Mining Transcriptomic Data to Uncover the Association between CBX Family Members and Cancer Stemness
Abstract
:1. Introduction
2. Results
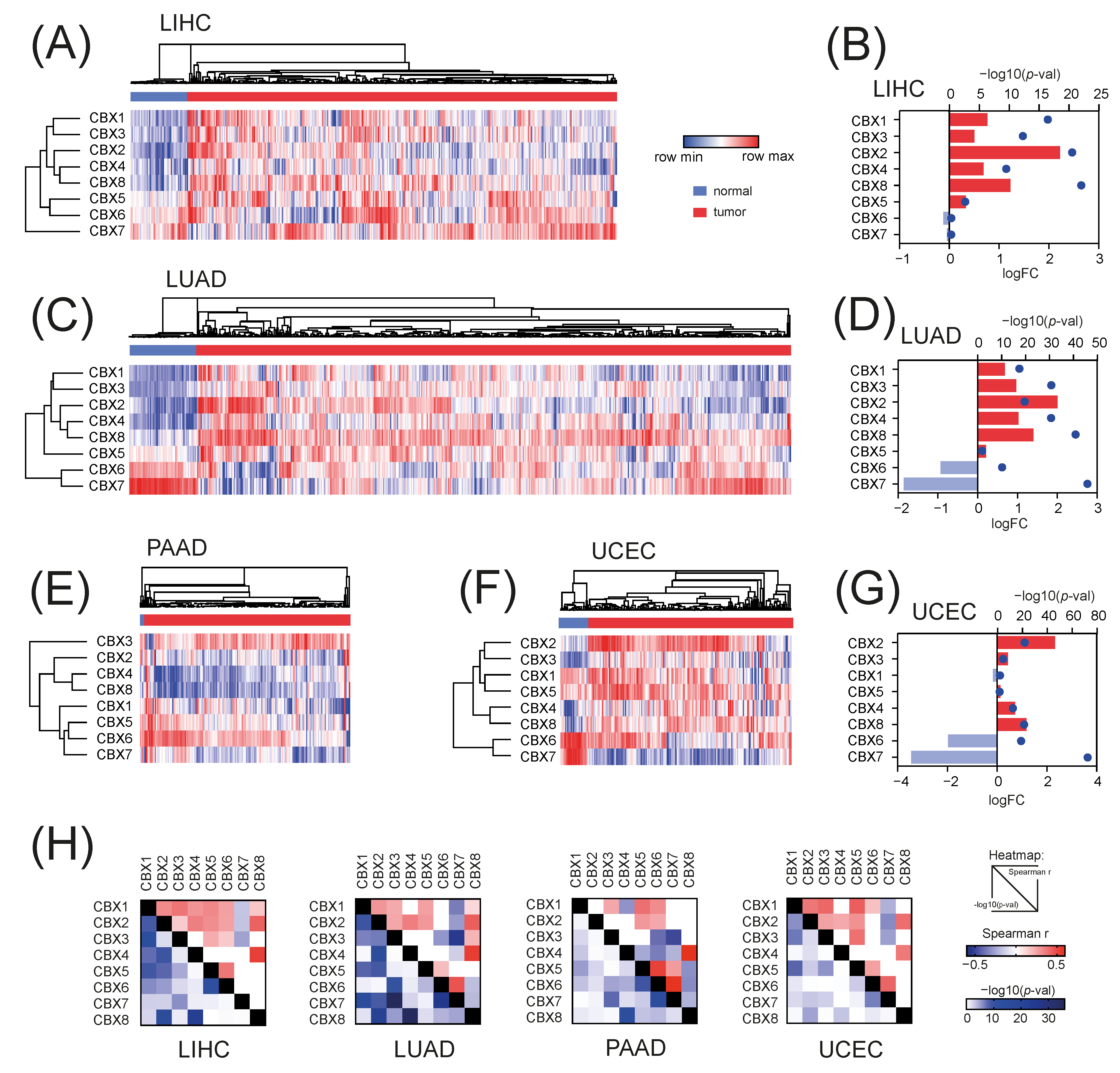
2.1. CBX Family Members Are Differentially Expressed in Solid Tumors and Possess Distinct Prognostic Values
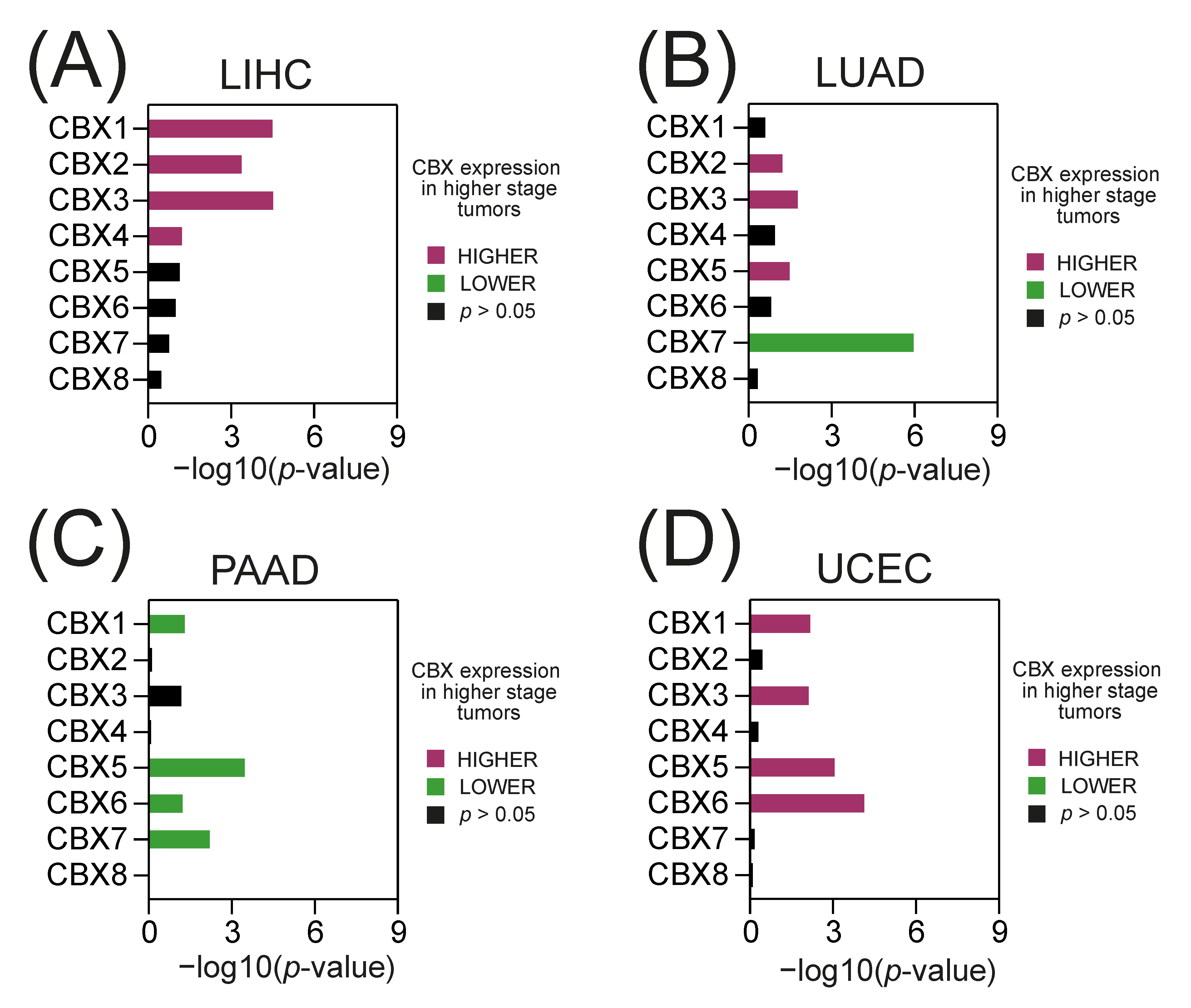
2.2. The Expression of Specific CBX Members Correlates with Tumor Stage and Tumor Grade
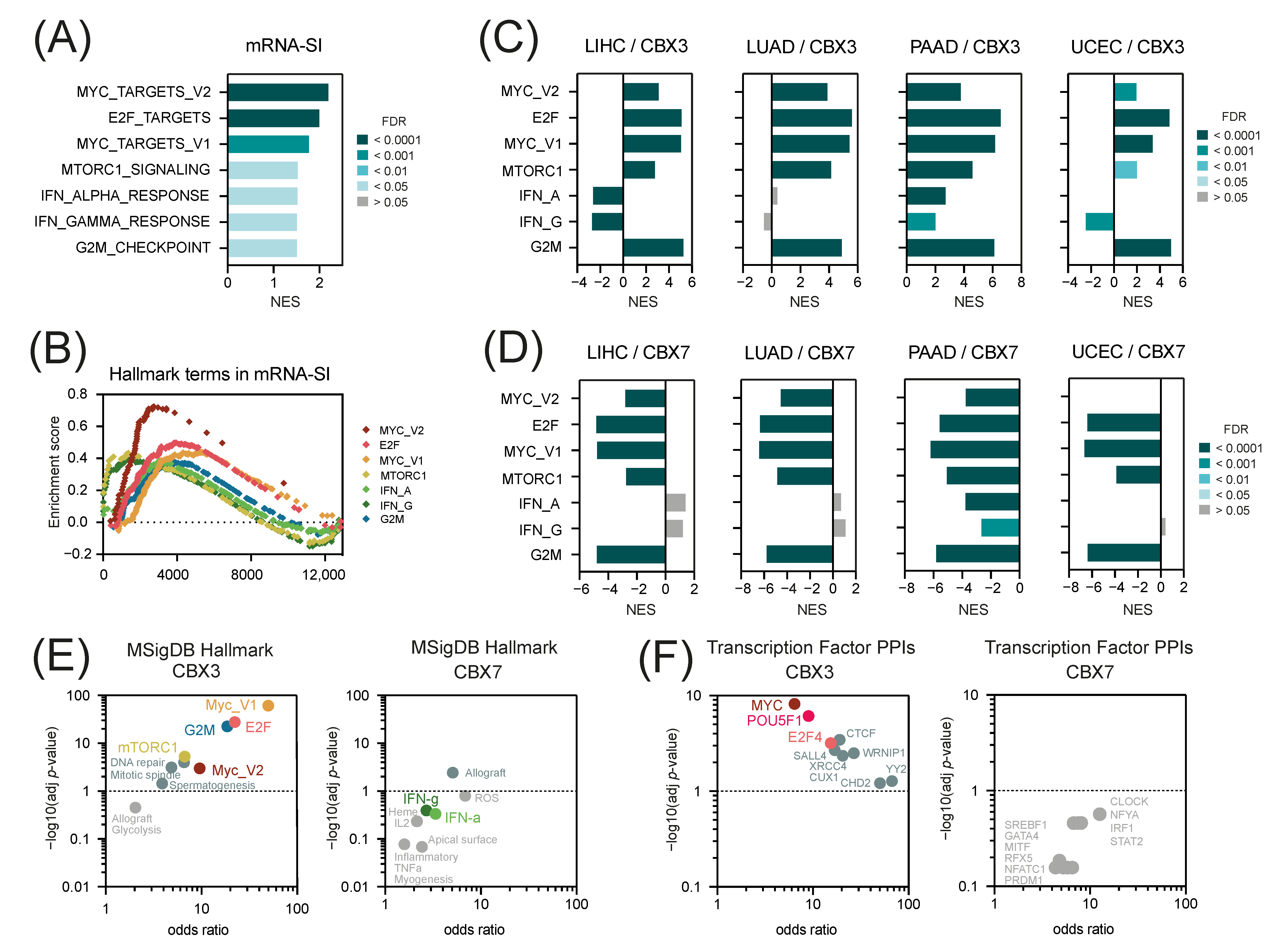
2.3. CBX3 Is Positively While CBX7 Is Negatively Associated with Tumor Stemness
2.4. The “Hallmark of Cancer” Terms in CBX3 and CBX7-Associated Gene Expression Profiles
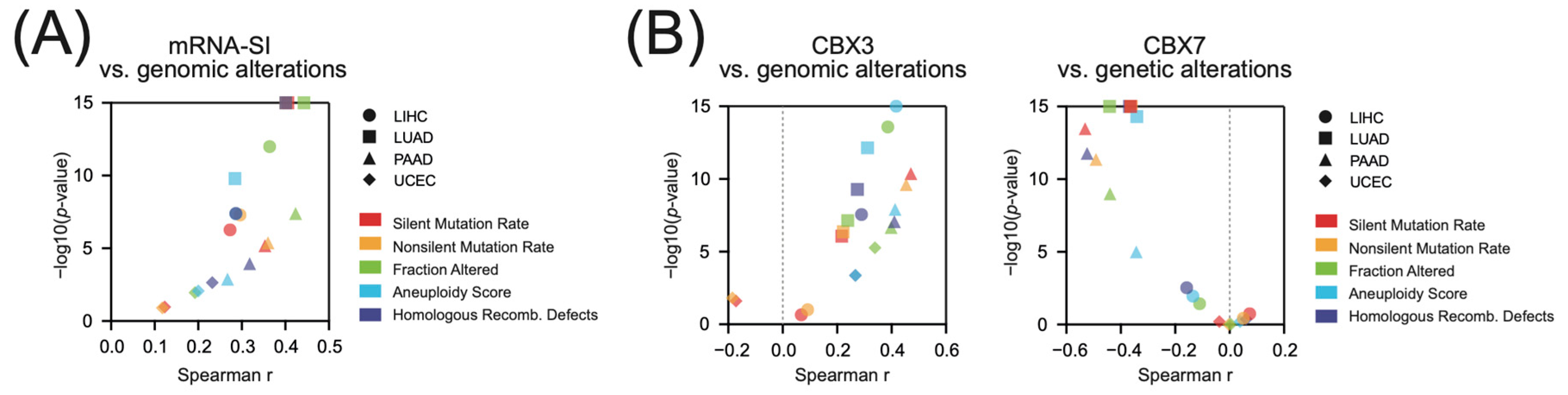
2.5. CBX3 Overexpression and CBX7 Downregulation Associate with the Abundance of Genomic Alterations
3. Discussion
4. Materials and Methods
4.1. TCGA Data
4.2. Transcriptomic Data
4.3. Oncomine Data
4.4. Mutation Status
4.5. Proteomic Data
4.6. Overall Survival and Relapse-Free Survival
4.7. Disease Stage and Histologic Tumor Grade
4.8. Stemness-Associated Scores
4.9. Gene Set Enrichment Analysis
4.10. Enrichr
4.11. Validation Sets
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBX | Chromobox |
| CPTAC | Clinical Proteomic Tumor Analysis Consortium |
| CSC | Cancer Stem Cell |
| CSD | Chromoshadow domain |
| EED | Embryonic Ectoderm Development |
| ESC | Embryonic Stem Cell |
| EZH1 | Enhancer Of Zeste 1 |
| EZH2 | Enhancer Of Zeste 2 |
| FDR | False Discovery Rate |
| GEO | Gene Expression Omnibus |
| GEPIA2 | Gene Expression Profiling Interactive Analysis 2.0 |
| GSCA | Gene Set Cancer Analysis platform |
| GSEA | Gene Set Enrichment Analysis |
| GTEx | Genotype-Tissue Expression Portal |
| HP1 | Heterochromatin Protein 1 |
| KRAB-ZNF | Kruppel-Associated Box Zinc Finger Protein |
| LIHC | Liver Hepatocellular Carcinoma |
| LUAD | Lung Adenocarcinoma |
| MRNA-SI | mRNA Stemness Index |
| NES | Normalized Enrichment Score |
| OS | Overall Survival |
| PAAD | Pancreatic Adenocarcinoma |
| Pc | Polycomb |
| PRC1 | Polycomb Repressive Complex 1 |
| PRC2 | Polycomb Repressive Complex 2 |
| RFS | Relapse-free survival |
| RING1A | Really Interesting New Gene 1A |
| RING1B | Really Interesting New Gene 1B |
| RNASeq | RNA Sequencing |
| RSEM | RNASeq by Expectation Maximization |
| SNV | Single Nucleotide Variation |
| SUZ12 | Suppressor Of Zeste 12 |
| TCGA | The Cancer Genome Atlas |
| TF | Transcription Factor |
| TIF1B | Transcription Intermediary Factor 1 Beta |
| TISIDB | Tumor-Immune System Interactions Database |
| TRAIL | Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand |
| UALCAN | The University of ALabama at Birmingham CANcer data analysis Portal |
| UCEC | Uterine Corpus Endometrial Carcinoma |
References
- Friedmann-Morvinski, D.; Verma, I.M. Dedifferentiation and reprogramming: Origins of cancer stem cells. EMBO Rep. 2014, 15, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.; Hall, R.R., 3rd; Ahmed, A.U. Cancer Stem Cells: Cellular Plasticity, Niche, and its Clinical Relevance. J. Stem. Cell Res. Ther. 2016, 6, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Malta, T.M.; Sokolov, A.; Gentles, A.J.; Burzykowski, T.; Poisson, L.; Weinstein, J.N.; Kamińska, B.; Huelsken, J.; Omberg, L.; Gevaert, O.; et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 2018, 173, 338–354.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Porath, I.; Thomson, M.W.; Carey, V.J.; Ge, R.; Bell, G.W.; Regev, A.; Weinberg, R.A. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008, 40, 499–507. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Miura, T.; Brandenberger, R.; Mejido, J.; Luo, Y.; Yang, A.X.; Joshi, B.H.; Ginis, I.; Thies, R.S.; Amit, M.; et al. Gene expression in human embryonic stem cell lines: Unique molecular signature. Blood 2004, 103, 2956–2964. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.J.; Liu, H.; Ridky, T.W.; Cassarino, D.; Segal, E.; Chang, H.Y. Module Map of Stem Cell Genes Guides Creation of Epithelial Cancer Stem Cells. Cell Stem Cell 2008, 2, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, J. Cell Reversal from a Differentiated to a Stem-Like State at Cancer Initiation. Front. Oncol. 2020, 10, 541. [Google Scholar] [CrossRef] [Green Version]
- Swygert, S.G.; Peterson, C.L. Chromatin dynamics: Interplay between remodeling enzymes and histone modifications. Biochim. Biophys. Acta 2014, 1839, 728–736. [Google Scholar] [CrossRef] [Green Version]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kingston, R.E. Perspectives the CBX family of proteins in transcriptional repression and memory. J. Biosci. 2020, 45, 16. [Google Scholar] [CrossRef]
- Liang, Y.-K.; Lin, H.-Y.; Chen, C.-F.; Zeng, D. Prognostic values of distinct CBX family members in breast cancer. Oncotarget 2017, 8, 92375–92387. [Google Scholar] [CrossRef]
- Zhou, H.; Xiong, Y.; Liu, Z.; Hou, S.; Zhou, T. Expression and prognostic significance of CBX2 in colorectal cancer: Database mining for CBX family members in malignancies and vitro analyses. Cancer Cell Int. 2021, 21, 402. [Google Scholar] [CrossRef]
- Xie, X.; Ning, Y.; Long, J.; Wang, H.; Chen, X. Diverse CBX family members as potential prognostic biomarkers in non-small-cell lung cancer. FEBS Open Bio 2020, 10, 2206–2215. [Google Scholar] [CrossRef]
- Kaustov, L.; Ouyang, H.; Amaya, M.; Lemak, A.; Nady, N.; Duan, S.; Wasney, G.A.; Li, Z.; Vedadi, M.; Schapira, M.; et al. Recognition and Specificity Determinants of the Human Cbx Chromodomains. J. Biol. Chem. 2011, 286, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomberk, G.; Wallrath, L.; Urrutia, R. The Heterochromatin Protein 1 family. Genome Biol. 2006, 7, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyengar, S.; Ivanov, A.V.; Jin, V.X.; Rauscher, F.J.; Farnham, P.J. Functional Analysis of KAP1 Genomic Recruitment. Mol. Cell. Biol. 2011, 31, 1833–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerwińska, P.; Mazurek, S.; Wiznerowicz, M. The complexity of TRIM28 contribution to cancer. J. Biomed. Sci. 2017, 24, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.-G.; Zhang, Y.; Sun, T.-T.; Cheng, B. Epigenetic regulation by polycomb group complexes: Focus on roles of CBX proteins. J. Zhejiang Univ. Sci. B 2014, 15, 412–428. [Google Scholar] [CrossRef] [Green Version]
- Piunti, A.; Shilatifard, A. The roles of Polycomb repressive complexes in mammalian development and cancer. Nat. Rev. Mol. Cell Biol. 2021, 22, 326–345. [Google Scholar] [CrossRef]
- Chan, H.L.; Morey, L. Emerging Roles for Polycomb-Group Proteins in Stem Cells and Cancer. Trends Biochem. Sci. 2019, 44, 688–700. [Google Scholar] [CrossRef]
- van Wijnen, A.J.; Bagheri, L.; Badreldin, A.A.; Larson, A.N.; Dudakovic, A.; Thaler, R.; Paradise, C.R.; Wu, Z. Biological functions of chromobox (CBX) proteins in stem cell self-renewal, lineage-commitment, cancer and development. Bone 2021, 143, 115659. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, M.; Czerwińska, P.; Mazurek, S.; Sozańska, B.; Biecek, P.; Mackiewicz, A.; Wiznerowicz, M. TRIM28 epigenetic corepressor is indispensable for stable induced pluripotent stem cell formation. Stem Cell Res. 2017, 23, 163–172. [Google Scholar] [CrossRef]
- Mazurek, S.; Oleksiewicz, U.; Czerwińska, P.; Wróblewska, J.; Klimczak, M.; Wiznerowicz, M. Disruption of RING and PHD Domains of TRIM28 Evokes Differentiation in Human iPSCs. Cells 2021, 10, 1933. [Google Scholar] [CrossRef] [PubMed]
- Addison, J.B.; Koontz, C.; Fugett, J.H.; Creighton, C.J.; Chen, D.; Farrugia, M.K.; Padon, R.R.; Voronkova, M.A.; McLaughlin, S.L.; Livengood, R.H.; et al. KAP1 Promotes Proliferation and Metastatic Progression of Breast Cancer Cells. Cancer Res. 2015, 75, 344–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerwińska, P.; Shah, P.K.; Tomczak, K.; Klimczak, M.; Mazurek, S.; Sozańska, B.; Biecek, P.; Korskis, K.; Filas, V.; Mackiewicz, A.; et al. TRIM28 multi-domain protein regulates cancer stem cell population in breast tumor development. Oncotarget 2016, 8, 863–882. [Google Scholar] [CrossRef] [Green Version]
- Czerwinska, P.; Jaworska, A.M.; Wlodarczyk, N.A.; Mackiewicz, A.A. Melanoma Stem Cell-Like Phenotype and Significant Suppression of Immune Response within a Tumor Are Regulated by TRIM28 Protein. Cancers 2020, 12, 2998. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pander, A.; Chinnaiyan, A.M. ONCOMINE: A Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Chandrashekar, D.S.; Varambally, S.; Creighton, C.J. Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers. Nat. Commun. 2019, 10, 5679. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-J.; Hu, F.-F.; Xia, M.-X.; Han, L.; Zhang, Q.; Guo, A.-Y. GSCALite: A web served for gene set cancer analysis. Bioinformatics 2018, 34, 3771–3772. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.P.; Kalathur, R.K.R.; Oliveira, D.; Barata, T.; Machado, R.; Machado, S.; Pacheco-Leyva, I.; Duarte, G.I.D.S.; Futschik, M.E. StemChecker: A web-based tool to discover and explore stemness signatures in gene sets. Nucleic Acids Res. 2015, 43, W72–W77. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vad-Nielsen, J.; Nielsen, A.L. Beyond the histone tale: HP1α deregulation in breast cancer epigenetics. Cancer Biol. Ther. 2015, 16, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Caillier, M.; Thénot, S.; Tribollet, V.; Birot, A.-M.; Samarut, J.; Mey, A. Role of the Epigenetic Regulator HP1γ in the Control of Embryonic Stem Cell Properties. PLoS ONE 2010, 5, e15507. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, A.L.; Ortiz, J.A.; You, J.; Oulad-Abdelghani, M.; Khechumian, R.; Gansmuller, A.; Chambon, P.; Losson, R. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 1999, 18, 6385–6395. [Google Scholar] [CrossRef]
- Vakoc, C.R.; Mandat, S.A.; Olenchock, B.A.; Blobel, G.A. Histone H3 Lysine 9 Methylation and HP1gamma Are Associated with Transcription Elongation through Mammalian Chromatin. Mol. Cell 2005, 19, 381–391. [Google Scholar] [CrossRef]
- Saint-André, V.; Batsché, E.; Rachez, C.; Muchardt, C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat. Struct. Mol. Biol. 2011, 18, 337–344. [Google Scholar] [CrossRef]
- Mateescu, B.; Bourachot, B.; Rachez, C.; Ogryzko, V.; Muchardt, C. Regulation of an inducible promoter by an HP1beta-HP1gamma switch. EMBO Rep. 2008, 9, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, H.; Li, N.; Dhar, S.S.; Wu, S.J.; Lv, J.; Chen, K.; Flores, E.R.; Baseler, L.; Lee, M.G. HP1γ Promotes Lung Adenocarcinoma by Downregulating the Transcription-Repressive Regulators NCOR2 and ZBTB7A. Cancer Res. 2018, 78, 3834–3848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.; Ma, N.; Chen, J.-L.; Tang, F.-X.; Zong, Z.; Yu, Z.-M.; Chen, S.; Zhou, T.-C. Expression and prognostic value of Chromobox family members in gastric cancer. J. Gastrointest. Oncol. 2020, 11, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-P.; Wang, F.; Yang, M.; Wang, X.-Y.; Jin, C.-L.; Ji, Q.-K.; Li, S.; Zhao, X.-L. CBX3 promotes glioma U87 cell proliferation and predicts an unfavorable prognosis. J. Neuro-Oncol. 2019, 145, 35–48. [Google Scholar] [CrossRef]
- Ma, C.; Nie, X.; Wang, Y.; Liu, X.; Liang, X.; Zhou, Q.; Wu, D. CBX3 predicts an unfavorable prognosis and promotes tumorigenesis in osteosarcoma. Mol. Med. Rep. 2019, 19, 4205–4212. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Kan, A.; Zhang, W.; Zhou, J.; Zhang, H.; Chen, J.; Tang, S. CBX3/HP1γ promotes tumor proliferation and predicts poor survival in hepatocellular carcinoma. Aging (Albany NY) 2019, 11, 5483–5497. [Google Scholar]
- Zhu, Y.; Pu, Z.; Li, Z.; Lin, Y.; Li, N.; Peng, F. Comprehensive Analysis of the Expression and Prognosis Value of Chromobox Family Members in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 700528. [Google Scholar] [CrossRef]
- Saini, V.; Hose, C.D.; Monks, A.; Nagashima, K.; Han, B.; Newton, D.L.; Millione, A.; Shah, J.; Hollingshead, M.G.; Hite, K.M.; et al. Identification of CBX3 and ABCA5 as Putative Biomarkers for Tumor Stem Cells in Osteosarcoma. PLoS ONE 2012, 7, e41401. [Google Scholar]
- He, Z.; Chen, J.; Chen, X.; Wang, H.; Tang, L.; Han, C. microRNA-377 acts as a suppressor in esophageal squamous cell carcinoma through CBX3-dependent P53/P21 pathway. J. Cell. Physiol. 2021, 236, 107–120. [Google Scholar]
- Sengupta, A.; Cancelas, J.A. Cancer stem cells: A stride towards cancer cure? J. Cell. Physiol. 2010, 225, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Miranda, A.; Hamilton, P.T.; Zhang, A.W.; Pattnaik, S.; Becht, E.; Mezheyeuski, A.; Bruun, J.; Micke, P.; de Reynies, A.; Nelson, B.H. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc. Natl. Acad. Sci. USA 2019, 116, 9020–9029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerwinska, P.; Wlodarczyk, N.; Jaworska, A.; Mackiewicz, A. The Association between TIF1 Family Members and Cancer Stemness in Solid Tumors. Cancers 2021, 13, 1528. [Google Scholar] [CrossRef] [PubMed]
- Morey, L.; Pascual, G.; Cozzuto, L.; Roma, G.; Wutz, A.; Benitah, S.A.; Di Croce, L. Nonoverlapping Functions of the Polycomb Group Cbx Family of Proteins in Embryonic Stem Cells. Cell Stem Cell 2012, 10, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ouyang, T.; Li, M.; Hong, T.; Alriashy, M.; Meng, W.; Zhang, N. CBX7 is Dualistic in Cancer Progression Based on its Function and Molecular Interactions. Front. Genet. 2021, 12, 740794. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, Y.; Zhu, Z.; Liu, J.; He, X.; Dalangood, S.; Li, M.; Tan, M.; Cai, J.; Tang, P.; et al. CBX7 suppresses urinary bladder cancer progression via modulating AKR1B10–ERK signaling. Cell Death Dis. 2021, 12, 537. [Google Scholar] [CrossRef]
- Li, X.; Gou, J.; Li, H.; Yang, X. Bioinformatic analysis of the expression and prognostic value of chromobox family proteins in human breast cancer. Sci. Rep. 2020, 10, 17739. [Google Scholar] [CrossRef]
- Ning, G.; Huang, Y.-L.; Zhen, L.-M.; Xu, W.-X.; Jiao, Q.; Yang, F.-J.; Wu, L.N.; Zheng, Y.Y.; Song, J.; Wang, Y.S.; et al. Transcriptional expressions of Chromobox 1/2/3/6/8 as independent indicators for survivals in hepatocellular carcinoma patients. Aging (Albany NY) 2018, 10, 3450–3473. [Google Scholar] [CrossRef]
- Pallante, P.; Terracciano, L.; Carafa, V.; Schneider, S.; Zlobec, I.; Lugli, A.; Bianco, M.; Ferraro, A.; Sacchetti, S.; Troncone, G.; et al. The loss of the CBX7 gene expression represents an adverse prognostic marker for survival of colon carcinoma patients. Eur. J. Cancer 2010, 46, 2304–2313. [Google Scholar] [CrossRef]
- Pallante, P.; Federico, A.; Berlingieri, M.T.; Bianco, M.; Ferraro, A.; Forzati, F.; Iaccarino, A.; Russo, M.; Pierantoni, G.M.; Leone, V.; et al. Loss of the CBX7 Gene Expression Correlates with a Highly Malignant Phenotype in Thyroid Cancer. Cancer Res. 2008, 68, 6770–6778. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, Z.; Patil, V.; Arora, A.; Hegde, A.S.; Arivazhagan, A.; Santosh, V.; Somasundaram, K. Cbx7 is epigenetically silenced in glioblastoma and inhibits cell migration by targeting YAP/TAZ-dependent transcription. Sci. Rep. 2016, 6, 27753. [Google Scholar] [CrossRef] [Green Version]
- Ni, S.; Wang, H.; Zhu, X.; Wan, C.; Xu, J.; Lu, C.; Xiao, L.; He, J.J.; Jiang, C.; Wang, W.; et al. CBX7 suppresses cell proliferation, migration, and invasion through the inhibition of PTEN/Akt signaling in pancreatic cancer. Oncotarget 2016, 8, 8010–8021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-W.; Zhang, L.; Qin, W.; Yao, X.-H.; Zheng, L.-Z.; Liu, X.; Li, J.; Guo, W.-J. Oncogenic role of the chromobox protein CBX7 in gastric cancer. J. Exp. Clin. Cancer Res. 2010, 29, 114–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, S.-J.; Zhao, L.-Q.; Wang, X.-F.; Wu, Z.-H.; Hua, R.-X.; Wan, C.-H.; Zhang, J.Y.; Zhang, X.W.; Huang, M.Z.; Gan, L.; et al. CBX7 regulates stem cell-like properties of gastric cancer cells via p16 and AKT-NF-κB-miR-21 pathways. J. Hematol. Oncol. 2018, 11, 17. [Google Scholar] [CrossRef]
- Shinjo, K.; Yamashita, Y.; Yamamoto, E.; Akatsuka, S.; Uno, N.; Kamiya, A.; Niimi, K.; Sakaguchi, Y.; Nagasaka, T.; Takahashi, T.; et al. Expression of chromobox homolog 7 (CBX7) is associated with poor prognosis in ovarian clear cell adenocarcinoma via TRAIL-induced apoptotic pathway regulation. Int. J. Cancer 2014, 135, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Park, J.-H.; Won, H.-Y.; Lee, J.-Y.; Kong, G. CBX7 inhibits breast tumorigenicity through DKK-1-mediated suppression of the Wnt/β-catenin pathway. FASEB J. 2015, 29, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, M.; Oikawa, K.; Fujita, K.; Kudo, M.; Kinoshita, M.; Kuroda, M. Heterochromatin Protein 1γ Epigenetically Regulates Cell Differentiation and Exhibits Potential as a Therapeutic Target for Various Types of Cancers. Am. J. Pathol. 2009, 174, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Yao, L.; Xu, Z.; Yan, Y.; Li, J. Prognostic Value and Therapeutic Potential of CBX Family Members in Ovarian Cancer. Front. Cell Dev. Biol. 2022, 10, 832354. [Google Scholar] [CrossRef]
- Fang, X.; Wang, J.; Chen, J.; Zhuang, M.; Huang, T.; Chen, Z.; Huang, Y.; Zheng, B.; Wang, X. Identification and Validation of Chromobox Family Members as Potential Prognostic Biomarkers and Therapeutic Targets for Human Esophageal Cancer. Front. Genet. 2022, 13, 851390. [Google Scholar] [CrossRef]
- Liu, M.; Huang, F.; Zhang, D.; Ju, J.; Wu, X.-B.; Wang, Y.; Wang, Y.; Wu, Y.; Nie, M.; Li, Z.; et al. Heterochromatin Protein HP1g Promotes Colorectal Cancer Progression and Is Regulated by miR-30a. Cancer Res. 2015, 75, 4593–4604. [Google Scholar] [CrossRef] [Green Version]
- Beshara, C.S.; Jones, C.E.; Daze, K.D.; Lilgert, B.J.; Hof, F. A Simple Calixarene Recognizes Post-translationally Methylated Lysine. ChemBioChem 2009, 11, 63–66. [Google Scholar] [CrossRef]
- I Stuckey, J.; Dickson, B.M.; Cheng, N.; Liu, Y.; Norris, J.L.; Cholensky, S.H.; Tempel, W.; Qin, S.; Huber, K.G.; Sagum, C.; et al. A cellular chemical probe targeting the chromodomains of Polycomb repressive complex 1. Nat. Chem. Biol. 2016, 12, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor–immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwinska, P.; Mackiewicz, A.A. Mining Transcriptomic Data to Uncover the Association between CBX Family Members and Cancer Stemness. Int. J. Mol. Sci. 2022, 23, 13083. https://doi.org/10.3390/ijms232113083
Czerwinska P, Mackiewicz AA. Mining Transcriptomic Data to Uncover the Association between CBX Family Members and Cancer Stemness. International Journal of Molecular Sciences. 2022; 23(21):13083. https://doi.org/10.3390/ijms232113083
Chicago/Turabian StyleCzerwinska, Patrycja, and Andrzej Adam Mackiewicz. 2022. "Mining Transcriptomic Data to Uncover the Association between CBX Family Members and Cancer Stemness" International Journal of Molecular Sciences 23, no. 21: 13083. https://doi.org/10.3390/ijms232113083




