Abstract
The association between APOE genotypes and cardiovascular disease (CVD) is partially mediated by LDL-cholesterol concentration but persists after adjusting for lipid levels and other cardiovascular risk factors. Data from the Aragon Workers Health Study (AWHS) (n = 4159) and the Lipid Unit at the Hospital Universitario Miguel Servet (HUMS) (n = 3705) were used to investigate the relationship between C-reactive protein (CRP) levels and APOE genotype. Lipoprotein particle and GlycA concentrations were analyzed in a subsample from AWHS. APOE genotyping was carried out by the Sanger method in both cohorts. APOE4 carriers had significantly lower levels of CRP than APOE3 carriers. Furthermore, APOE4 carriers had cholesterol-enriched LDL particles compared to APOE2 carriers. APOE4 carriers also had higher concentrations of small, medium, and large LDL particles. CRP levels were not associated with lipoprotein particle number, size, or composition. GlycA levels were not associated with APOE genotypes. However, GlycA levels were significantly associated with the size and the amount of cholesterol contained in HDL, VLDL, and LDL particles. APOE genotype influences CRP concentration regardless of lipid profile. APOE2 carriers showed the highest CRP levels, followed by APOE3 and APOE4. A more atherogenic lipid profile, but not inflammatory markers could partly explain the higher CVD risk observed in APOE4 carriers.
1. Introduction
Apolipoprotein E (ApoE) is a structural component of chylomicrons, as well as very-low-density (VLDL) and high-density (HDL) lipoproteins, which mediates the clearance of triglyceride-rich remnants, participates in the flow of cholesterol in macrophages in tissues, and modulates the generation of new HDL particles [1,2]. Thus, it plays a central function in lipid metabolism. ApoE is codified by the APOE gene, located in chromosome 19, q13.32, constituting three common alleles (E2, E3, and E4). The most common ApoE isoform is ApoE3, which has cysteine at residue 130 (SNV rs429358) and arginine at residue 176. ApoE2 has cysteine at residues 130 (SNV rs429358) and 176 (SNV rs7412). In contrast, the less common isoform, ApoE4, has arginine at both residues [3,4]. The receptor-binding function and lipid affinity of ApoE is particular to each isoform. Consequently, individuals carrying the ApoE4 isoform have higher low-density lipoprotein (LDL) cholesterol than those carrying the ApoE2 allele, whose binding capability to the LDL receptor is less than 2% [5]. ApoE3 is considered the wildtype reference isoform. In addition, ApoE2 has been associated with dysbetalipoproteinemia, while ApoE4 has been associated with Alzheimer’s disease and a higher risk of atherosclerosis and cardiovascular disease [2,4]. The association of the APOE genotype with cardiovascular disease is partially mediated by its influence on low-density lipoprotein (LDL) cholesterol concentrations. However, it persists despite adjustment for plasma lipids and classical cardiovascular risk factors [6].
One of the potential mechanisms for the association of the APOE locus with cardiovascular disease could be through inflammation-related mechanisms [7,8,9]. APOE gene variation has been associated with the concentration of C-reactive protein (CRP), a well-established marker of inflammation and an independent risk factor for cardiovascular disease (CVD) [10]. However, the mechanism of this association needs to be determined. In addition to CRP, a new biomarker of systemic inflammation, the glycoprotein acetyls (GlycA), was recently discovered for early cardiovascular risk in both young and older people [11,12]. In addition to knowing the central role of APOE in lipid metabolism, we hypothesize that this relationship between the APOE genotype and markers of inflammation could be related to lipid metabolism. Therefore, the objective of this study was to investigate the association of the APOE locus with CRP levels in two cohorts: patients from a Lipid Unit undergoing regular follow-up and subjects from the general population. Furthermore, this work analyzes the relationship between CRP and APOE genotype, which may depend on the lipid profile, by studying the composition and quantity of lipoparticles, as well as other markers of inflammation, such as GlycA, in a large group of individuals from the general population.
2. Results
Data from 7864 subjects were collected after applying exclusion criteria from both cohorts, including 3705 subjects from Lipid Unit from Hospital Universitario Miguel Servet (HUMS) and 4159 subjects from Aragon Workers’ Health Study (AWHS). As expected, HUMS subjects had significantly higher levels of total and LDL-cholesterol, TG, ApoA1, ApoB, and Lp(a) and lower HDL-cholesterol levels than the AWHS general population. HUMS subjects were significantly younger and had a significantly higher prevalence of diabetes. AWHS subjects were predominantly men (94.3%) in contrast to HUMS (49.4%). The APOE genotype distribution was significantly different between cohorts. The HUMS cohort had a higher prevalence of APOE2 and APOE4 alleles than the AWHS cohort (Table 1).

Table 1.
Clinical and biochemical characteristics in both cohorts.
Clinical and biochemical characteristics according to APOE genotype are summarized in Table 2. As expected, levels of TC, TG, ApoA1, and ApoB were significantly different according to the APOE genotype. APOE4 carriers had higher total and LDL-cholesterol values and lower TG levels (p < 0.001 in all cases). The Lp(a) levels were significantly different according to APOE genotype: higher in APOE4 carriers than APOE3 carriers and APOE2, with the lowest Lp(a) being observed in ApoE2/E2 subjects (p < 0.001 and p = 0.036, 4159, and 3705 for AWHS and HUMS, respectively, Table 2).

Table 2.
Clinical and biochemical characteristics according to APOE genotype in both cohorts.
2.1. APOE Genotype and C-Reactive Protein
Table 3 shows CRP levels according to APOE genotype in HUMS and AWHS cohorts. Both cohorts showed that APOE3/E3 subjects had greater levels of CRP than APOE3/E4 and APOE4/E4, respectively, (p < 0.001, in both cohorts), while higher values of CRP were found in E2 carriers.

Table 3.
CRP levels according to APOE genotype in HUMS and AWHS cohorts.
CRP levels were inverse weakly correlated to HDL-cholesterol levels and directly correlated to TG levels. Moreover, in the AWHS cohort, we found a positive weak correlation of LDL-cholesterol and TC levels with CRP (Figure 1).
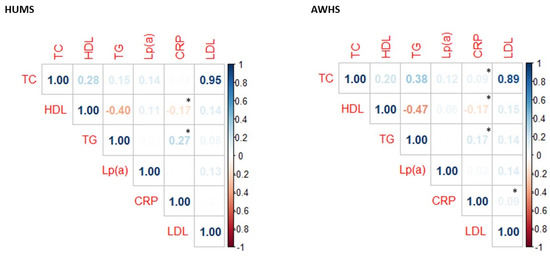
Figure 1.
Correlation between CRP levels and lipid profile in AWHS and HUMS cohorts. * p < 0.05 calculated using the Spearman method. TC: total cholesterol, HDL: high-density lipoprotein cholesterol, TG: triglyceride, Lp(a): lipoprotein (a), CRP: C-reactive protein, LDL: low-density lipoprotein cholesterol.
2.2. Association of APOE Genotype with Lipoprotein Particle Number, Composition, and Size
Among participants from the AWHS cohort, 1128 random serum samples were analyzed by NMR. A total of 132 subjects were carriers of at least one APOE2 allele, including APOE2/E2 and APOE2/E3 genotypes, 813 subjects were APOE3/E3, and 183 subjects were APOE3/E4 or APOE4/E4. HDL and VLDL lipoprotein subclasses did not show significant differences according to the APOE genotype (Tables S1 and S2). Nevertheless, LDL composition was significantly different according to APOE genotype. Subjects carrying APOE4 alleles presented a higher amount of cholesterol within LDL than carriers of the APOE2 allele, including small, medium, and large LDL particles (p = 0.004, p = 0.003, p = 0.003, and p = 0.005, respectively). APOE4 carriers also had a higher concentration of the small, medium, and large LDL particles (p = 0.006, p = 0.004, and p = 0.008, respectively, Table 4). These differences in LDL composition were maintained when subjects were distributed into five groups according to APOE genotype (Table S3). However, HDL and VLDL subclasses did not show differences when subjects were distributed into five groups according to APOE genotype (Tables S4 and S5).

Table 4.
LDL composition and fractions according to APOE allele in a subsample from AWHS cohort.
2.3. Association of APOE Genotype with Inflammatory Markers
Table 5 shows CRP and GlycA levels according to APOE genotype. As seen above, APOE genotype influenced CRP concentration regardless of lipid profile, showing that APOE2 carriers had the highest CRP levels, followed by APOE3 and APOE4 carriers. This latter group had the lowest levels of CRP (p = 0.009, Table 5). Moreover, we found that CRP levels were associated with APOE genotype independently of any lipoprotein particle number size or composition, suggesting that the association between CRP and APOE genotype seems independent of the lipid profile (Table 5). GlycA did not show any association with the APOE genotype. However, GlycA showed a significant association with the size and the amount of cholesterol contained in HDL, VLDL, and LDL (p = 0.002, p = 0.002, p = 0.027, p < 0.001, p < 0.001, and p < 0.001, respectively). However, it must be taken into account that the AWHS cohort was predominantly male, and that these associations were confirmed in a population with a homogeneous distribution of both sexes.

Table 5.
CRP and GlycA levels according to APOE genotype in in a subsample from AWHS cohort.
3. Discussion
This study showed that the CRP serum levels and APOE genotype are associated, and that the lipoprotein particle profile does not modulate this association. APOE4 carriers have significantly higher plasma TC concentrations due to higher levels of all LDL particle subfractions and a higher cholesterol concentration in each one of them. Conversely, the APOE genotype does not seem related to GlycA concentrations. However, this systemic inflammatory biomarker was associated in our cohort with the size and the amount of cholesterol contained in VLDL, LDL, and HDL.
The relationship between APOE genotype and CVD has been well established. In the Framingham Study, Lahoz et al. [13] concluded that the presence of the APOE2 or APOE4 alleles in men was associated with significantly greater CVD risk than APOE3/3 carriers. More recently, Dankner et al. [14] and Weiss et al. [6] demonstrated that, in men, the presence of the APOE4 allele was associated with CVD after adjusting for several confounding factors, such as age, ethnicity, physical activity, hypertension, diabetes, lipid levels, and lipid-lowering drugs. This association was also demonstrated in patients with type 2 diabetes and end-stage renal disease [15]. However, the origin of this association has not yet been completely elucidated. In a meta-analysis, Sofat et al. [16] concluded that there is no evidence of an association of circulating ApoE concentration with CVD events, indicating that isoform-specific functions may explain the established association between APOE genotype and CVD events rather than circulating concentrations of ApoE.
Two potential mechanisms behind the association of the APOE genotypes with CVD are the variation in lipid concentrations and the inflammation response to the different APOE alleles [17]. It is well established that APOE4 carriers have higher LDL-cholesterol concentration, probably via downregulation of the LDL receptor due to a higher affinity of APOE4-rich VLDL particles. In our study, APOE4 carriers had significantly higher cholesterol values and a higher number of all subclasses of LDL particles, associated with a more atherogenic lipid profile.
Numerous studies have identified inflammatory biomarkers associated with the development of CVD, including CRP, TNF-α, and several interleukins, or dysfunctional biomarkers of adiposity tissue, such as leptin or adiponectin [18,19], which improve the CVD risk classification, especially CRP [20,21]. Moreover, some studies have suggested that the association between APOE and CVD risk is partially mediated by a significant impact on inflammatory biomarkers, such as CRP levels, additionally modulated by factors such as sex and adiposity [22].
CRP is a short pentraxin found mainly as a pentamer in circulation or as non-soluble CRP monomers (mCRP). It is synthesized in the liver and induced by proinflammatory stimuli, such as IL-6, IL-1β, and TNF-α. CRP has a role in the humoral innate immune response but also contributes to the progression of cardiovascular disease by recognizing and binding to multiple intrinsic ligands. Although mCRP is not present in the healthy vessel wall, it becomes detectable in the early stages of atherogenesis. It accumulates during the progression of atherosclerosis, contributing to plaque instability by increasing the expression of endothelial cell adhesion molecules [23]. CRP plasma levels are also determined by polymorphisms in the CRP gene. These polymorphisms also modulate CVD risk [24]. Previous studies have shown that CRP levels are associated with the APOE genotype, indicating that APOE4 carriers have significantly lower and APOE2 carriers have significantly higher values of CRP than APOE3/E3 subjects [25,26]. This association is in contrast with the positive association between APOE4 and CVD. However, Tziakas et al. reported that APOE4 carriers had lower values of the atheroprotective IL-10, hypothesizing that perhaps this mechanism was the cause of the association between the APOE and CVD [27]. Another study reported significantly higher levels of lipoprotein-associated phospholipase A2, a vascular inflammation marker, in APOE4 carriers [28]. Our study found the same previously described relationship between CRP and APOE genotype. In addition, we found that this relationship seems to be independent of the lipid profile.
GlycA represents the integrated concentration and glycosylation of numerous acute-phase proteins (predominantly α-1-acid glycoprotein, haptoglobin, and α-1-antitrypsin) released in the inflammatory state. This novel inflammatory biomarker has a positive predictive value for the future development of type 2 diabetes and CVD independently of CRP in different cohorts [12,29], especially in young individuals [11]. GlycA was consistently associated with impaired endothelial function and increased diastolic blood pressure, suggesting a link between chronic inflammatory processes. GlycA is also associated with lifestyle, including physical activity, smoking habits, or socioeconomic status, and metabolic disorders [11]. We found that GlycA is significantly positively associated with the amount of cholesterol in VLDL, LDL, and HDL and the number of all these particles. No previous studies have reported the relationship between GlycA and APOE genotype. Our study found that the APOE genotype seems unrelated to the glycoprotein acetyl levels, suggesting that APOE4 carriers present a high risk of CVD due to a pathogenic mechanism unrelated to GlycA.
Our study had some limitations. First, our data were observational and do not imply causality. Second, 1% of the subjects from the HUMS were on lipid-lowering treatment at the time of the analysis, and it is known that reducing LDL-cholesterol with drugs, especially statins [30], reduces the concentration of CRP. However, the fact that the lipid-lowering treatment was homogeneously distributed among the different APOE genotypes and that the results were replicated in the AWHS cohort makes it unlikely that this fact had a relevant influence on the results. Thirdly, the analysis carried out generating small groups based on the APOE genotype may mean that the findings found are not generalizable. However, for this very reason, we also carried out the distribution of the individuals according to the APOE allele, observing that the relationship between the APOE genotype and CRP was also maintained, while it did not exist in the case of GlycA. Lastly, the AHWS cohort was predominantly male, which means that the results could have been partially biased. However, the HUMS cohort had a fairly homogeneous distribution between women and men, and we obtained the same results in both regarding the relationship between APOE genotype and CRP values. In addition, in both cases, the association studied was adjusted for age, sex, and BMI.
4. Materials and Methods
4.1. Design
This research was a cross-sectional, observational study that used data from two sources: the Aragon Workers’ Health Study (AWHS) [31] and the Hospital Universitario Miguel Servet (HUMS), both in Zaragoza (Spain).
4.2. Participants
4.2.1. AWHS
The AWHS is a longitudinal Caucasian cohort study started in 2009 based on an automobile assembly plant in Zaragoza, Spain. AWHS involves annual medical examinations and biological samples [31]. All workers were offered to participate in the study, and the response rate was 94.5%. The sample is predominantly male (>95%). Exclusion criteria included a history of personal CVD or the presence of clinical conditions that limited survival to less than 3 years.
4.2.2. HUMS
All consecutive unrelated studied patients with hyperlipidemia aged 18 to 80 from the Lipid Unit of HUMS from January 2006 to July 2022 were recruited for lipid research. This Lipid Unit is located in Zaragoza (Spain), and practically all of the individuals that comprise it are Caucasian. Subjects were excluded for the presence of secondary causes, including nephrotic syndrome, uncontrolled hypothyroidism (thyroid-stimulating hormone >6 mU/L), cholestasis (direct bilirubin >1 mg/dL), high alcohol intake (>30 g/day), or use of drugs that promote disorders of lipid metabolism (anabolic steroids, protease inhibitors, cyclosporine, mTOR kinase inhibitors, or cyclophosphamide). Most HUMS patients were removed from their lipid-lowering medication before their first visit to the Lipid Unit. After 6 weeks without medication, a complete biochemical analysis was performed, except for subjects with prior CVD or very high cardiovascular risk.
All AWHS participants and HUMS patients included in the study followed a complete clinical exam, analytic laboratory tests, and APOE genotyping. Exclusion criteria included a prior history of CVD, acute infectious or inflammatory disease, chronic anti-inflammatory drug use, or CRP serum levels higher than 10 mg/L.
4.3. Biochemical Analysis
AWHS and HUMS used the same biochemical protocols. Blood samples were drawn after overnight fasting without lipid-lowering treatment for at least 5 weeks, except in patients from HUMS with very high CVD risk. Laboratory tests were performed on the same day as blood sampling.
Serum glucose, triglycerides (TG), total cholesterol (TC), and HDL-cholesterol were measured by spectrophotometry (AU5800 Beckman Coulter Inc), and ApoAI and ApoB were measured by kinetic nephelometry (Immunochemistry Analyzer IMMAGE 800, Beckman Coulter, Brea, CA, USA). Lipoprotein(a) (Lp(a)) concentration was measured by rate nephelometry using LPAX reagent in conjunction with IMMAGE Immunochemistry Systems and Lp(a) Calibrator (OMS/IFCC SRM 2 B) (Beckman Coulter), following the manufacturer’s instructions. LDL-cholesterol was calculated using the Friedewald equation. Whole-blood HbA1c was measured by reverse-phase cationic exchange chromatography with double wave-length colorimetry quantification (Analyzer ADAMS A1c HA-810, Arkray Factory). High-sensitivity CRP was measured by latex-enhanced immunoturbidimetry (DXC 700 Au, Beckman Coulter).
4.4. APOE Genotype
Genomic DNA from whole-blood samples was isolated using standard methods. Exon 4 of the APOE gene was amplified by polymerase chain reaction and purified by ExoSap-IT (USB), as previously described [32]. Amplified fragments were sequenced by the Sanger method using the BigDye 3.1 sequencing kit (Applied Biosystems, Waltham, MA, USA) in an automated ABI 3500xL sequencer (Applied Biosystems). DNA sequences were analyzed using Variant Reporter software (Applied Biosystems).
4.5. Lipoprotein Particles and GlycA Concentration
Lipoprotein particles and GlycA were quantified from serum samples in a subset of 1128 volunteers from AWHS using high-throughput nuclear magnetic resonance (NMR) metabolomics (Nightingale Health Ltd., Helsinki, Finland). This method provides simultaneous quantification of lipids and lipoprotein subclass profiling with lipid concentrations within 14 subclasses. Details of the experimentation and applications of the NMR metabolomics platform were described previously [33,34]. In this study, for each lipoprotein subclass in the LDL, VLDL, and HDL range (small, medium, and large LDL; very small, small, medium, large, very large, and extremely very large VLDL; small, medium, large, and very large HDL), we determined particle concentration (nmol/L), size, and cholesterol and TG concentration (mmol/L).
4.6. Statistical Analysis
Continuous variables were expressed as the mean ± SD or median [25th percentile–75th percentile] as applicable, and categorical (nominal) variables were reported as percentages of the total sample. The p-value was calculated by ANOVA test or Kruskal–Wallis and chi-square tests, as appropriate. The relationship between CRP and APOE genotype was studied adjusted by age, sex, body mass index (BMI), TC, TG, ApoA1, and ApoB using linear regression models. The composition and fractions of VLDL, LDL, and HDL according to APOE alleles were adjusted by age, sex and BMI using linear regression models. All statistical analyses were performed with R version 3.5.0, including tidyverse, xlsx, dlpyr, gglot2, GGally, Hmsic, corrplot, and performanceAnalytics packages; significance was set at p < 0.05 [35].
5. Conclusions
In summary, we found a relationship between APOE genotypes and CRP plasma concentration in dyslipidemic subjects and the general population. APOE4 carriers had significantly lower levels of CRP than APOE3 carriers, who had lower levels than APOE2 carriers independently of age, sex, and BMI. This relationship between CRP and APOE genotypes seems to be independent of the lipid profile. Therefore, the higher risk of CVD associated with the ApoE4 allele could be partly explained by the higher levels of cholesterol-enriched LDL particles. Lastly, we did not observe significant independent associations between GlycA and APOE genotypes in our cohorts.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms232112947/s1.
Author Contributions
Conceptualization, F.C. and I.L.-M.; methodology, M.C.-M., A.M.B. and I.L.-M.; software, I.L.-M. and M.C.-M.; validation, I.L.-M., A.C. and M.L.; formal analysis, I.L.-M. and M.C.-M.; investigation, F.C. and A.C.; resources, J.M.O. and F.C.; data curation, V.M.-B., M.L. and B.M.-F.; writing—original draft preparation, I.L.-M., M.C.-M. and F.C.; writing—review and editing, A.C., R.M.-G., J.M.O. and F.C.; visualization, A.C., F.C. and R.M.-G.; supervision, F.C. and A.C.; project administration, F.C.; funding acquisition, F.C. and J.M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the Gobierno de Aragon, B14-7R, Spain, and the Spanish Ministry of Economy and competitiveness PI18/01777, PI19/0694, and CIBERCV. These projects were co-financed by Instituto de Salud Carlos III and the European Regional Development Fund (ERDF) of the European Union “A way to make Europe”. CIBERCV is a project of Instituto de Salud Carlos III.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the central Institutional Review Board of Aragón (Comité Ético de Investigación Clínica de Aragón, CEICA).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to carry out this study can be provided upon request to the principal investigators.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tudorache, I.F.; Trusca, V.G.; Gafencu, A.V. Apolipoprotein E—A Multifunctional Protein with Implications in Various Pathologies as a Result of Its Structural Features. Comput. Struct. Biotechnol. J. 2017, 15, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and Function in Lipid Metabolism, Neurobiology, and Alzheimer’s Diseases. Neurobiol. Dis. 2014, 72 Pt A, 3–12. [Google Scholar] [CrossRef]
- Greenow, K.; Pearce, N.J.; Ramji, D.P. The Key Role of Apolipoprotein E in Atherosclerosis. J. Mol. Med. 2005, 83, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Bu, G. Apolipoprotein E and Its Receptors in Alzheimer’s Disease: Pathways, Pathogenesis and Therapy. Nat. Rev. Neurosci. 2009, 10, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Bennet, A.M.; Angelantonio, E.D.; Ye, Z.; Wensley, F.; Dahlin, A.; Ahlbom, A.; Keavney, B.; Collins, R.; Wiman, B.; de Faire, U.; et al. Association of Apolipoprotein E Genotypes With Lipid Levels and Coronary Risk. JAMA 2007, 298, 1300–1311. [Google Scholar] [CrossRef]
- Weiss, J.; Hossain, S.; Maldonado, A.I.; Shen, B.; Beydoun, H.A.; Kivimaki, M.; Evans, M.K.; Zonderman, A.B.; Beydoun, M.A. Associations between Race, APOE Genotype, Cognition, and Mortality among Urban Middle-Aged White and African American Adults. Sci. Rep. 2021, 11, 19849. [Google Scholar] [CrossRef]
- Schönknecht, Y.B.; Crommen, S.; Stoffel-Wagner, B.; Coenen, M.; Fimmers, R.; Stehle, P.; Ramirez, A.; Egert, S. APOE Ɛ4 Is Associated with Postprandial Inflammation in Older Adults with Metabolic Syndrome Traits. Nutrients 2021, 13, 3924. [Google Scholar] [CrossRef]
- Jofre-Monseny, L.; Minihane, A.-M.; Rimbach, G. Impact of ApoE Genotype on Oxidative Stress, Inflammation and Disease Risk. Mol. Nutr. Food Res. 2008, 52, 131–145. [Google Scholar] [CrossRef]
- Hasel, P.; Liddelow, S.A. Isoform-Dependent APOE Secretion Modulates Neuroinflammation. Nat. Rev. Neurol. 2021, 17, 265–266. [Google Scholar] [CrossRef]
- Chai, J.T.; Ruparelia, N.; Goel, A.; Kyriakou, T.; Biasiolli, L.; Edgar, L.; Handa, A.; Farrall, M.; Watkins, H.; Choudhury, R.P. Differential Gene Expression in Macrophages From Human Atherosclerotic Plaques Shows Convergence on Pathways Implicated by Genome-Wide Association Study Risk Variants. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2718–2730. [Google Scholar] [CrossRef]
- Lawler, P.R.; Akinkuolie, A.O.; Chandler, P.D.; Moorthy, M.V.; Vandenburgh, M.J.; Schaumberg, D.A.; Lee, I.-M.; Glynn, R.J.; Ridker, P.M.; Buring, J.E.; et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ. Res. 2016, 118, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.T.; Charakida, M.; Georgiopoulos, G.; Roberts, J.D.; Stafford, S.J.; Park, C.; Mykkänen, J.; Kähönen, M.; Lehtimäki, T.; Ala-Korpela, M.; et al. Glycoprotein Acetyls: A Novel Inflammatory Biomarker of Early Cardiovascular Risk in the Young. J. Am. Heart Assoc. 2022, 11, e024380. [Google Scholar] [CrossRef] [PubMed]
- Lahoz, C.; Schaefer, E.J.; Cupples, L.A.; Wilson, P.W.; Levy, D.; Osgood, D.; Parpos, S.; Pedro-Botet, J.; Daly, J.A.; Ordovas, J.M. Apolipoprotein E Genotype and Cardiovascular Disease in the Framingham Heart Study. Atherosclerosis 2001, 154, 529–537. [Google Scholar] [CrossRef]
- Dankner, R.; Ben Avraham, S.; Harats, D.; Chetrit, A. ApoE Genotype, Lipid Profile, Exercise, and the Associations With Cardiovascular Morbidity and 18-Year Mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Hoffmann, M.M.; Krane, V.; März, W.; Drechsler, C.; Wanner, C. Apolipoprotein E Genotype Predicts Cardiovascular Endpoints in Dialysis Patients with Type 2 Diabetes Mellitus. Atherosclerosis 2010, 208, 197–202. [Google Scholar] [CrossRef]
- Sofat, R.; Cooper, J.A.; Kumari, M.; Casas, J.P.; Mitchell, J.P.; Acharya, J.; Thom, S.; Hughes, A.D.; Humphries, S.E.; Hingorani, A.D. Circulating Apolipoprotein E Concentration and Cardiovascular Disease Risk: Meta-Analysis of Results from Three Studies. PLoS Med. 2016, 13, e1002146. [Google Scholar] [CrossRef]
- Corsetti, J.P.; Bakker, S.J.L.; Sparks, C.E.; Dullaart, R.P.F. Apolipoprotein A-II Influences Apolipoprotein E-Linked Cardiovascular Disease Risk in Women with High Levels of HDL Cholesterol and C-Reactive Protein. PLoS ONE 2012, 7, e39110. [Google Scholar] [CrossRef]
- Li, J.; Lee, D.H.; Hu, J.; Tabung, F.K.; Li, Y.; Bhupathiraju, S.N.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; et al. Dietary Inflammatory Potential and Risk of Cardiovascular Disease Among Men and Women in the U.S. J. Am. Coll. Cardiol. 2020, 76, 2181–2193. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Yin, X.; Larson, M.G.; Yamamoto, J.F.; Fontes, J.D.; Kathiresan, S.; Rong, J.; Levy, D.; Keaney, J.F.; Wang, T.J.; et al. Multiple Inflammatory Biomarkers in Relation to Cardiovascular Events and Mortality in the Community. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1728–1733. [Google Scholar] [CrossRef]
- Woodward, M.; Welsh, P.; Rumley, A.; Tunstall-Pedoe, H.; Lowe, G.D.O. Do Inflammatory Biomarkers Add to the Discrimination of Cardiovascular Disease after Allowing for Social Deprivation? Results from a 10-Year Cohort Study in Glasgow, Scotland. Eur. Heart J. 2010, 31, 2669–2675. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinician’s Guide to Reducing Inflammation to Reduce Atherothrombotic Risk: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 3320–3331. [Google Scholar] [CrossRef] [PubMed]
- Kofler, B.M.; Miles, E.A.; Curtis, P.; Armah, C.K.; Tricon, S.; Grew, J.; Napper, F.L.; Farrell, L.; Lietz, G.; Packard, C.J.; et al. Apolipoprotein E Genotype and the Cardiovascular Disease Risk Phenotype: Impact of Sex and Adiposity (the FINGEN Study). Atherosclerosis 2012, 221, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Peña, E.; Arderiu, G.; Padró, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.A.; Carlson, C.S.; Hindorff, L.A.; Lange, E.M.; Walston, J.; Durda, J.P.; Cushman, M.; Bis, J.C.; Zeng, D.; Lin, D.; et al. Association of Polymorphisms in the CRP Gene with Circulating C-Reactive Protein Levels and Cardiovascular Events. JAMA 2006, 296, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Schick, U.M.; Auer, P.L.; Bis, J.C.; Lin, H.; Wei, P.; Pankratz, N.; Lange, L.A.; Brody, J.; Stitziel, N.O.; Kim, D.S.; et al. Association of Exome Sequences with Plasma C-Reactive Protein Levels in >9000 Participants. Hum. Mol. Genet. 2015, 24, 559–571. [Google Scholar] [CrossRef]
- Hubacek, J.A.; Peasey, A.; Pikhart, H.; Stavek, P.; Kubinova, R.; Marmot, M.; Bobak, M. APOE Polymorphism and Its Effect on Plasma C-Reactive Protein Levels in a Large General Population Sample. Hum. Immunol. 2010, 71, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Tziakas, D.N.; Chalikias, G.K.; Antonoglou, C.O.; Veletza, S.; Tentes, I.K.; Kortsaris, A.X.; Hatseras, D.I.; Kaski, J.C. Apolipoprotein E Genotype and Circulating Interleukin-10 Levels in Patients with Stable and Unstable Coronary Artery Disease. J. Am. Coll. Cardiol. 2006, 48, 2471–2481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gungor, Z.; Anuurad, E.; Enkhmaa, B.; Zhang, W.; Kim, K.; Berglund, L. Apo E4 and Lipoprotein-Associated Phospholipase A2 Synergistically Increase Cardiovascular Risk. Atherosclerosis 2012, 223, 230–234. [Google Scholar] [CrossRef]
- Akinkuolie, A.O.; Pradhan, A.D.; Buring, J.E.; Ridker, P.M.; Mora, S. Novel Protein Glycan Side-Chain Biomarker and Risk of Incident Type 2 Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1544–1550. [Google Scholar] [CrossRef]
- Kinlay, S. Low-Density Lipoprotein-Dependent and -Independent Effects of Cholesterol-Lowering Therapies on C-Reactive Protein: A Meta-Analysis. J. Am. Coll. Cardiol. 2007, 49, 2003–2009. [Google Scholar] [CrossRef]
- Casasnovas, J.A.; Alcaide, V.; Civeira, F.; Guallar, E.; Ibañez, B.; Borreguero, J.J.; Laclaustra, M.; León, M.; Peñalvo, J.L.; Ordovás, J.M.; et al. Aragon Workers’ Health Study—Design and Cohort Description. BMC Cardiovasc. Disord. 2012, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Bartens, W.; Rader, D.J.; Talley, G.; Brewer, H.B. Decreased Plasma Levels of Lipoprotein(a) in Patients with Hypertriglyceridemia. Atherosclerosis 1994, 108, 147–149. [Google Scholar] [CrossRef]
- Würtz, P.; Kangas, A.J.; Soininen, P.; Lawlor, D.A.; Davey Smith, G.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2017, 186, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Tukiainen, T.; Tynkkynen, T.; Laatikainen, R.; Järvelin, M.-R.; Kähönen, M.; Lehtimäki, T.; Viikari, J.; et al. High-Throughput Serum NMR Metabonomics for Cost-Effective Holistic Studies on Systemic Metabolism. Analyst 2009, 134, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R development core team. RA Lang Environ. Stat. Comput. 2013, 55, 275–286. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).