Analysis of Bone Histomorphometry in Rat and Guinea Pig Animal Models Subject to Hypoxia
Abstract
1. Introduction
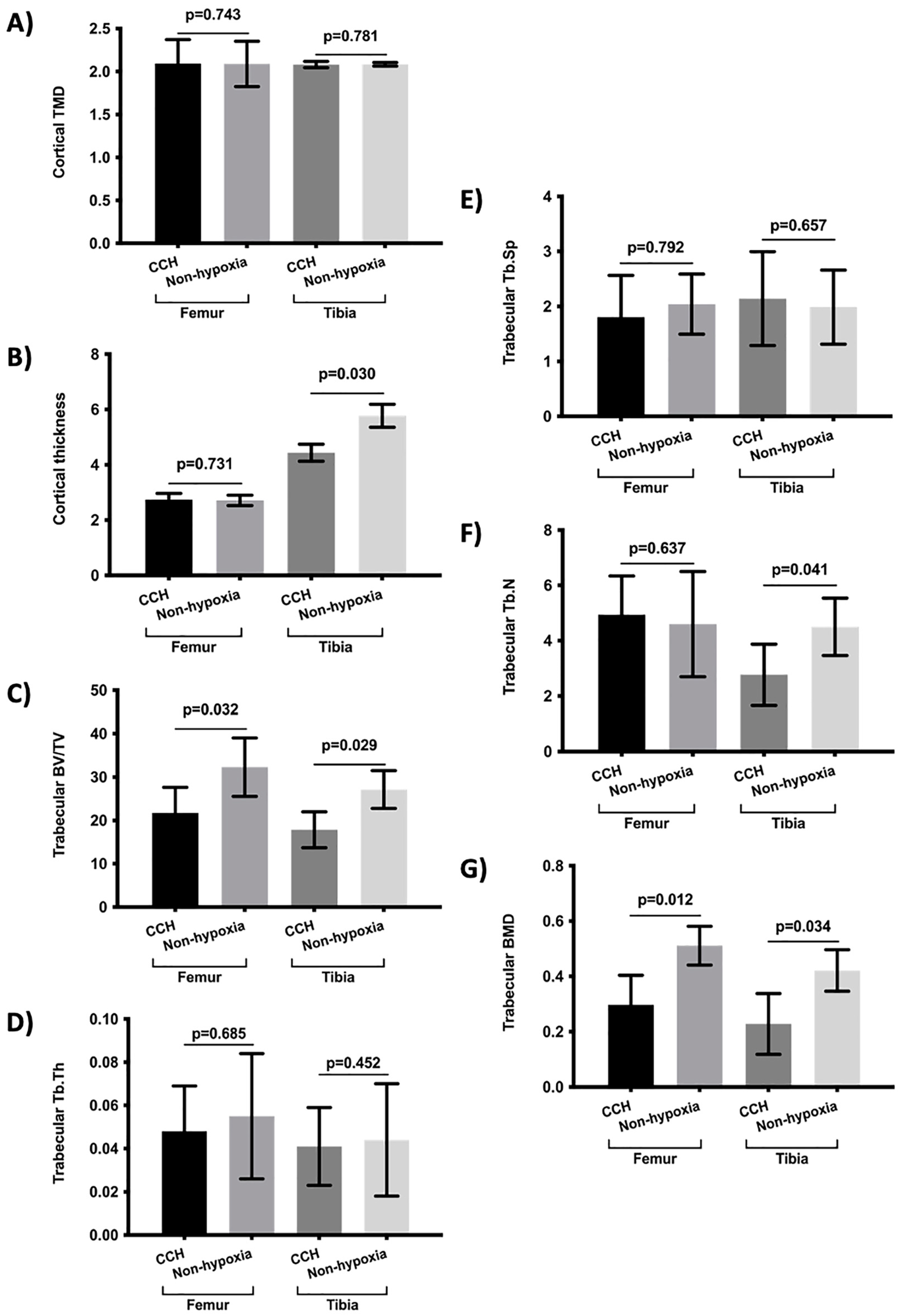
2. Results
3. Discussion
4. Material and Methods
4.1. Animals and Experimental Design
4.2. Micro-Computed Tomography (μCT)
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ralston, S.H. Bone structure and metabolism. Medicine 2013, 41, 581–585. [Google Scholar] [CrossRef]
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. Bonekey Rep. 2014, 3, 481. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Davis, D.A.; Haque, M.; Huang, L.E.; Yarchoan, R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005, 65, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Malhotra, A.S.; Pal, K.; Chatterjee, T.; Ghosh, D.; Haldar, K.; Verma, S.K.; Kumar, S.; Sharma, Y.K.; Sawhney, R.C. Determination of bone mass using multisite quantitative ultrasound and biochemical markers of bone turnover during residency at extreme altitude: A longitudinal study. High Alt. Med. Biol. 2013, 14, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Malhotra, A.S.; Pal, K.; Kumar, R.; Bajaj, R.; Verma, S.K.; Ghosh, D.; Sharma, Y.K.; Sawhney, R.C. Alterations in different indices of skeletal health after prolonged residency at high altitude. High Alt. Med. Biol. 2014, 15, 170–175. [Google Scholar] [CrossRef]
- O’Brien, K.A.; Pollock, R.D.; Stroud, M.; Lambert, R.J.; Kumar, A.; Atkinson, R.A.; Green, D.A.; Anton-Solanas, A.; Edwards, L.M.; Harridge, S.D.R. Human physiological and metabolic responses to an attempted winter crossing of Antarctica: The effects of prolonged hypobaric hypoxia. Physiol. Rep. 2018, 6, e13613. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; Rigual, R.; Ruiz-Mambrilla, M.; Fernández-Gómez, J.M.; Dueñas, A.; Pérez-Castrillón, J.L. Molecular Mechanisms Involved in Hypoxia-Induced Alterations in Bone Remodeling. Int. J. Mol. Sci. 2022, 23, 3233. [Google Scholar] [CrossRef]
- Rittweger, J.; Debevec, T.; Frings-Meuthen, P.; Lau, P.; Mittag, U.; Ganse, B.; Ferstl, P.G.; Simpson, E.J.; Macdonald, I.A.; Eiken, O.; et al. On the combined effects of normobaric hypoxia and bed rest upon bone and mineral metabolism: Results from the PlanHab study. Bone 2016, 91, 130–138. [Google Scholar] [CrossRef]
- Brent, M.B.; Emmanuel, T.; Simonsen, U.; Brüel, A.; Thomsen, J.S. Hypobaric hypoxia deteriorates bone mass and strength in mice. Bone 2022, 154, 116203. [Google Scholar] [CrossRef] [PubMed]
- Brent, M.B. A review of the skeletal effects of exposure to high altitude and potential mechanisms for hypobaric hypoxia-induced bone loss. Bone 2022, 154, 116258. [Google Scholar] [CrossRef] [PubMed]
- Brent, M.B.; Simonsen, U.; Thomsen, J.S.; Brüel, A. Effect of Acetazolamide and Zoledronate on Simulated High Altitude-Induced Bone Loss. Front. Endocrinol. 2022, 13, 831369. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Utting, J.C.; Robins, S.P.; Brandao-Burch, A.; Orriss, I.R.; Behar, J.; Arnett, T.R. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp. Cell Res. 2006, 312, 1693–1702. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Sun, D.; Xin, J.; Wang, L.; Huang, D.; Wu, W.; Xian, C.J. Short-Term Hypoxia Accelerates Bone Loss in Ovariectomized Rats by Suppressing Osteoblastogenesis but Enhancing Osteoclastogenesis. Med. Sci. Monit. 2016, 22, 2962–2971. [Google Scholar] [CrossRef]
- Arnett, T.R.; Gibbons, D.C.; Utting, J.C.; Orriss, I.R.; Hoebertz, A.; Rosendaal, M.; Meghji, S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell. Physiol. 2003, 196, 2–8. [Google Scholar] [CrossRef]
- Utting, J.C.; Flanagan, A.M.; Brandao-Burch, A.; Orriss, I.R.; Arnett, T.R. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem. Funct. 2010, 28, 374–380. [Google Scholar] [CrossRef]
- Ontiveros, C.; Irwin, R.; Wiseman, R.W.; McCabe, L.R. Hypoxia suppresses runx2 independent of modeled microgravity. J. Cell. Physiol. 2004, 200, 169–176. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, K.J. TWIST activation by hypoxia inducible factor-1 (HIF-1): Implications in metastasis and development. Cell Cycle 2008, 7, 2090–2096. [Google Scholar] [CrossRef]
- Merceron, C.; Ranganathan, K.; Wang, E.; Tata, Z.; Makkapati, S.; Khan, M.P.; Mangiavini, L.; Yao, A.Q.; Castellini, L.; Levi, B.; et al. Hypoxia-inducible factor 2α is a negative regulator of osteoblastogenesis and bone mass accrual. Bone Res. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Yang, S.; Zhang, T.; Sun, H.; Wang, Y.; Xue, H.; Zhou, D. Hypoxia enhances glucocorticoid-induced apoptosis and cell cycle arrest via the PI3K/Akt signaling pathway in osteoblastic cells. J. Bone Miner. Metab. 2015, 33, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, K.H.; Yu, H.-G.; Kook, E.; Song, W.-H.; Lee, G.; Koh, J.-T.; Shin, H.-I.; Choi, J.-Y.; Huh, Y.H.; et al. Controlling hypoxia-inducible factor-2α is critical for maintaining bone homeostasis in mice. Bone Res. 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Shirakura, M.; Tanimoto, K.; Eguchi, H.; Miyauchi, M.; Nakamura, H.; Hiyama, K.; Tanimoto, K.; Tanaka, E.; Takata, T.; Tanne, K. Activation of the hypoxia-inducible factor-1 in overloaded temporomandibular joint, and induction of osteoclastogenesis. Biochem. Biophys. Res. Commun. 2010, 393, 800–805. [Google Scholar] [CrossRef]
- Knowles, H.J. Distinct roles for the hypoxia-inducible transcription factors HIF-1α and HIF-2α in human osteoclast formation and function. Sci. Rep. 2020, 10, 21072. [Google Scholar] [CrossRef]
- Hulley, P.A.; Bishop, T.; Vernet, A.; Schneider, J.E.; Edwards, J.R.; Athanasou, N.A.; Knowles, H.J. Hypoxia-inducible factor 1-alpha does not regulate osteoclastogenesis but enhances bone resorption activity via prolyl-4-hydroxylase 2. J. Pathol. 2017, 242, 322–333. [Google Scholar] [CrossRef]
- Hiram-Bab, S.; Liron, T.; Deshet-Unger, N.; Mittelman, M.; Gassmann, M.; Rauner, M.; Franke, K.; Wielockx, B.; Neumann, D.; Gabet, Y. Erythropoietin directly stimulates osteoclast precursors and induces bone loss. FASEB J. 2015, 29, 1890–1900. [Google Scholar] [CrossRef]
- Hiram-Bab, S.; Neumann, D.; Gabet, Y. Erythropoietin in bone—Controversies and consensus. Cytokine 2017, 89, 155–159. [Google Scholar] [CrossRef]
- Deshet-Unger, N.; Hiram-Bab, S.; Haim-Ohana, Y.; Mittelman, M.; Gabet, Y.; Neumann, D. Erythropoietin treatment in murine multiple myeloma: Immune gain and bone loss. Sci Rep. 2016, 6, 30998. [Google Scholar] [CrossRef]
- Won, S.; Sayeed, I.; Peterson, B.L.; Wali, B.; Kahn, J.S.; Stein, D.G. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS ONE 2015, 10, e0122821. [Google Scholar] [CrossRef]
- López-Gómez, J.J.; Pérez Castrillón, J.L.; de Luis Román, D.A. Impact of obesity on bone metabolism. Endocrinol. Nutr. 2016, 63, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Fassio, A.; Idolazzi, L.; Rossini, M.; Gatti, D.; Adami, G.; Giollo, A.; Viapiana, O. The obesity paradox and osteoporosis. Eat. Weight Disord. 2018, 23, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Gonnelli, S.; Caffarelli, C.; Nuti, R. Obesity and fracture risk. Clin. Cases Miner. Bone Metab. 2014, 11, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.A.; Lenzi, A.; Migliaccio, S. The obesity of bone. Ther. Adv. Endocrinol. Metab. 2015, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.J.; Pérez-Castrillón, J.L.; García de Santos, I.; Pérez-Alonso, M.; Izaola-Jauregui, O.; Primo-Martín, D.; De Luis-Román, D.A. Influence of Obesity on Bone Turnover Markers and Fracture Risk in Postmenopausal Women. Nutrients 2022, 14, 1617. [Google Scholar] [CrossRef]
- Holecki, M.; Wiecek, A. Relationship between body fat mass and bone metabolism. Pol. Arch. Med. Wewn. 2010, 120, 361–367. [Google Scholar] [CrossRef]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A.; Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef]
- Olea, E.; Agapito, M.T.; Gallego-Martin, T.; Rocher, A.; Gomez-Niño, A.; Obeso, A.; Gonzalez, C.; Yubero, S. Intermittent hypoxia and diet-induced obesity: Effects on oxidative status, sympathetic tone, plasma glucose and insulin levels, and arterial pressure. J. Appl. Physiol. 2014, 117, 706–719. [Google Scholar] [CrossRef]
- Gonzalez-Obeso, E.; Docio, I.; Olea, E.; Cogolludo, A.; Obeso, A.; Rocher, A.; Gomez-Niño, A. Guinea Pig Oxygen-Sensing and Carotid Body Functional Properties. Front. Physiol. 2017, 8, 285. [Google Scholar] [CrossRef]
- Olea, E.; Docio, I.; Quintero, M.; Rocher, A.; Obeso, A.; Rigual, R.; Gomez-Niño, A. Peripheral Dopamine 2-Receptor Antagonist Reverses Hypertension in a Chronic Intermittent Hypoxia Rat Model. Int. J. Mol. Sci. 2020, 21, 4893. [Google Scholar] [CrossRef]
- Quintero, M.; Olea, E.; Conde, S.V.; Obeso, A.; Gallego-Martin, T.; Gonzalez, C.; Monserrat, J.M.; Gómez-Niño, A.; Yubero, S.; Agapito, T. Age protects from harmful effects produced by chronic intermittent hypoxia. J. Physiol. 2016, 594, 1773–1790. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.M.; Sophocleous, A. Quantitative analysis of bone and soft tissue by micro-computed tomography: Applications to ex vivo and in vivo studies. Bonekey Rep. 2014, 3, 564. [Google Scholar] [CrossRef] [PubMed]
- Van ’t Hof, R.J. Analysis of bone architecture in rodents using microcomputed tomography. Methods Mol. Biol. 2012, 816, 461–476. [Google Scholar] [PubMed]


| Bone Parameters | CCH | CIH | Non-Hypoxia | p-Value 1 | ||
|---|---|---|---|---|---|---|
| Cortical | TMD | Femur | 2.092 (0.278) | 1.912 (0.075) | 2.192 (0.141) * | 0.239 |
| Tibia | 2.080 (0.036) | 2.035 (0.093) | 2.087 (0.074) | 0.491 | ||
| Thickness | Femur | 2.739 (0.225) | 2.607 (0.141) | 2.681 (0.190) | 0.712 | |
| Tibia | 4.335 (0.306) | 4.440 (0.681) | 5.105 (0.231) # | 0.842 | ||
| Trabecular | BV/TV | Femur | 21.747 (6.883) | 24.429 (7.763) | 28.877 (7.112) # | 0.089 |
| Tibia | 17.842 (5.160) | 18.070 (5.846) | 30.194 (5.909) # * | 0.101 | ||
| Tb.Th | Femur | 0.048 (0.021) | 0.051 (0.008) | 0.050 (0.020) | 0.078 | |
| Tibia | 0.054 (0.018) | 0.041 (0.006) | 0.049 (0.023) * | 0.198 | ||
| Tb.Sp | Femur | 1.804 (0.761) | 1.720 (0.022) | 1.828 (0.327) | 0.823 | |
| Tibia | 2.142 (0.854) | 1.951 (0.716) | 1.988 (0.377) | 0.389 | ||
| Tb.N | Femur | 4.935 (1.401) | 5.027 (1.485) | 5.055 (1.226) | 0.506 | |
| Tibia | 3.166 (1.896) | 3.201 (1.392) | 5.025 (1.009) # * | 0.319 | ||
| BMD | Femur | 0.377 (0.127) | 0.417 (0.092) | 0.437 (0.119) # | 0.713 | |
| Tibia | 0.228 (0.110) | 0.275 (0.177) | 0.318 (0.092) # | 0.861 | ||
| Bone Parameters | Hypoxia | Non-Hypoxia | p-Value | ||
|---|---|---|---|---|---|
| Cortical | TMD | Femur | 2.019 (0.192) | 2.145 (0.281) | 0.439 |
| Tibia | 1.892 (0.313) | 2.023 (0.118) | 0.210 | ||
| Thickness | Femur | 0.612 (0.060) | 0.636 (0.129) | 0.089 | |
| Tibia | 0.560 (0.026) | 0.601 (0.293) | 0.218 | ||
| Trabecular | BV/TV | Femur | 48.849 (1.292) | 49.189 (3.371) | 0.591 |
| Tibia | 47.182 (2.032) | 48.293 (2.301) | 0.189 | ||
| Tb.Th | Femur | 0.110 (0.012) | 0.111 (0.002) | 0.742 | |
| Tibia | 0.089 (0.006) | 0.092 (0.003) | 0.087 | ||
| Tb.Sp | Femur | 0.098 (0.007) | 0.121 (0.017) | 0.421 | |
| Tibia | 0.162 (0.179) | 0.129 (0.025) | 0.761 | ||
| Tb.N | Femur | 3.886 (0.419) | 4.145 (0.211) | 0.139 | |
| Tibia | 3.379 (0.388) | 3.894 (0.510) | 0.201 | ||
| BMD | Femur | 0.561 (0.132) | 0.589 (0.244) | 0.459 | |
| Tibia | 0.490 (0.198) | 0.514 (0.291) | 0.278 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usategui-Martín, R.; Del Real, Á.; Sainz-Aja, J.A.; Prieto-Lloret, J.; Olea, E.; Rocher, A.; Rigual, R.J.; Riancho, J.A.; Pérez-Castrillón, J.L. Analysis of Bone Histomorphometry in Rat and Guinea Pig Animal Models Subject to Hypoxia. Int. J. Mol. Sci. 2022, 23, 12742. https://doi.org/10.3390/ijms232112742
Usategui-Martín R, Del Real Á, Sainz-Aja JA, Prieto-Lloret J, Olea E, Rocher A, Rigual RJ, Riancho JA, Pérez-Castrillón JL. Analysis of Bone Histomorphometry in Rat and Guinea Pig Animal Models Subject to Hypoxia. International Journal of Molecular Sciences. 2022; 23(21):12742. https://doi.org/10.3390/ijms232112742
Chicago/Turabian StyleUsategui-Martín, Ricardo, Álvaro Del Real, José A. Sainz-Aja, Jesús Prieto-Lloret, Elena Olea, Asunción Rocher, Ricardo J. Rigual, José A. Riancho, and José Luis Pérez-Castrillón. 2022. "Analysis of Bone Histomorphometry in Rat and Guinea Pig Animal Models Subject to Hypoxia" International Journal of Molecular Sciences 23, no. 21: 12742. https://doi.org/10.3390/ijms232112742
APA StyleUsategui-Martín, R., Del Real, Á., Sainz-Aja, J. A., Prieto-Lloret, J., Olea, E., Rocher, A., Rigual, R. J., Riancho, J. A., & Pérez-Castrillón, J. L. (2022). Analysis of Bone Histomorphometry in Rat and Guinea Pig Animal Models Subject to Hypoxia. International Journal of Molecular Sciences, 23(21), 12742. https://doi.org/10.3390/ijms232112742








