An Inflamed and Infected Reconstructed Human Epidermis to Study Atopic Dermatitis and Skin Care Ingredients
Abstract
:1. Introduction
- The inside-out signal, where chronic inflammation, driven by T helper (Th)2 cytokines, including interleukin (IL)-4 and IL-13, secondarily alters keratinocyte differentiation and reduces the expression of several epidermal barrier proteins [9];
2. Results
2.1. AD-like RHEs Treated with S. aureus and/or Cytokines
2.2. Treatment of RHE with Cytokines Alters Its Morphology at the Ultrastructural Level
2.3. Cytokine Treatment Promotes S. aureus Adhesion and Growth
2.4. Topically Applied S. aureus Increases the Secretion of IL-8
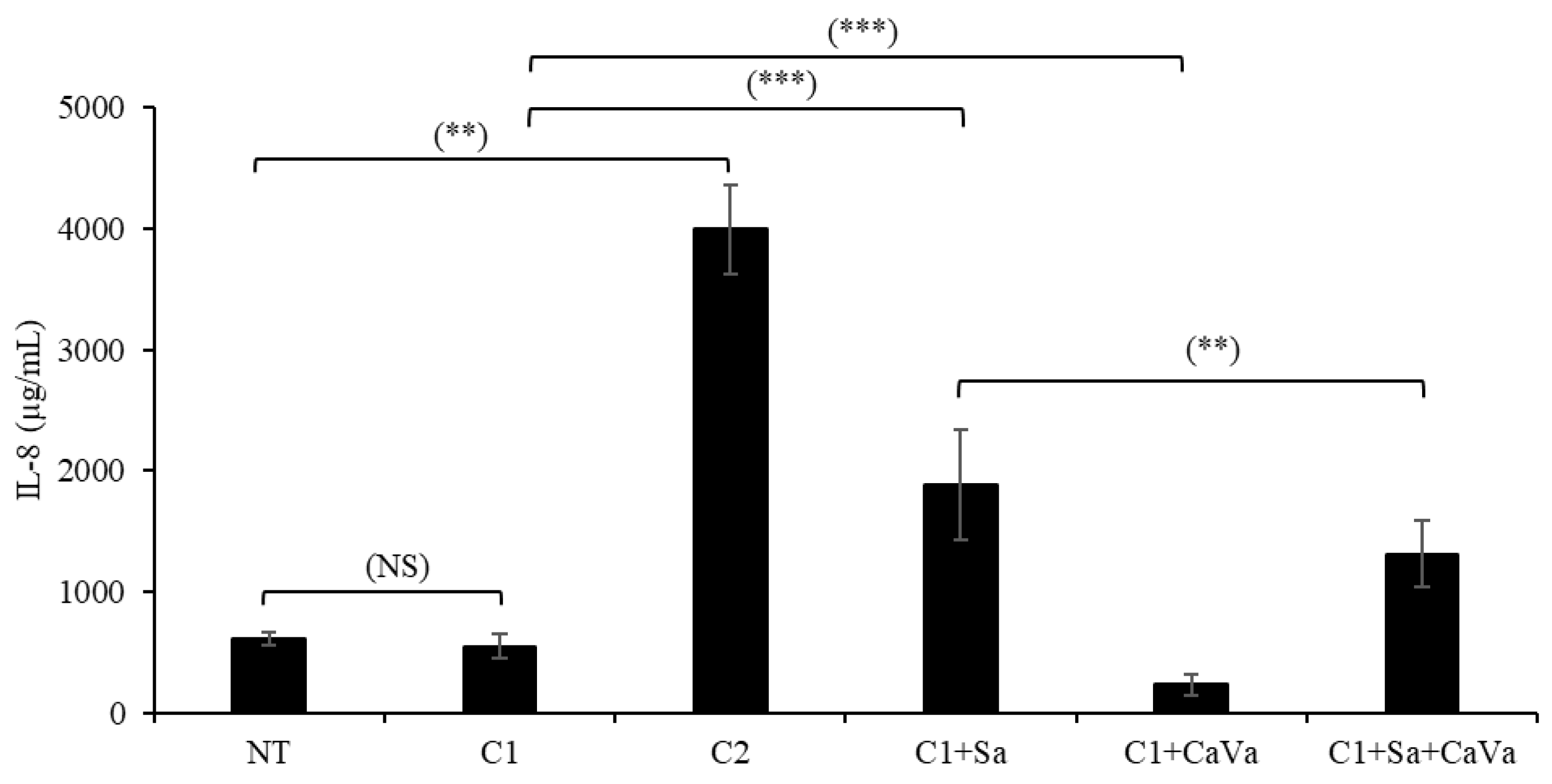
2.5. A Castanea Sativa Extract Reverses the AD-like Phenotype of Th2-Cytokine- and S. aureus-Treated RHEs
2.6. The Castanea Ssativa Extract Reduced S. aureus Virulence
3. Discussion
4. Materials and Methods
4.1. Castanea sativa Extract
4.2. RHE Production and Treatments with Cytokines, Castanea sativa Extract and Bacteria
4.3. Histological and Immunohistological Analyses of RHE Sections
4.4. Transmission Electron Microscopy
4.5. Western Blotting Analysis
4.6. IL-8 Measurement
4.7. S. aureus Biofilm Formation
4.8. Quantification of Enzymatic Activities Released by S. aureus
4.9. Cytokine Production by Human Keratinocytes
4.10. IL-8 Released by Human Macrophages
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bieber, T. Atopic Dermatitis: An Expanding Therapeutic Pipeline for a Complex Disease. Nat. Rev. Drug. Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Leshem, Y.A.; Hajar, T.; Hanifin, J.M.; Simpson, E.L. What the Eczema Area and Severity Index Score Tells Us about the Severity of Atopic Dermatitis: An Interpretability Study. Br. J. Dermatol. 2015, 172, 1353–1357. [Google Scholar] [CrossRef]
- Abdayem, R.; Haftek, M. The Epidermal Barrier. Ann. Dermatol. Venereol. 2018, 145, 293–301. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Epidermal Barrier in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2012, 4, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Elias, P.M. Structure and Function of the Stratum Corneum Extracellular Matrix. J. Investig. Dermatol. 2012, 132, 2131–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feingold, K.; Elias, P. The Important Role of Lipids in the Epidermis and Their Role in the Formation and Maintenance of the Cutaneous Barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 279. [Google Scholar] [CrossRef] [PubMed]
- Le Lamer, M.; Pellerin, L.; Reynier, M.; Cau, L.; Pendaries, V.; Leprince, C.; Méchin, M.-C.; Serre, G.; Paul, C.; Simon, M. Defects of Corneocyte Structural Proteins and Epidermal Barrier in Atopic Dermatitis. Biol. Chem. 2015, 396, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Multifactorial Skin Barrier Deficiency and Atopic Dermatitis: Essential Topics to Prevent the Atopic March. J. Allergy Clin. Immunol. 2016, 138, 350–358.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokura, Y. Drug Photoallergy. J. Cutan. Immunol. Allergy 2018, 1, 48–57. [Google Scholar] [CrossRef]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y.M. Cytokine Modulation of Atopic Dermatitis Filaggrin Skin Expression. J. Allergy Clin. Immunol. 2009, 124, R7–R12. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Leung, D.Y.M.; Boguniewicz, M.; Howell, M.D. Loricrin and Involucrin Expression Is Down-Regulated by Th2 Cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [Green Version]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight Junction Defects in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e7. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Moriyama, M.; Feld, M.; Buddenkotte, J.; Buhl, T.; Szöllösi, A.; Zhang, J.; Miller, P.; Ghetti, A.; Fischer, M.; et al. New Mechanism Underlying IL-31-Induced Atopic Dermatitis. J. Allergy Clin. Immunol. 2018, 141, 1677–1689.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smieszek, S.P.; Welsh, S.; Xiao, C.; Wang, J.; Polymeropoulos, C.; Birznieks, G.; Polymeropoulos, M.H. Correlation of Age-of-Onset of Atopic Dermatitis with Filaggrin Loss-of-Function Variant Status. Sci. Rep. 2020, 10, 2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanillas, B.; Novak, N. Atopic Dermatitis and Filaggrin. Curr. Opin. Immunol. 2016, 42, 1–8. [Google Scholar] [CrossRef]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the Frontline: Role in Skin Barrier Function and Disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Tokumasu, R.; Tamura, A.; Tsukita, S. Time- and Dose-Dependent Claudin Contribution to Biological Functions: Lessons from Claudin-1 in Skin. Tissue Barriers 2017, 5, e1336194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igawa, S.; Kishibe, M.; Minami-Hori, M.; Honma, M.; Tsujimura, H.; Ishikawa, J.; Fujimura, T.; Murakami, M.; Ishida-Yamamoto, A. Incomplete KLK7 Secretion and Upregulated LEKTI Expression Underlie Hyperkeratotic Stratum Corneum in Atopic Dermatitis. J. Investig. Dermatol. 2017, 137, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M.; Wakefield, J.S. Mechanisms of Abnormal Lamellar Body Secretion and the Dysfunctional Skin Barrier in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2014, 134, 781–791.e1. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The Important Role of Stratum Corneum Lipids for the Cutaneous Barrier Function. Biochim. Biophys. Acta 2014, 1841, 295–313. [Google Scholar] [CrossRef]
- Powers, C.E.; McShane, D.B.; Gilligan, P.H.; Burkhart, C.N.; Morrell, D.S. Microbiome and Pediatric Atopic Dermatitis. J. Dermatol. 2015, 42, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.R.; Gallo, R.L. The Role of the Skin Microbiome in Atopic Dermatitis. Curr. Allergy Asthma Rep. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus Aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef]
- De Vuyst, E.; Salmon, M.; Evrard, C.; Lambert de Rouvroit, C.; Poumay, Y. Atopic Dermatitis Studies through In Vitro Models. Front. Med. 2017, 4, 119. [Google Scholar] [CrossRef] [Green Version]
- Berroth, A.; Kühnl, J.; Kurschat, N.; Schwarz, A.; Stäb, F.; Schwarz, T.; Wenck, H.; Fölster-Holst, R.; Neufang, G. Role of Fibroblasts in the Pathogenesis of Atopic Dermatitis. J. Allergy Clin. Immunol. 2013, 131, 1547–1554.e6. [Google Scholar] [CrossRef]
- Kamsteeg, M.; Bergers, M.; de Boer, R.; Zeeuwen, P.L.J.M.; Hato, S.V.; Schalkwijk, J.; Tjabringa, G.S. Type 2 Helper T-Cell Cytokines Induce Morphologic and Molecular Characteristics of Atopic Dermatitis in Human Skin Equivalent. Am. J. Pathol. 2011, 178, 2091–2099. [Google Scholar] [CrossRef] [Green Version]
- Danso, M.O.; van Drongelen, V.; Mulder, A.; van Esch, J.; Scott, H.; van Smeden, J.; El Ghalbzouri, A.; Bouwstra, J.A. TNF-α and Th2 Cytokines Induce Atopic Dermatitis-like Features on Epidermal Differentiation Proteins and Stratum Corneum Lipids in Human Skin Equivalents. J. Investig. Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, H.; Nagao, K.; Kubo, A.; Hata, T.; Shimizu, A.; Mizuno, H.; Yamada, T.; Amagai, M. Altered Stratum Corneum Barrier and Enhanced Percutaneous Immune Responses in Filaggrin-Null Mice. J. Allergy Clin. Immunol. 2012, 129, 1538–1546.e6. [Google Scholar] [CrossRef] [Green Version]
- Pendaries, V.; Malaisse, J.; Pellerin, L.; Le Lamer, M.; Nachat, R.; Kezic, S.; Schmitt, A.-M.; Paul, C.; Poumay, Y.; Serre, G.; et al. Knockdown of Filaggrin in a Three-Dimensional Reconstructed Human Epidermis Impairs Keratinocyte Differentiation. J. Investig. Dermatol. 2014, 134, 2938–2946. [Google Scholar] [CrossRef]
- Elias, M.S.; Long, H.A.; Newman, C.F.; Wilson, P.A.; West, A.; McGill, P.J.; Wu, K.C.; Donaldson, M.J.; Reynolds, N.J. Proteomic Analysis of Filaggrin Deficiency Identifies Molecular Signatures Characteristic of Atopic Eczema. J. Allergy Clin. Immunol. 2017, 140, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Bäsler, K.; Galliano, M.-F.; Bergmann, S.; Rohde, H.; Wladykowski, E.; Vidal-y-Sy, S.; Guiraud, B.; Houdek, P.; Schüring, G.; Volksdorf, T.; et al. Biphasic Influence of Staphylococcus Aureus on Human Epidermal Tight Junctions: S. Aureus and Tight Junctions. Ann. N. Y. Acad. Sci. 2017, 1405, 53–70. [Google Scholar] [CrossRef]
- Pellerin, L.; Henry, J.; Hsu, C.-Y.; Balica, S.; Jean-Decoster, C.; Méchin, M.-C.; Hansmann, B.; Rodriguez, E.; Weindinger, S.; Schmitt, A.-M.; et al. Defects of Filaggrin-like Proteins in Both Lesional and Nonlesional Atopic Skin. J. Allergy Clin. Immunol. 2013, 131, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Omori-Miyake, M.; Yamashita, M.; Tsunemi, Y.; Kawashima, M.; Yagi, J. In Vitro Assessment of IL-4- or IL-13-Mediated Changes in the Structural Components of Keratinocytes in Mice and Humans. J. Investig. Dermatol. 2014, 134, 1342–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Jegga, A.G.; Shanmukhappa, K.S.; Edukulla, R.; Khurana, G.H.; Medvedovic, M.; Dillon, S.R.; Madala, S.K. IL-31-Driven Skin Remodeling Involves Epidermal Cell Proliferation and Thickening That Lead to Impaired Skin-Barrier Function. PLoS ONE 2016, 11, e0161877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatano, Y.; Terashi, H.; Arakawa, S.; Katagiri, K. Interleukin-4 Suppresses the Enhancement of Ceramide Synthesis and Cutaneous Permeability Barrier Functions Induced by Tumor Necrosis Factor-Alpha and Interferon-Gamma in Human Epidermis. J. Investig. Dermatol. 2005, 124, 786–792. [Google Scholar] [CrossRef] [Green Version]
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; et al. Lipid Abnormalities in Atopic Skin Are Driven by Type 2 Cytokines. JCI Insight 2018, 3, 98006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauber, M.; Balica, S.; Hsu, C.-Y.; Jean-Decoster, C.; Lauze, C.; Redoules, D.; Viodé, C.; Schmitt, A.-M.; Serre, G.; Simon, M.; et al. Staphylococcus Aureus Density on Lesional and Nonlesional Skin Is Strongly Associated with Disease Severity in Atopic Dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1272–1274.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Domenico, E.G.; Cavallo, I.; Capitanio, B.; Ascenzioni, F.; Pimpinelli, F.; Morrone, A.; Ensoli, F. Staphylococcus Aureus and the Cutaneous Microbiota Biofilms in the Pathogenesis of Atopic Dermatitis. Microorganisms 2019, 7, E301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus Aureus Biofilm: A Complex Developmental Organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Sieprawska-Lupa, M.; Mydel, P.; Krawczyk, K.; Wójcik, K.; Puklo, M.; Lupa, B.; Suder, P.; Silberring, J.; Reed, M.; Pohl, J.; et al. Degradation of Human Antimicrobial Peptide LL-37 by Staphylococcus Aureus -Derived Proteinases. Antimicrob. Agents Chemother. 2004, 48, 4673–4679. [Google Scholar] [CrossRef] [Green Version]
- Tam, K.; Torres, V.J. Staphylococcus Aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7, 7-2. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xiong, N.; Zhang, Y.; Rayner, S.; Chen, S. Functional Characterization of Lipase in the Pathogenesis of Staphylococcus Aureus. Biochem. Biophys. Res. Commun. 2012, 419, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Bokarewa, M.I.; Jin, T.; Tarkowski, A. Staphylococcus Aureus: Staphylokinase. Int. J. Biochem. Cell Biol. 2006, 38, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Ibberson, C.B.; Jones, C.L.; Singh, S.; Wise, M.C.; Hart, M.E.; Zurawski, D.V.; Horswill, A.R. Staphylococcus Aureus Hyaluronidase Is a CodY-Regulated Virulence Factor. Infect. Immun. 2014, 82, 4253–4264. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, M.; Nagasaki, S.; Ito, R.; Ohta, T. Sesquiterpene Farnesol as a Competitive Inhibitor of Lipase Activity of Staphylococcus Aureus. FEMS Microbiol. Lett. 2007, 273, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peetermans, M.; Vanassche, T.; Liesenborghs, L.; Claes, J.; Vande Velde, G.; Kwiecinksi, J.; Jin, T.; De Geest, B.; Hoylaerts, M.F.; Lijnen, R.H.; et al. Plasminogen Activation by Staphylokinase Enhances Local Spreading of S. Aureus in Skin Infections. BMC Microbiol. 2014, 14, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, I.D.; Cho, S.H.; Leung, D.Y.M. Role of Bacterial Superantigens in Atopic Dermatitis: Implications for Future Therapeutic Strategies. Am. J. Clin. Dermatol. 2006, 7, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Mempel, M.; Voelcker, V.; Köllisch, G.; Plank, C.; Rad, R.; Gerhard, M.; Schnopp, C.; Fraunberger, P.; Walli, A.K.; Ring, J.; et al. Toll-like Receptor Expression in Human Keratinocytes: Nuclear Factor KappaB Controlled Gene Activation by Staphylococcus Aureus Is Toll-like Receptor 2 but Not Toll-like Receptor 4 or Platelet Activating Factor Receptor Dependent. J. Investig. Dermatol. 2003, 121, 1389–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiekens, R.C.M.; Thepen, T.; Oosting, A.J.; Bihari, I.C.; Van De Winkel, J.G.J.; Bruijnzeel-Koomen, C.A.F.M.; Knol, E.F. Heterogeneity within Tissue-Specific Macrophage and Dendritic Cell Populations during Cutaneous Inflammation in Atopic Dermatitis. Br. J. Dermatol. 2001, 145, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Kasraie, S.; Werfel, T. Role of Macrophages in the Pathogenesis of Atopic Dermatitis. Mediat. Inflamm. 2013, 2013, 942375. [Google Scholar] [CrossRef] [Green Version]
- Ha, A.T.; Rahmawati, L.; You, L.; Hossain, M.A.; Kim, J.-H.; Cho, J.Y. Anti-Inflammatory, Antioxidant, Moisturizing, and Antimelanogenesis Effects of Quercetin 3-O-β-D-Glucuronide in Human Keratinocytes and Melanoma Cells via Activation of NF-κB and AP-1 Pathways. Int. J. Mol. Sci. 2022, 23, 433. [Google Scholar] [CrossRef]
- Mi, X.J.; Kim, J.K.; Lee, S.; Moon, S.K.; Kim, Y.J.; Kim, H. In vitro assessment of the anti-inflammatory and skin-moisturizing effects of Filipendula palmata (Pall.) Maxim. On human keratinocytes and identification of its bioactive phytochemicals. J. Ethnopharmacol. 2022, 296, 115523. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, S.H. Inhibitory effect of astragalin on expression of lipopolysaccharide-induced inflammatory mediators through NF-κB in macrophages. Arch. Pharm. Res. 2011, 34, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Soromou, L.W.; Chen, N.; Jiang, L.; Huo, M.; Wei, M.; Chu, X.; Millimouno, F.M.; Feng, H.; Sidime, Y.; Deng, X. Astragalin attenuates lipopolysaccharide-induced inflammatory responses by down-regulating NF-κB signaling pathway. Biochem. Biophys. Res. Commun. 2012, 419, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Frankart, A.; Malaisse, J.; De Vuyst, E.; Minner, F.; de Rouvroit, C.L.; Poumay, Y. Epidermal Morphogenesis during Progressive in Vitro 3D Reconstruction at the Air-Liquid Interface. Exp. Dermatol. 2012, 21, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Reynier, M.; Allart, S.; Gaspard, E.; Moga, A.; Goudounèche, D.; Serre, G.; Simon, M.; Leprince, C. Rab11a Is Essential for Lamellar Body Biogenesis in the Human Epidermis. J. Investig. Dermatol. 2016, 136, 1199–1209. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, D.; Hidalgo-Cantabrana, C.; Rodríguez, A.; García, P.; Ruas-Madiedo, P. Monitoring in Real Time the Formation and Removal of Biofilms from Clinical Related Pathogens Using an Impedance-Based Technology. PLoS ONE 2016, 11, e0163966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, R.H. The Turbidimetric Estimation of Hyaluronidase. Biochem. J. 1953, 55, 467–472. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadau, S.; Gault, M.; Berthelemy, N.; Hsu, C.-Y.; Danoux, L.; Pelletier, N.; Goudounèche, D.; Pons, C.; Leprince, C.; André-Frei, V.; et al. An Inflamed and Infected Reconstructed Human Epidermis to Study Atopic Dermatitis and Skin Care Ingredients. Int. J. Mol. Sci. 2022, 23, 12880. https://doi.org/10.3390/ijms232112880
Cadau S, Gault M, Berthelemy N, Hsu C-Y, Danoux L, Pelletier N, Goudounèche D, Pons C, Leprince C, André-Frei V, et al. An Inflamed and Infected Reconstructed Human Epidermis to Study Atopic Dermatitis and Skin Care Ingredients. International Journal of Molecular Sciences. 2022; 23(21):12880. https://doi.org/10.3390/ijms232112880
Chicago/Turabian StyleCadau, Sébastien, Manon Gault, Nicolas Berthelemy, Chiung-Yueh Hsu, Louis Danoux, Nicolas Pelletier, Dominique Goudounèche, Carole Pons, Corinne Leprince, Valérie André-Frei, and et al. 2022. "An Inflamed and Infected Reconstructed Human Epidermis to Study Atopic Dermatitis and Skin Care Ingredients" International Journal of Molecular Sciences 23, no. 21: 12880. https://doi.org/10.3390/ijms232112880





