Pediatric Multisystem Syndrome Associated with SARS-CoV-2 (MIS-C): The Interplay of Oxidative Stress and Inflammation
Abstract
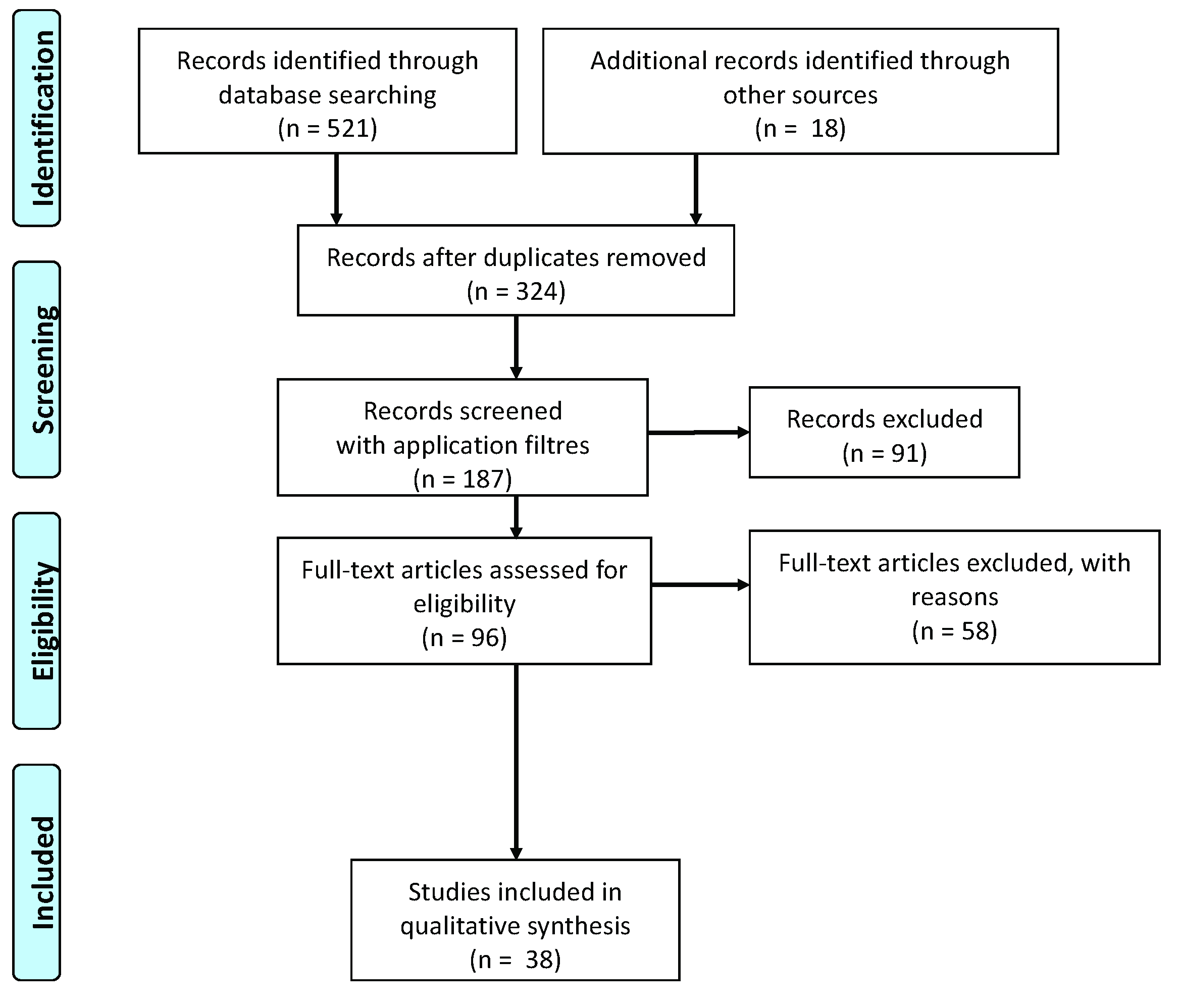
1. Introduction
2. MIS-C and Hyperinflammatory Syndromes
3. Immune Response in MIS-C
4. Oxidative Stress in MIS-C
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
List of Abbreviations
| COVID-19 | Coronavirus disease of 2019 |
| CRP | C-reactive protein |
| ESR | erythrocyte sedimentation rate |
| H2O2 | Hydrogen peroxide |
| IL | interleukin |
| LDH | lactate dehydrogenase |
| MAS | Macrophage activation syndrome |
| MIS-C | Multisystem inflammatory syndrome in children |
| NT-proBNP | N-terminal probrain natriuretic peptide |
| KD | Kawasaki disease |
| OS | oxidative stress |
| PT | prothrombin |
| PTT | partial thromboplastin time |
| RCT | randomized controlled trials |
| RT-PCR | reverse transcription-polymerase chain reaction |
| ROS | reactive oxygen species |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SIRS | systemic inflammatory response syndrome |
| TFH | T-follicular helper |
| TSS | toxic shock syndrome |
References
- Centers for Disease Control and Prevention. MIS-C and COVID-19. Available online: https://www.cdc.gov/mis-c/ (accessed on 29 March 2021).
- Rothan, H.A.; Byrareddy, S.N. The potential threat of multisystem inflammatory syndrome in children during the COVID-19 pandemic. Pediatr. Allergy Immunol. 2021, 32, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- WHO. Novel Coronavirus Situation Dashboard; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Paradowska-Stolarz, A.M. Oral manifestations of COVID-19: Brief review. Dent. Med. Probl. 2021, 58, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Maji, S.; Kumar, S.; Bhattacharya, S.; Chakraborty, P.; Sarkar, J. Clinical aspects and presumed etiology of multisystem inflammatory syndrome in children (MIS-C): A review. Clin. Epidemiol. Glob. Health 2022, 14, 100966. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, M.S.; Burrows, H.; Joseph, J.P.; Leveille, J.; Nihtianova, S.; Amirian, E.S. Multisystem inflammatory syndrome in children (MIS-C) and the coronavirus pandemic: Current knowledge and implications for public health. J. Infect. Public Health 2021, 14, 484–494. [Google Scholar] [CrossRef]
- Martynowicz, H.; Jodkowska, A.; Poręba, R.; Mazur, G.; Więckiewicz, M. Demographic, clinical, laboratory, and genetic risk factors associated with COVID-19 severity in adults: A narrative review. Dent. Med. Probl. 2021, 58, 115–121. [Google Scholar] [CrossRef]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem Inflammatory Syndrome in Children Related to COVID-19: A Systematic Review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- Carter, M.J.; Shankar-Hari, M.; Tibby, S.M. Paediatric Inflammatory Multisystem Syndrome Temporally-Associated with SARS-CoV-2 Infection: An Overview. Intensive Care Med. 2021, 47, 90–93. [Google Scholar] [CrossRef]
- Alonso de Vega, J.M.; Díaz, J.; Serrano, E.; Carbonell, L.F. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit. Care Med. 2002, 30, 1782–1786. [Google Scholar] [CrossRef]
- Tsal, K.; Hsu, T.G.; Kong, C.W.; Lin, K.; Lu, F. Is the endogenous peroxyl-radical scavenging capacity of plasma protective in systemic inflammatory disorders in human? Free Radic. Biol. Med. 2000, 28, 926–933. [Google Scholar]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Oxygen metabolism and the toxic properties of phagocytes. Ann. Intern. Med. 1980, 93, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Graciano-Machuca, O.; Villegas-Rivera, G.; López-Pérez, I.; Macías-Barragán, J.; Sifuentes-Franco, S. Multisystem Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Role of Oxidative Stress. Front. Immunol. 2021, 12, 723654. [Google Scholar] [CrossRef]
- Mondal, N.K.; Sorensen, E.N.; Pham, S.M.; Koenig, S.C.; Griffith, B.P.; Slaughter, M.S.; Wu, Z.J. Systemic Inflammatory Response Syndrome in End-Stage Heart Failure Patients Following Continuous-Flow Left Ventricular Assist Device Implantation: Differences in Plasma Redox Status and Leukocyte Activation. Artif. Organs 2016, 40, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Sharma, S.; Sood, I.; Sharma, K.; Kaushik, A. Emerging Evidence on Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection: A Systematic Review with Meta-analysis. SN Compr. Clin. Med. 2021, 3, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Yahata, T.; Hamaoka, K. Oxidative Stress and Kawasaki Disease: How Is Oxidative Stress Involved from the Acute Stage to the Chronic Stage? Rheumatology 2017, 56, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Seki, K. The Association between Oxidative Stress and Endothelial Dysfunction in Early Childhood Patients with Kawasaki Disease. BMC Cardiovasc. Disord. 2018, 18, 30. [Google Scholar] [CrossRef]
- Straface, E.; Gambardella, L.; Metere, A.; Marchesi, A.; Palumbo, G.; Cortis, E.; Villani, A.; Pietraforte, D.; Viora, M.; Malorni, W.; et al. Oxidative Stress and Defective Platelet Apoptosis in Naïve Patients with Kawasaki Disease. Biochem. Biophys. Res. Commun. 2010, 392, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.; Brodsky, N.N.; Sumida, T.S.; Comi, M.; Asashima, H.; Hoehn, K.B.; Li, N.; Liu, Y.; Shah, A.; Ravindra, N.G.; et al. Post-Infectious Inflammatory Disease in MIS-C Features Elevated Cytotoxicity Signatures and Autoreactivity That Correlates with Severity. Immunity 2021, 54, 1083–1095.e7. [Google Scholar] [CrossRef]
- Morise, T.; Takeuchi, Y.; Takeda, R.; Karayalcin, U.; Yachie, A.; Miyawaki, T. Increased Plasma Endothelin Levels in Kawasaki Disease: A Possible Marker for Kawasaki Disease. Angiology 1993, 44, 719–723. [Google Scholar] [CrossRef]
- Piechota, A.; Polanńczyk, A.; Goraca, A. Role of Endothelin-1 Receptor Blockers on Hemodynamic Parameters and Oxidative Stress. Pharmacol. Rep. PR 2010, 62, 28–34. [Google Scholar] [CrossRef]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981.e7. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Sole, A.; Anton, J.; Pino-Ramirez, R.M.; Sanchez-Manubens, J.; Fumadó, V.; Fortuny, C.; Rios-Barnes, M.; Sanchez-de-Toledo, J.; Girona-Alarcón, M.; Mosquera, J.M.; et al. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease. J. Clin. Investig. 2021, 131, e144554. [Google Scholar] [CrossRef] [PubMed]
- Maiese, A.; Frati, P.; Del Duca, F.; Santoro, P.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics 2021, 11, 1647. [Google Scholar] [CrossRef]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Mesta, F. Oxidative stress as key player in severe acute respiratory syndrome Coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020, 51, 384–387. Available online: https://www.sciencedirect.com/science/article/pii/S0188440920305403?via%3Dihub (accessed on 15 September 2022). [CrossRef]
- Baqi, H.R.M.; Farag, H.A.; El Bilbeisi, A.H.H.; Askandar, R.H.; El Afifi, A.M. Oxidative stress and its association with COVID-19: A narrative review. Kurd. J. Appl. Res. 2020, 5, 97–105. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem inflammatory syndrome in children in New York state. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef]
- Vella, L.A.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Diorio, C.; Kuri-Cervantes, L.; Alanio, C.; Pampena, M.B.; Wu, J.E.; Chen, Z.; et al. Deep Immune Profiling of MIS-C Demonstrates Marked But Transient Immune Activation Compared to Adult and Pediatric COVID-19. Sci. Immunol. 2021, 6, eabf7570. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Tataranno, M.L. Oxygen toxicity: Chemistry and biology of reactive oxygen species. Semin. Fetal Neonatal. Med. 2010, 15, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Sozio, C.; La Rosa, G.; Santillo, M. NOX-Dependent Signaling Dysregulation in Severe COVID-19: Clues to Effective Treatments. Front. Cell. Infect. Microbiol. 2020, 10, 608435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Li, G.Z.; Chen, S.Z.; Wang, J.J.; Olson, J.E.; Xia, H.J.; Lazartigues, E.; Zhu, Y.L.; Chen, Y.F. Angiotensin converting enzyme 2/Ang-(1-7)/mas axis protects brain from ischemic injury with a tendency of age-dependence. CNS Neurosci. Ther. 2014, 20, 452–459. [Google Scholar] [CrossRef]
- Katsuyama, M. NOX/NADPH oxidase, the superoxide-generating enzyme: Its transcriptional regulation and physiological roles. J. Pharmacol. Sci. 2010, 114, 134–146. [Google Scholar] [CrossRef]
- Ogier-Denis, E.; Mkaddem, S.B.; Vandewalle, A. NOX enzymes and Toll-like receptor signaling. Semin. Immunopathol. 2008, 30, 291–300. [Google Scholar] [CrossRef]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Laurindo, F.R.; Araujo, T.L.; Abrahão, T.B. Nox NADPH oxidases and the endoplasmic reticulum. Antioxid. Redox Signal. 2014, 20, 2755–2775. [Google Scholar] [CrossRef]
- Nauseef, W.M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004, 122, 277–291. [Google Scholar] [CrossRef]
- Ellson, C.; Davidson, K.; Anderson, K.; Stephens, L.R.; Hawkins, P.T. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006, 25, 4468–4478. [Google Scholar] [CrossRef]
- Suh, C.I.; Stull, N.D.; Li, X.J.; Tian, W.; Price, M.O.; Grinstein, S.; Yaffe, M.B.; Atkinson, S.; Dinauer, M.C. The phosphoinositide-bind- ing protein p40phox activates the NADPH oxidase during FcgIIA receptor-induced phagocytosis. J. Exp. Med. 2006, 203, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K. Novel NAD(P)H oxidases in the cardiovascular system. Heart 2004, 90, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Lardy, B.; Krause, K.H. NOX family NADPH oxidases: Not just in mammals. Biochimie 2007, 89, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Dinauer, M.C.; Newburger, P.E. The Phagocyte System and Disorders of Granulopoiesis and Granulocyte Function. In Nathan and Oski’s Hematology of Infancy and Childhood, 7th ed.; Orkin, S.H., Nathan, D.G., Ginsburg, D., Look, A.T., Fisher, D.E., Lux, S., Eds.; Elsevier Saunders: London, UK, 2009; pp. 1109–1217. [Google Scholar]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Vogel, T.P.; Top, K.A.; Karatzios, C.; Hilmers, D.C.; Tapia, L.I.; Moceri, P.; Giovannini-Chami, L.; Wood, N.; Chandler, R.E.; Klein, N.P.; et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021, 39, 3037–3049. [Google Scholar] [CrossRef]
- Maiese, A.; Russa, R.; Santoro, P.; Matteis, A.; Paolo, M.D. Future litigation after Covid-19 pandemic in Italy. Med. Leg. J. 2021, 89, 148–149. [Google Scholar] [CrossRef]

| World Health Organization [4] | Royal College of Pediatrics and Child Health (UK) | Centers for Disease Control and Prevention (US) [1] | |
|---|---|---|---|
| Age | 0–19 years of age. | 0–18 years of age. | Individual aged <21 years. |
| Clinical feature | Fever >3 days and 2 of the following: (a) Rash or bilateral nonpurulent conjunctivitis or muco-cutaneous inflammation signs (oral, hands or feet). (b) Hypotension or shock. (c) Features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including ECHO findings or elevated Troponin/NT-proBNP), (e) Acute gastrointestinal problems (diarrhea, vomiting, or abdominal pain). | Persistent fever > 38.5 °C. Evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal or neurological disorder) with additional features, which may include children fulfilling full or partial criteria for KD. | Fever ≥38.0 °C for ≥24 h, or report of subjective fever lasting ≥24 h. Severe illness necessitating hospitalization. 2 or more organ systems affected (e.g., cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, and neurological. |
| Laboratoristic criteria | Evidence of coagulopathy (by PT, PTT, elevated D-dimer). Elevated ESR, C-reactive protein, or procalcitonin. No other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal, or streptococcal shock syndromes. | Neutrophilia, elevated CRP, and lymphopenia | Elevated CRP, ESR, fibrinogen, procalcitonin, D-dimer, ferritin, LDH, or IL-6, elevated neutrophils, reduced lymphocytes, and low albumin. |
| COVID-19 relationship | Evidence of COVID-19 (RT-PCR, antigen test or serology positive), or likely contact with patients with COVID-19. | SARS-CoV-2 PCR testing may be positive or negative. | Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or COVID-19 exposure within the 4 weeks prior to onset of symptoms. |
| MIS-C | Kawasaki Disease | Hemophagocytic Lymphohis-Tiocytosis/Macrophage Activation Syndrome (HLH/MAS) | Toxic Shock Syndrome | |
|---|---|---|---|---|
| Age of affected persons | 4–15 years | <5years | <10 years | |
| Ethnicity | Hispanic/Latino/African American | Est Asian | No difference | No difference |
| Symptoms | ||||
| Hypotension | Present or absent | Generally absent | Generally absent | Present |
| Rash | Generally present | Generally present | Present or absent | Generally present |
| Fever | Present | Present | Present | Present |
| Vomiting/Diarrhea/or abdominal pain | Generally present | Rare | Present or absent | Generally present |
| Respiratory distress | Generally present | Rare | Generally present | Present |
| Myocarditis and pericarditis | Generally present | Rare | Present or absent | Rare |
| Hemodynamic instability | Present or absent | Rare | Present or absent | Present or absent |
| Coronary artery dilatation and aneu- rysms | Present or absent | Present | Generally absent | Generally absent |
| Heart involvement | Generally present | Present | Present or absent | Present or absent |
| Mucous Membrane Involvement | Present or absent | Generally present | Generally present | Rare |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, S.; Cannavò, L.; Manti, S.; Rulli, I.; Buonocore, G.; Esposito, S.M.R.; Gitto, E. Pediatric Multisystem Syndrome Associated with SARS-CoV-2 (MIS-C): The Interplay of Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2022, 23, 12836. https://doi.org/10.3390/ijms232112836
Perrone S, Cannavò L, Manti S, Rulli I, Buonocore G, Esposito SMR, Gitto E. Pediatric Multisystem Syndrome Associated with SARS-CoV-2 (MIS-C): The Interplay of Oxidative Stress and Inflammation. International Journal of Molecular Sciences. 2022; 23(21):12836. https://doi.org/10.3390/ijms232112836
Chicago/Turabian StylePerrone, Serafina, Laura Cannavò, Sara Manti, Immacolata Rulli, Giuseppe Buonocore, Susanna Maria Roberta Esposito, and Eloisa Gitto. 2022. "Pediatric Multisystem Syndrome Associated with SARS-CoV-2 (MIS-C): The Interplay of Oxidative Stress and Inflammation" International Journal of Molecular Sciences 23, no. 21: 12836. https://doi.org/10.3390/ijms232112836
APA StylePerrone, S., Cannavò, L., Manti, S., Rulli, I., Buonocore, G., Esposito, S. M. R., & Gitto, E. (2022). Pediatric Multisystem Syndrome Associated with SARS-CoV-2 (MIS-C): The Interplay of Oxidative Stress and Inflammation. International Journal of Molecular Sciences, 23(21), 12836. https://doi.org/10.3390/ijms232112836








