In Vivo Assessments of Mesoblastic Nephroma (Ne/De) and Myelomonoblastic Leukaemia (My1/De) Tumour Development in Hypercholesterolemia Rat Models
Abstract
1. Introduction
2. Results
2.1. Effect of Butter and Cholesterol-Rich (BCR) Diet on Healthy Rats
2.2. Effect of BCR Animal Diet on the Blood Cholesterol Levels of Mesoblastic Nephroma (Ne/De)-Transplanted F-344 Rats
2.3. Growth of Ne/De Tumours in Normolipidaemic and Dyslipidaemic Rats
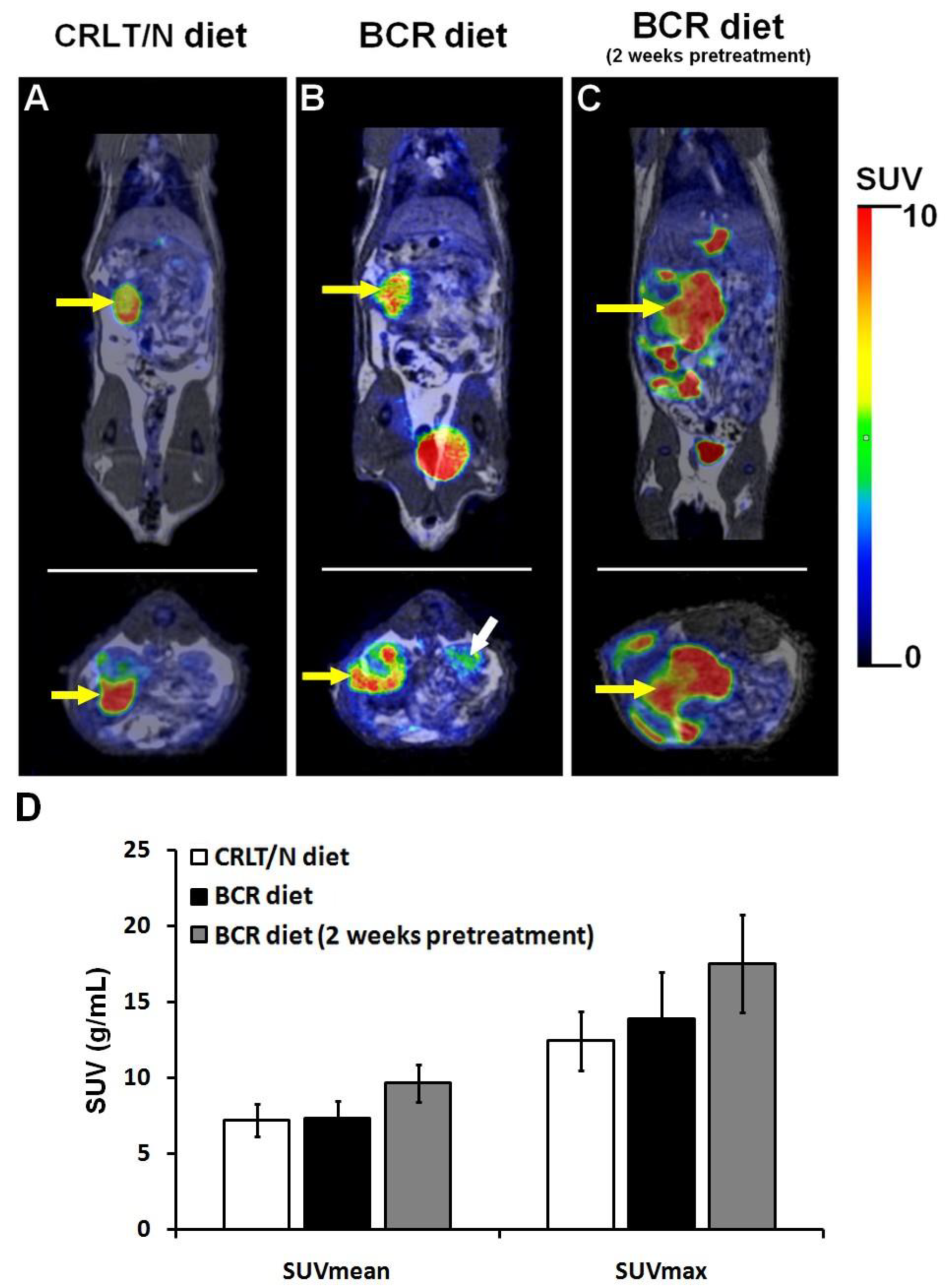
2.4. 2-Deoxy-2-[18F]Fluoro-D-Glucose Positron Emission Tomography/Magnetic Resonance Imaging ([18F]F-FDG PET/MRI) Examinations of Ne/De Tumours in Normolipidaemic and Dyslipidaemic Rats
2.5. Impact of BCR Diet on Blood Cholesterol Levels of Myelomonoblastic Leukaemia (My1/De) Tumour-Bearing Long–Evans Rats
2.6. Growth of My1/De Tumours in Normolipidaemic and Dyslipidaemic Rats
2.7. [18F]F-FDG PET/MRI Examinations of My1/De Tumours in Normolipidaemic and Dyslipidaemic Rats
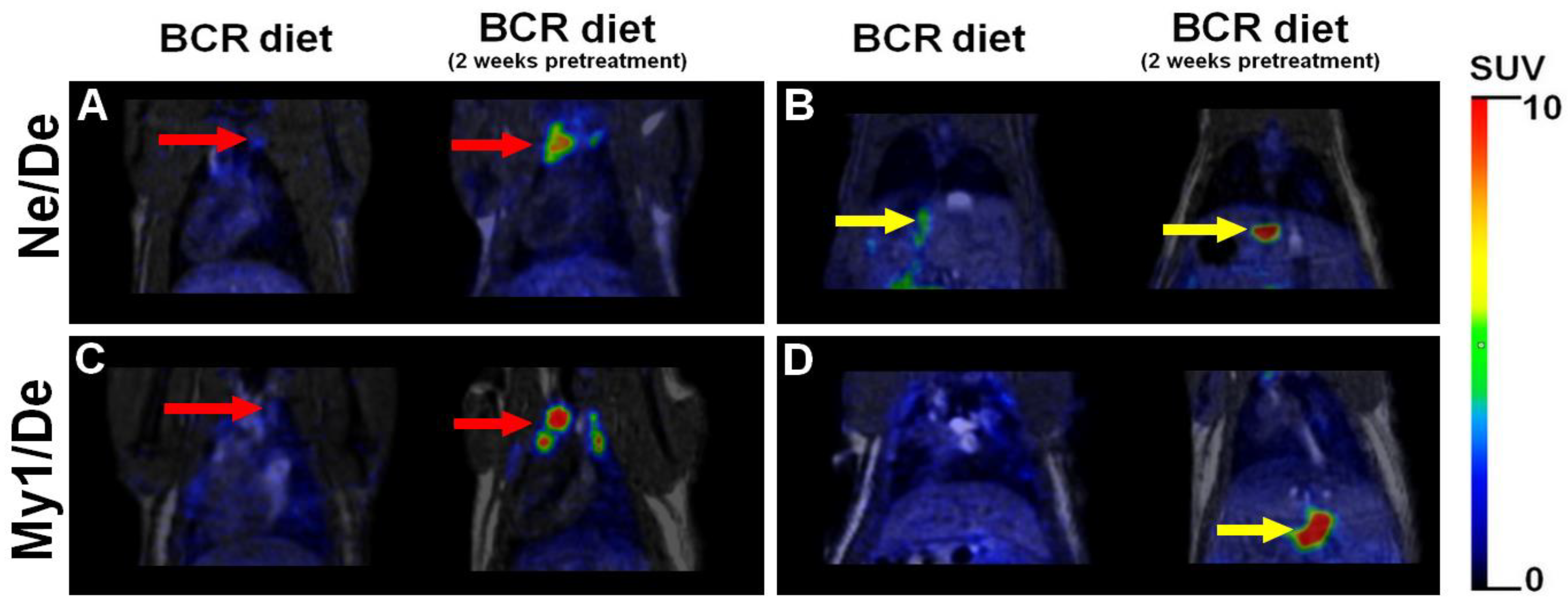
2.8. [18F]F-FDG PET/MRI Investigation on Metastases in Tumour-Bearing Dyslipidaemic Rats
3. Discussion
4. Materials and Methods
4.1. Cell Culturing
4.2. Experimental Animals
4.3. Animal Feed
4.4. Surgical Procedure
4.5. Blood Tests
4.6. A case of [18F]F-FDG PET/MRI: In Vivo PET/MRI Examinations
- (a)
- SUV (g/mL), which determines the concentration of the tracer within the region of interest, that is, the volume of interest (VOI) based on the administered dose and the weight of the animal.
- (b)
- SUVmean, which is the average activity concentration of the VOI.
- (c)
- SUVmax, which refers to the voxel with the highest activity within the VOI.
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCR | Butter and cholesterol rich |
| CK | Creatine kinase |
| CRD | Cholesterol-rich diet |
| CRLT/N | Charles River Laboratories feed/normal |
| [18F]FDG | [18F] labelled fluoro-deoxy-glucose |
| HDL | High-density lipoprotein |
| He/De | Hepatocellular cell line |
| i.p. | Intraperitoneal |
| i.v. | Intravenous |
| LDL | Low-density lipoprotein |
| LDL-R | Low-density lipoprotein receptor |
| NAFLD-HCC | Non-alcoholic fatty liver disease-related hepatocellular carcinoma |
| Ne/De | Mesoblastic nephroma |
| My1/De | Myelomonoblastic leukaemia |
| PET/MRI | Positron emission tomography/magnetic resonance imaging |
| SRCA | Subrenal capsule assay |
| SUV | Standardised uptake value |
| VOI | Volume of interest |
References
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.A.; Pan, L.; Abney, M.; Ober, C. The sex-specific genetic architecture of quantitative traits in humans. Nat. Genet. 2006, 38, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, R.A. Inherited metabolic disorders and dyslipidaemia. J. Clin. Pathol. 2020, 73, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Bakiri, L.; Hamacher, R.; Graña, O.; Guío-Carrión, A.; Campos-Olivas, R.; Martinez, L.; Dienes, H.P.; Thomsen, M.K.; Hasenfuss, S.C.; Wagner, E.F. Liver carcinogenesis by FOS-dependent inflammation and cholesterol dysregulation. J. Exp. Med. 2017, 214, 1387–1409. [Google Scholar] [CrossRef]
- Du, Q.; Wang, Q.; Fan, H.; Wang, J.; Liu, X.; Wang, H.; Wang, Y.; Hu, R. Dietary cholesterol promotes AOM-induced colorectal cancer through activating the NLRP3 inflammasome. Biochem. Pharm. 2016, 105, 42–54. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Yang, L.; Sun, J.; Li, M.; Long, Y.; Zhang, D.; Guo, H.; Huang, R.; Yan, J. Oxidized Low-Density Lipoprotein Links Hypercholesterolemia and Bladder Cancer Aggressiveness by Promoting Cancer Stemness. Cancer Res. 2021, 81, 5720–5732. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Moholdt, T.; Lavie, C.J.; Nauman, J. Sustained Physical Activity, Not Weight Loss, Associated with Improved Survival in Coronary Heart Disease. J. Am. Coll. Cardiol. 2018, 71, 1094–1101. [Google Scholar] [CrossRef]
- Abid, Z.; Cross, A.J.; Sinha, R. Meat, dairy, and cancer. Am. J. Clin. Nutr. 2014, 100, 386S–393S. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose–response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, M.A.M.; Malhotra, N.; Mukherjee, S.D.; Sanger, S.; Dhesy-Thind, S.K.; Ellis, P.; Leong, D.P. Statin therapy in the treatment of active cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0209486. [Google Scholar] [CrossRef] [PubMed]
- Trencsenyi, G.; Kertai, P.; Bako, F.; Hunyadi, J.; Marian, T.; Hargitai, Z.; Pocsi, I.; Muranyi, E.; Hornyak, L.; Banfalvi, G. Renal capsule-parathymic lymph node complex: A new In Vivo metastatic model in rats. Anticancer. Res. 2009, 29, 2121–2126. [Google Scholar] [PubMed]
- Padra, J.; Kertai, P.; Fóris, G.; Fülöp, P.; Seres, I.; Oláh, A.; Balogh, Z.; Paragh, G. MS488 Effects of fluvastatin on tumor development and paraoxonase-1 activity in rats fed with cholesterol-rich diet. Atheroscler. Suppl. 2010, 2, 208. [Google Scholar] [CrossRef]
- Paragh, G.; Seres, I.; Olah, A.; Balogh, Z.; Foris, G.; Kertai, P. Animal model for simultaneuos study of statin-effects against hypercholesterolemia and tumor development. Atheroscler. Suppl. 2008, 1, 196. [Google Scholar] [CrossRef]
- You, W.; Henneberg, M. Cancer incidence increasing globally: The role of relaxed natural selection. Evol. Appl. 2018, 11, 140–153. [Google Scholar] [CrossRef]
- Paragh, G.; Foris, G.; Paragh, G., Jr.; Serest, I.; Karanyi, Z.; Fulop, P.; Balogh, Z.; Kosztaczky, B.A.; Kertai, P. W16-P-067 Different anticancer effects of fluvastatin on primary hepatocellular tumors and metastases in rats. Atheroscler. Suppl. 2005, 1, 118. [Google Scholar] [CrossRef]
- Füzi, M.; Palicz, Z.; Vincze, J.; Cseri, J.; Szombathy, Z.; Kovács, I.; Oláh, A.; Szentesi, P.; Kertai, P.; Paragh, G.; et al. Fluvastatin-induced alterations of skeletal muscle function in hypercholesterolaemic rats. J. Muscle. Res. Cell Motil. 2012, 32, 391–401. [Google Scholar] [CrossRef][Green Version]
- Paragh, G.; Kertai, P.; Kovacs, P.; Paragh, G., Jr.; Fülöp, P.; Foris, G. HMG CoA reductase inhibitor fluvastatin arrests the development of implanted hepatocarcinoma in rats. Anticancer. Res. 2003, 23, 3949–3954. [Google Scholar]
- Fillios, L.C.; Andrus, S.B.; Mann, G.V.; Stare, F.J. Experimental production of gross Atherosclerosis in the rat. J. Exp. Med. 1956, 104, 539–554. [Google Scholar] [CrossRef]
- Padra, J.T.; Seres, I.; Oláh, A.; Fenyvesi, F.; Paragh, G.; Paragh, G.; Csernoch, L.; Fóris, G.; Kertai, P. A comparative study on dyslipidaemia inducing diets in various rat strains. Acta. Physiol. Hung. 2014, 101, 250–258. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; López-Vilaró, L.; Nasarre, L.; Perez-Olabarria, M.; Vázquez, T.; Escuin, D.; Badimon, L.; Barnadas, A.; Lerma, E.; Llorente-Cortés, V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer 2015, 15, 460. [Google Scholar] [CrossRef] [PubMed]
- Khallouki, F.; Owen, R.W.; Silvente-Poirot, S.; Poirot, M. Bryonolic Acid Blocks Cancer Cell Clonogenicity and Invasiveness through the Inhibition of Fatty Acid: Cholesteryl Ester Formation. Biomedicines 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- de Medina, P.; Diallo, K.; Huc-Claustre, E.; Attia, M.; Soulès, R.; Silvente-Poirot, S.; Poirot, M. The 5,6-epoxycholesterol metabolic pathway in breast cancer: Emergence of new pharmacological targets. Br. J. Pharmacol. 2021, 178, 3248–3260. [Google Scholar] [CrossRef] [PubMed]
- Poirot, M.; Soules, R.; Mallinger, A.; Dalenc, F.; Silvente-Poirot, S. Chemistry, biochemistry, metabolic fate and mechanism of action of 6-oxo-cholestan-3β, 5α-diol (OCDO), a tumor promoter and cholesterolmetabolite. Biochimie 2018, 153, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Silvente-Poirot, S.; Dalenc, F.; Poirot, M. The Effects of Cholesterol-Derived Oncometabolites on Nuclear Receptor Functionin Cancer. Cancer Res. 2018, 78, 4803–4808. [Google Scholar] [CrossRef]
- Voisin, M.; de Medina, P.; Mallinger, A.; Dalenc, F.; Huc-Claustre, E.; Leignadier, J.; Serhan, N.; Soules, R.; Ségala, G.; Mougel, A.; et al. Identification of a tumor-promoter cholesterol metabolite in human breast cancers acting through the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 2017, 114, E9346–E9355. [Google Scholar] [CrossRef]
- Jasim, S.A.; Kzar, H.H.; Haider, H.M.; Ahmad, I.; Al-Gazally, M.E.; Ziyadullaev, S.; Sivaraman, R.; Abed, J.M.; Thaeer, H.A.; Oudaha, K.H.; et al. The emerging role of 27-hydroxycholesterol in cancer development and progression: An update. Int. Immunopharmacol. 2022, 110, 109074. [Google Scholar] [CrossRef]
- Sós, J.; Szelényi, I. Mineral supply in experimental diets. In Diets for Animal Experiments; Sós, J., Szelényi, I., Eds.; Akadémiai Kiadó, Budapest: Budapest, Hungary, 1974; pp. 139–157. [Google Scholar]
- Uzvolgyi, E.; Katona, A.; Kertai, P. Tumor cell implantation with the use of Gelaspon® gelatin sponge disc. Cancer Lett. 1990, 51, 1–5. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Sugiuchi, H.; Uji, Y.; Okabe, H.; Irie, T.; Uekama, K.; Kayahara, N.; Miyauchi, K. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated alpha-cyclodextrin. Clin. Chem. 1995, 41, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Sugiuchi, H.; Irie, T.; Uji, Y.; Ueno, T.; Chaen, T.; Uekama, K.; Okabe, H. Homogeneous assay for measuring low-density lipoprotein cholesterol in serum with triblock copolymer and α-cyclodextrin sulfate. Clin. Chem. 1998, 44, 522–531. [Google Scholar] [CrossRef] [PubMed]
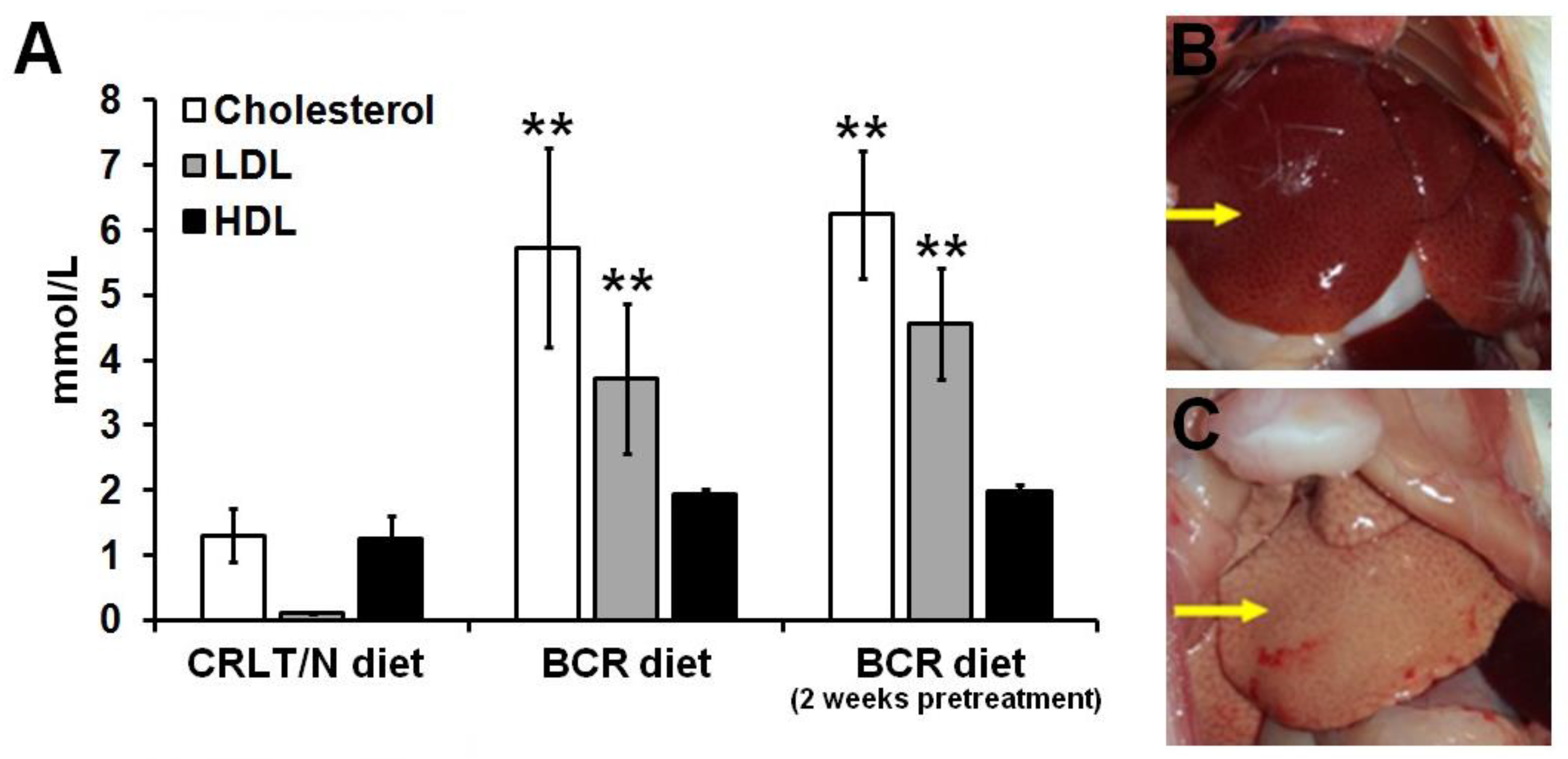



| Ne/De Tumorous Rats | Left Kidney (g) | Right Kidney (g) | Tumour Mass (g) |
|---|---|---|---|
| CRLT/N | 7.46 ± 1.31 | 0.82 ± 0.20 | 6.74 ± 0.81 |
| BCR diet | 8.31 ± 0.73 | 0.75 ± 0.17 | 7.28 ± 0.73 |
| BCR diet (2 weeks of pretreatment) | 13.42 ± 1.09 * | 0.78 ± 0.13 | 12.93 ± 1.27 ** |
| My1/De | Left Kidney (g) | Right Kidney (g) | Tumour Mass (g) |
|---|---|---|---|
| CRLT/N | 3.22 ± 1.05 | 1.12 ± 0.24 | 2.47 ± 0.64 |
| BCR diet | 4.62 ± 0.89 | 0.99 ± 0.31 | 3.45 ± 0.39 |
| pretreated BCR diet | 7.46 ± 1.14 * | 1.06 ± 0.24 | 6.26 ± 0.89 ** |
| Groups | Status | Diet | Administration of Rodent Chow (Number of Weeks) |
|---|---|---|---|
| 1 | Healthy | Standard CRLT/N | 2 |
| 2 | Healthy | BCR | 2 (baseline) |
| 3 | Healthy | BCR | 2 + 2 (pretreatment + maintenance) |
| 4 | Tumorous | Standard CRLT/N | 2 |
| 5 | Tumorous | BCR | 2 (baseline) |
| 6 | Tumorous | BCR | 2 + 2 (pretreatment + maintenance) |
| Standard CRLT/N Diet | |
|---|---|
| Component | Composition (%) |
| Dry matter | 86.00 |
| Crude protein | 20.00 |
| Digested protein | 18.00 |
| Lysine | 0.97 |
| Methionine | 0.30 |
| Methionine + cysteine | 0.64 |
| Crude fat | 4.00 |
| Crude fibre | 4.30 |
| Calcium | 0.96 |
| Phosphorus | 0.67 |
| Sodium | 0.20 |
| BCR Diet | |
|---|---|
| Component | Composition (%) |
| Standard CRLT/N feed | 60.00 |
| Casein | 9.50 |
| Butter | 20.00 |
| Cholesterol | 4.00 |
| Sodium cholate | 1.00 |
| “SM8” salt mixture, according to Sós [29] | 5.00 |
| 6-Methyl-2-thio-uracil | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Képes, Z.; Barkóczi, A.; Szabó, J.P.; Kálmán-Szabó, I.; Arató, V.; Garai, I.; Árkosy, P.; Jószai, I.; Deák, Á.; Kertész, I.; et al. In Vivo Assessments of Mesoblastic Nephroma (Ne/De) and Myelomonoblastic Leukaemia (My1/De) Tumour Development in Hypercholesterolemia Rat Models. Int. J. Mol. Sci. 2022, 23, 13060. https://doi.org/10.3390/ijms232113060
Képes Z, Barkóczi A, Szabó JP, Kálmán-Szabó I, Arató V, Garai I, Árkosy P, Jószai I, Deák Á, Kertész I, et al. In Vivo Assessments of Mesoblastic Nephroma (Ne/De) and Myelomonoblastic Leukaemia (My1/De) Tumour Development in Hypercholesterolemia Rat Models. International Journal of Molecular Sciences. 2022; 23(21):13060. https://doi.org/10.3390/ijms232113060
Chicago/Turabian StyleKépes, Zita, Alexandra Barkóczi, Judit P. Szabó, Ibolya Kálmán-Szabó, Viktória Arató, Ildikó Garai, Péter Árkosy, István Jószai, Ádám Deák, István Kertész, and et al. 2022. "In Vivo Assessments of Mesoblastic Nephroma (Ne/De) and Myelomonoblastic Leukaemia (My1/De) Tumour Development in Hypercholesterolemia Rat Models" International Journal of Molecular Sciences 23, no. 21: 13060. https://doi.org/10.3390/ijms232113060
APA StyleKépes, Z., Barkóczi, A., Szabó, J. P., Kálmán-Szabó, I., Arató, V., Garai, I., Árkosy, P., Jószai, I., Deák, Á., Kertész, I., Hajdu, I., & Trencsényi, G. (2022). In Vivo Assessments of Mesoblastic Nephroma (Ne/De) and Myelomonoblastic Leukaemia (My1/De) Tumour Development in Hypercholesterolemia Rat Models. International Journal of Molecular Sciences, 23(21), 13060. https://doi.org/10.3390/ijms232113060







