Synergistic Effects of Silicon and Preservative on Promoting Postharvest Performance of Cut Flowers of Peony (Paeonia lactiflora Pall.)
Abstract
:1. Introduction
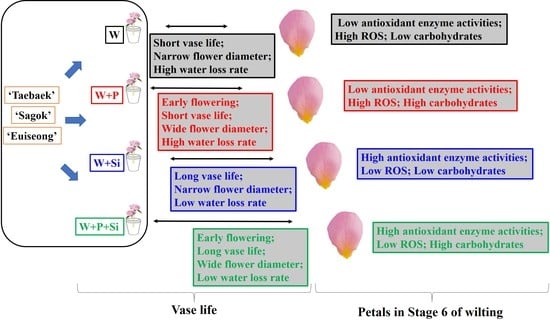
2. Results
2.1. Vase Life and Diameters of Cut Peony Flowers as Affected by the Four Treatments
2.2. Fresh Weight Loss of Cut Stems during the Vase Life as Affected by the Four Treatments
2.3. Effects of the Four Treatments on the Major Antioxidant Enzyme Activities
2.4. Effects of the Four Treatments on the ROS (Reactive Oxygen Species) Accumulation
2.5. Responses of the Starch and Soluble Sugar Contents to the Four Treatments
2.6. Differences in the Mechanisms Regarding Si and the Commercial Preservative Are Supported by the PCA
3. Discussion
3.1. Combined Use of Si and a Preservative Promotes Early Flowering but Prolongs the Vase Life
3.2. Si Application Reduces Water Loss and Delays the Senescence of Cut Stems
3.3. Si Application Intensified the Antioxidant Defense System but Did Not Significantly Increase the Carbohydrate Content
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Treatments and Experimental Design
4.3. Flower Opening Stage Definitions and Observations
4.4. Measurement of the Daily Fresh Weight Loss
4.5. Assays of the Key Antioxidant Enzyme Activities
4.6. Quantification of Superoxide Radical (O2.−), Hydrogen Peroxide (H2O2), and Malondialdehyde (MDA)
4.7. Determination of the Carbohydrate Level
4.8. Statistics and Graph
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhao, D.; Cheng, M.; Tang, W.; Liu, D.; Zhou, S.; Meng, J.; Tao, J. Nano-silver modifies the vase life of cut herbaceous peony (Paeonia lactiflora Pall.) flowers. Protoplasma 2018, 255, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Kamenetsky, R.; Dole, J. Herbaceous peony (Paeonia): Genetics, physiology and cut flower production. Floric. Ornam. Biotechnol 2012, 6, 62–77. [Google Scholar]
- Du, G.; Xu, J.; Gao, C.; Lu, J.; Li, Q.; Du, J.; Lv, M.; Sun, X. Effect of low storage temperature on pollen viability of fifteen herbaceous peonies. Biotechnol. Rep. 2019, 21, e00309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xu, C.; Luan, Y.; Shi, W.; Tang, Y.; Tao, J. Silicon enhances stem strength by promoting lignin accumulation in herbaceous peony (Paeonia lactiflora Pall.). Int. J. Biol. Macromol. 2021, 190, 769–779. [Google Scholar] [CrossRef]
- Rhie, Y.H.; Jung, H.H.; Kim, K.S. Chilling requirement for breaking dormancy and flowering in Paeonia lactiflora ‘Taebaek’ and ‘Mulsurae’. Hortic. Environ. Biotechnol. 2012, 53, 277–282. [Google Scholar] [CrossRef]
- Bae, S.-G.; Kim, J.-H.; Park, S.-J.; Kim, J.-C. Influence of forcing cultivation time on cut flower, root quality, and yield in Peony (Paeonia Lactiflora Pall. cv. Taebaek). Korean J. Med. Crop Sci. 2008, 16, 421–426. (In Korean) [Google Scholar]
- Rabiza-Świder, J.; Skutnik, E.; Jędrzejuk, A.; Łukaszewska, A. Postharvest treatments improve quality of cut peony flowers. Agronomy 2020, 10, 1583. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Hu, J.; Lee, J.; Jeong, B.R. Pre- and/or postharvest silicon application prolongs the vase life and enhances the quality of cut peony (Paeonia lactiflora Pall.) flowers. Plants 2021, 10, 1742. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Abadie, P.; Belde, P.J. Alkylethoxylate surfactants for rehydration of roses and Bouvardia flowers. Postharvest Biol. Tec. 2002, 24, 327–333. [Google Scholar] [CrossRef]
- Alaey, M.; Babalar, M.; Naderi, R.; Kafi, M. Effect of pre-and postharvest salicylic acid treatment on physio-chemical attributes in relation to vase-life of rose cut flowers. Postharvest Biol. Tec. 2011, 61, 91–94. [Google Scholar] [CrossRef]
- Geerdink, G.M.; Orsi, B.; Tezotto-Uliana, J.V.; Pessoa, C.O.; Sasaki, F.F.; Kluge, R.A. Pre-harvest silicon treatment improves quality of cut rose stems and maintains postharvest vase life. J. Plant Nutr. 2020, 43, 1418–1426. [Google Scholar] [CrossRef]
- Dole, J.; Stamps, B.; Carlson, A.; Ahmad, I.; Greer, L.; Laushman, J. Postharvest Handling of Cut Flowers and Greens; ASCFG Press: Oberlin, OH, USA, 2017. [Google Scholar]
- Ahmad, I.; Dole, J.M.; Amjad, A.; Ahmad, S. Dry storage effects on postharvest performance of selected cut flowers. HortTechnology 2012, 22, 463–469. [Google Scholar] [CrossRef]
- Elhindi, K.M. Evaluation of several holding solutions for prolonging vase-life and keeping quality of cut sweet pea flowers (Lathyrus odoratus L.). Saudi J. Biol. Sci. 2012, 19, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.K.; Lim, J.H. Do Eco-Friendly Floral Preservative Solutions Prolong Vase Life Better than Chemical Solutions? Horticulturae 2021, 7, 415. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Silicon mitigates ammonium toxicity in cabbage (Brassica campestris L. ssp. pekinensis) ‘Ssamchu’. Front. Sustain. Food Syst. 2022, 6, 922666. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How does silicon mediate plant water uptake and loss under water deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef]
- Chérif, M.; Benhamou, N.; Menzies, J.G.; Bélanger, R. Silicon induced resistance in cucumber plants against Pythium ultimum. Physiol. Mol. Plant Pathol. 1992, 41, 411–425. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; De Vleesschauwer, D.; Höfte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef]
- Farooq, M.A.; Saqib, Z.A.; Akhtar, J.; Bakhat, H.F.; Pasala, R.-K.; Dietz, K.-J. Protective role of silicon (Si) against combined stress of salinity and boron (B) toxicity by improving antioxidant enzymes activity in rice. Silicon 2019, 11, 2193–2197. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. R. 2015, 22, 2837–2845. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotox. Environ. Safe. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Ahmad, I.; Dole, J.M. Homemade floral preservatives affect postharvest performance of selected specialty cut flowers. HortTechnology 2014, 24, 384–393. [Google Scholar] [CrossRef]
- Karimian, N.; Nazari, F.; Samadi, S. Morphological and biochemical properties, leaf nutrient content, and vase life of tuberose (Polianthes tuberosa L.) affected by root or foliar applications of silicon (Si) and silicon nanoparticles (SiNPs). J. Plant Growth Regul. 2021, 40, 2221–2235. [Google Scholar] [CrossRef]
- Tian, S.; Qin, G.; Xu, Y. Synergistic effects of combining biocontrol agents with silicon against postharvest diseases of jujube fruit. J. Food Protect. 2005, 68, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, L.; Zhao, J.; Bi, Y. Use of silicon oxide and sodium silicate for controlling Trichothecium roseum postharvest rot in Chinese cantaloupe (Cucumis melo L.). Int. J. Food Sci. Technol. 2007, 42, 1012–1018. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Taher, M.A. Silicon induces resistance to postharvest rot of carrot caused by Sclerotinia sclerotiorum and the possible of defense mechanisms. Postharvest Biol. Tec. 2018, 140, 11–17. [Google Scholar] [CrossRef]
- Sacala, E. Role of silicon in plant resistance to water stress. J. Elementol. 2009, 14, 619–630. [Google Scholar] [CrossRef]
- Sidi, M.; Omar, D.; Nahrawi, H.; Elias, H.; Wasli, H. Effect of NPK and silicon fertilizer on growth, flowering, and nectar of Turnera ulmifolia L. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; p. 012022. [Google Scholar] [CrossRef]
- Asrar, A.-W.A. Effects of some preservative solutions on vase life and keeping quality of snapdragon (Antirrhinum majus L.) cut flowers. J. Saudi Soc. Agric. Sci. 2012, 11, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Dung, C.D.; Seaton, K.; Singh, Z. Influence of type and concentration of sugars, supplemented with 8-hydroxyquinoline sulphate, on the vase life of waxflower. Folia Hortic. 2017, 29, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Norikoshi, R.; Shibata, T.; Niki, T.; Ichimura, K. Sucrose treatment enlarges petal cell size and increases vacuolar sugar concentrations in cut rose flowers. Postharvest Biol. Tec. 2016, 116, 59–65. [Google Scholar] [CrossRef]
- Anjum, M.A.; Naveed, F.; Shakeel, F.; Amin, S. Effect of some chemicals on keeping quality and vase-life of tuberose (Polianthes tuberosa L.) cut flowers. Life 2001, 12, 23–65. [Google Scholar]
- Thwala, M.; Wahome, P.K.; Oseni, T.O.; Masarirambi, M.T. Effects of floral preservatives on the vase life of Orchid (Epidendrum radicans L.) cut flowers. Hortic. Sci. Ornam. Plants 2013, 5, 22–29. [Google Scholar] [CrossRef]
- Jamali, B.; Rahemi, M. Carnation flowers senescence as influenced by nickel, cobalt and silicon. J. Biol. Environ. Sci. 2011, 5, 15. Available online: http://hdl.handle.net/11452/17316 (accessed on 18 July 2021).
- Kazemi, M. Effect of cobalt, silicon, acetylsalicylic acid and sucrose as novel agents to improve vase-life of Argyranthemum flowers. Trends Appl. Sci. Res. 2012, 7, 579. [Google Scholar] [CrossRef] [Green Version]
- Bayat, H.; Aminifard, M. Effects of different preservative solutions on vase life of Narcissus tazetta cut flowers. J. Ornam. Plants 2018, 8, 13–21. Available online: http://jornamental.iaurasht.ac.ir/article_538642.html (accessed on 6 August 2022).
- Kazemi, M.; Gholami, M.; Asadi, M.; Aghdasi, S. Efficiency of silicon, nickel and acetylsalicylic acid reduced senescence and extended vase life of cut rose flowers. Trends Appl. Sci. Res. 2012, 7, 590. [Google Scholar] [CrossRef]
- Kamiab, F.; Shahmoradzadeh Fahreji, S.; Zamani Bahramabadi, E. Antimicrobial and physiological effects of silver and silicon nanoparticles on vase life of lisianthus (Eustoma grandiflora cv. Echo) flowers. Int. J. Hortic. Sci. Technol. 2017, 4, 135–144. [Google Scholar]
- Liu, J.; Zong, Y.; Qin, G.; Li, B.; Tian, S. Plasma membrane damage contributes to antifungal activity of silicon against Penicillium digitatum. Curr. Microbial. 2010, 61, 274–279. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- PUN, U.K.; Ichimura, K. Role of sugars in senescence and biosynthesis of ethylene in cut flowers. Jpn. Agric. Res. Q. 2003, 37, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Arrom, L.; Munné-Bosch, S. Sucrose accelerates flower opening and delays senescence through a hormonal effect in cut lily flowers. Plant Sci. 2012, 188, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S. Role of sugar in the vase solution on postharvest flower and leaf quality of oriental lily ‘Stargazer’. HortScience 2003, 38, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Frew, A.; Weston, L.A.; Reynolds, O.L.; Gurr, G.M. The role of silicon in plant biology: A paradigm shift in research approach. Ann. Bot. 2018, 121, 1265–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Yang, J.; Jeong, B.R. Alleviation of Ammonium Toxicity in Salvia splendens ‘Vista Red’ with Silicon Supplementation. Toxics 2022, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Jaiganesh, V.; Devi Shanthini, V.; Kannan, C.; Darwin Christdhas Henry, L. Role of Silicon Nutrient Mediated Plant Disease Resistance. Curr. Res. Innov. Plant Pathol. 2019, 3, 63–81. [Google Scholar]
- Bartoli, C.G.; Simontacchi, M.; Montaldi, E.; Puntarulo, S. Oxidative stress, antioxidant capacity and ethylene production during ageing of cut carnation (Dianthus caryophyllus) petals. J. Exp. Bot. 1996, 47, 595–601. [Google Scholar] [CrossRef]
- Dušková, E.; Dušek, K.; Indrák, P.; Smékalová, K. Postharvest changes in essential oil content and quality of lavender flowers. Ind. Crops Prod. 2016, 79, 225–231. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 11. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Jiang, W.; Liu, D. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ. Sci. Pollut. R. 2013, 20, 1117–1123. [Google Scholar] [CrossRef]
- Vasanthi, N.; Saleena, L.M.; Raj, S.A. Silicon in crop production and crop protection-A review. Agric. Rev. 2014, 35, 14–23. [Google Scholar] [CrossRef]
- El-Serafy, R.S. Silica nanoparticles enhances physio-biochemical characters and postharvest quality of Rosa hybrida L. cut flowers. J. Hortic. Res. 2019, 27, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-H.; Khan, A.; Waqas, M.; Shahzad, R.; Lee, I.-J. Silicon-mediated mitigation of wounding stress acts by up-regulating the rice antioxidant system. Cereal Res. Commun. 2016, 44, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Kiany, T.; Pishkar, L.; Sartipnia, N.; Iranbakhsh, A.; Barzin, G. Effects of silicon and titanium dioxide nanoparticles on arsenic accumulation, phytochelatin metabolism, and antioxidant system by rice under arsenic toxicity. Environ. Sci. Pollut. R. 2022, 29, 34725–34737. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Ali, S.; Ahmad, R.; Ercisli, S.; Anjum, M.A. Foliar application of silicon enhances growth, flower yield, quality and postharvest life of tuberose (Polianthes tuberosa L.) under saline conditions by improving antioxidant defense mechanism. Silicon 2022, 14, 1511–1518. [Google Scholar] [CrossRef]
- Li, W.; Bi, Y.; Ge, Y.; Li, Y.; Wang, J.; Wang, Y. Effects of postharvest sodium silicate treatment on pink rot disease and oxidative stress-antioxidative system in muskmelon fruit. Eur. Food Res. Technol. 2012, 234, 137–145. [Google Scholar] [CrossRef]
- Ichimura, K. Improvement of postharvest life in several cut flowers by the addition of sucrose. Jap. Agric. Res. Q. 1998, 32, 275–280. [Google Scholar]
- Shahri, W.; Tahir, I. Flower senescence: Some molecular aspects. Planta 2014, 239, 277–297. [Google Scholar] [CrossRef]
- Ichimura, K.; Kawabata, Y.; Kishimoto, M.; Goto, R.; Yamada, K. Shortage of soluble carbohydrates is largely responsible for short vase life of cut ‘Sonia’ rose flowers. J. Japan. Soc. Hortic. Sci. 2003, 72, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Zaheer, M.M.; Yasin, N.A.; Ahmad, S.R.; Khan, W.U.; Ahmad, A.; Ali, A.; Rehman, S.U. Amelioration of cadmium stress in gladiolus (Gladiolus grandiflora L.) by application of potassium and silicon. J. Plant Nutr. 2018, 41, 461–476. [Google Scholar] [CrossRef]
- 2010–2022 World Weather & Climate Information. Available online: https://weather-and-climate.com/ (accessed on 26 July 2022).
- Eason, J.; Pinkney, T.; Heyes, J.; Brash, D.; Bycroft, B. Effect of storage temperature and harvest bud maturity on bud opening and vase life of Paeonia lactiflora cultivars. N. Z. J. Crop Hort. 2002, 30, 61–67. [Google Scholar] [CrossRef]
- Available online: http://www.oasisfloral.com.cn/index.php/product/show-194.html (accessed on 18 June 2022).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Amako, K.; Chen, G.-X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar] [CrossRef]
- Zhao, J.; Thi, L.T.; Park, Y.G.; Jeong, B.R. Light quality affects growth and physiology of Carpesium triste maxim. cultured in vitro. Agriculture 2020, 10, 258. [Google Scholar] [CrossRef]
- Wu, Y.-x.; von Tiedemann, A. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ. Pollut. 2002, 116, 37–47. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choudhuri, M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Knight, J.A.; Pieper, R.K.; McClellan, L. Specificity of the thiobarbituric acid reaction: Its use in studies of lipid peroxidation. Clin. Chem. 1988, 34, 2433–2438. [Google Scholar] [CrossRef]
- Li, N.; Wang, K.; Lv, Y.; Zhang, Z.; Cao, B.; Chen, Z.; Xu, K. Silicon enhanced the resistance of Chinese cabbage (Brassica rapa L. ssp. pekinensis) to ofloxacin on the growth, photosynthetic characteristics and antioxidant system. Plant Physiol. Bioch. 2022, 175, 44–57. [Google Scholar] [CrossRef]
- McCready, R.; Guggolz, J.; Silviera, V.; Owens, H. Determination of starch and amylose in vegetables. Anal. Chem. 1950, 22, 1156–1158. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Growth, Quality, and Nitrogen Assimilation in Response to High Ammonium or Nitrate Supply in Cabbage (Brassica campestris L.) and Lettuce (Lactuca sativa L.). Agronomy 2021, 11, 2556. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Yang, J.; Jeong, B.R. Synergistic Effects of Silicon and Preservative on Promoting Postharvest Performance of Cut Flowers of Peony (Paeonia lactiflora Pall.). Int. J. Mol. Sci. 2022, 23, 13211. https://doi.org/10.3390/ijms232113211
Song J, Yang J, Jeong BR. Synergistic Effects of Silicon and Preservative on Promoting Postharvest Performance of Cut Flowers of Peony (Paeonia lactiflora Pall.). International Journal of Molecular Sciences. 2022; 23(21):13211. https://doi.org/10.3390/ijms232113211
Chicago/Turabian StyleSong, Jinnan, Jingli Yang, and Byoung Ryong Jeong. 2022. "Synergistic Effects of Silicon and Preservative on Promoting Postharvest Performance of Cut Flowers of Peony (Paeonia lactiflora Pall.)" International Journal of Molecular Sciences 23, no. 21: 13211. https://doi.org/10.3390/ijms232113211
APA StyleSong, J., Yang, J., & Jeong, B. R. (2022). Synergistic Effects of Silicon and Preservative on Promoting Postharvest Performance of Cut Flowers of Peony (Paeonia lactiflora Pall.). International Journal of Molecular Sciences, 23(21), 13211. https://doi.org/10.3390/ijms232113211