossRef]
- Lawrence, A.J.; Coote, J.G.; Kazi, Y.F.; Lawrence, P.D.; MacDonald-Fyall, J.; Orr, B.M.; Parton, R.; Riehle, M.; Sinclair, J.; Young, J.; et al. A Direct Pyrophosphatase-coupled Assay Provides New Insights into the Activation of the Secreted Adenylate Cyclase from Bordetella pertussis by Calmodulin. J. Biol. Chem. 2002, 277, 22289–22296. [Google Scholar] [CrossRef] [Green Version]
- Hua, G.; Hu, Y.; Yang, C.; Liu, D.; Mao, Z.; Zhang, L.; Zhang, Y. Characterization of santalene synthases using an inorganic pyrophosphatase coupled colorimetric assay. Anal. Biochem. 2018, 547, 26–36. [Google Scholar] [CrossRef]
- Baykov, A.; Anashkin, V.; Malinen, A. Good-Practice Non-Radioactive Assays of Inorganic Pyrophosphatase Activities. Molecules 2021, 26, 2356. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Schutt, C.E. The enhancement of PCR amplification by low molecular-weight sulfones. Gene 2001, 274, 293–298. [Google Scholar] [CrossRef]
- Zheng, Z.; Liebers, M.; Zhelyazkova, B.; Cao, Y.; Panditi, D.; Lynch, K.D.; Chen, J.; Robinson, H.E.; Shim, H.S.; Chmielecki, J.; et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat. Med. 2014, 20, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, B.A.; Rejzek, M.; Pesnot, T.; Tedaldi, L.M.; Caputi, L.; O’Neill, E.C.; Benini, S.; Wagner, G.K.; Field, R.A. Enzymatic synthesis of nucleobase-modified UDP-sugars: Scope and limitations. Carbohydr. Res. 2015, 404, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, Y.; Wan, Y.; Li, Y.; Chen, X.; Zhao, W.; Wang, P.G. Efficient Enzymatic Synthesis of Guanosine 5′-Diphosphate-Sugars and Derivatives. Org. Lett. 2013, 15, 5528–5530. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zou, Y.; Guan, W.; Zhai, Y.; Xue, M.; Jin, L.; Zhao, X.; Dong, J.; Wang, W.; Shen, J.; et al. Biosynthesis of nucleotide sugars by a promiscuous UDP-sugar pyrophosphorylase from Arabidopsis thaliana (AtUSP). Bioorg. Med. Chem. Lett. 2013, 23, 3764–3768. [Google Scholar] [CrossRef]
- Chien, W.T.; Liang, C.F.; Yu, C.C.; Lin, J.H.; Wu, H.T.; Lin, C.C. Glucose 1-Phosphate Thymidylyltransferase in the Synthesis of Uridine 5′-Diphosphate Galactose and its Application in the Synthesis of N-Acetyllactosamine. Adv. Synth. Catal. 2012, 354, 123–132. [Google Scholar] [CrossRef]



| Enzyme | Source of Species | pH | Temperature (°C) | t1/2 (h) | Kcat (s−1) | Km (M) | kcat/Km (s−1M−1) |
|---|---|---|---|---|---|---|---|
| Ton1914 | Thermococcus onnurineus NA1 | 9.0 | 80 | 2.5 (90 °C) | 2.995 × 104 | 1.116 × 10−3 | 2.68 × 107 |
| PhPPase [23] | Pyrococcus horikoshii | 7.5 | 70 | 0.83 (105 °C) | 744 | 1.13 × 10−4 | 6.584 × 106 |
| MePPase [26] | Methanobacterium thernoautotrophicum | 8.5 | 70 | —— | 0.962 × 103 | 1.6 × 10−4 | 6.01 × 106 |
| PfPPase [6] | Pyrococcus furiosus | 9.5 | 95 | 46 (95 °C) | —— | 1.73 × 10−4 | —— |
| ThPPase [27] | Thermus aquaticus | 8.3 | 80 | —— | —— | 6 × 10−4 | —— |
| AaePPase [28] | Aquifex aeolicus | 8.0 | 80 | 1.5 (95 °C) | —— | —— | —— |
| S-PPase [29] | Sulfolobus acidocaldarius | 7.0 | 75 | 2.5 (95 °C) | 1.08 × 103 | 5.4 × 10−6 | 2.0 × 108 |
| TON_0002 [30] | Thermococcus onnurineus NA1 | 6.5 | 80 | —— | 0.16 | 0.35 × 10−3 | 6.4 × 102 |
| TON_1705 [31] | Thermococcus onnurineus NA1 | 9.5 | —— | —— | 2.1 | 18.8 × 10−6 | 0.11 × 106 |
| Substrate | Relative Activity (%) |
|---|---|
| PPi | 100 |
| PPPi | 6.9 |
| UTP | 5.0 |
| ATP | <1 |
| ADP | <1 |
| Glc-1P | <1 |
| Glc-6P | <1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, X.; Gao, R. Thermophilic Inorganic Pyrophosphatase Ton1914 from Thermococcus onnurineus NA1 Removes the Inhibitory Effect of Pyrophosphate. Int. J. Mol. Sci. 2022, 23, 12735. https://doi.org/10.3390/ijms232112735
Li Y, Yang X, Gao R. Thermophilic Inorganic Pyrophosphatase Ton1914 from Thermococcus onnurineus NA1 Removes the Inhibitory Effect of Pyrophosphate. International Journal of Molecular Sciences. 2022; 23(21):12735. https://doi.org/10.3390/ijms232112735
Chicago/Turabian StyleLi, Yajing, Xue Yang, and Renjun Gao. 2022. "Thermophilic Inorganic Pyrophosphatase Ton1914 from Thermococcus onnurineus NA1 Removes the Inhibitory Effect of Pyrophosphate" International Journal of Molecular Sciences 23, no. 21: 12735. https://doi.org/10.3390/ijms232112735
APA StyleLi, Y., Yang, X., & Gao, R. (2022). Thermophilic Inorganic Pyrophosphatase Ton1914 from Thermococcus onnurineus NA1 Removes the Inhibitory Effect of Pyrophosphate. International Journal of Molecular Sciences, 23(21), 12735. https://doi.org/10.3390/ijms232112735







