Mitochondrial Epilepsy, a Challenge for Neurologists
Abstract
1. Introduction
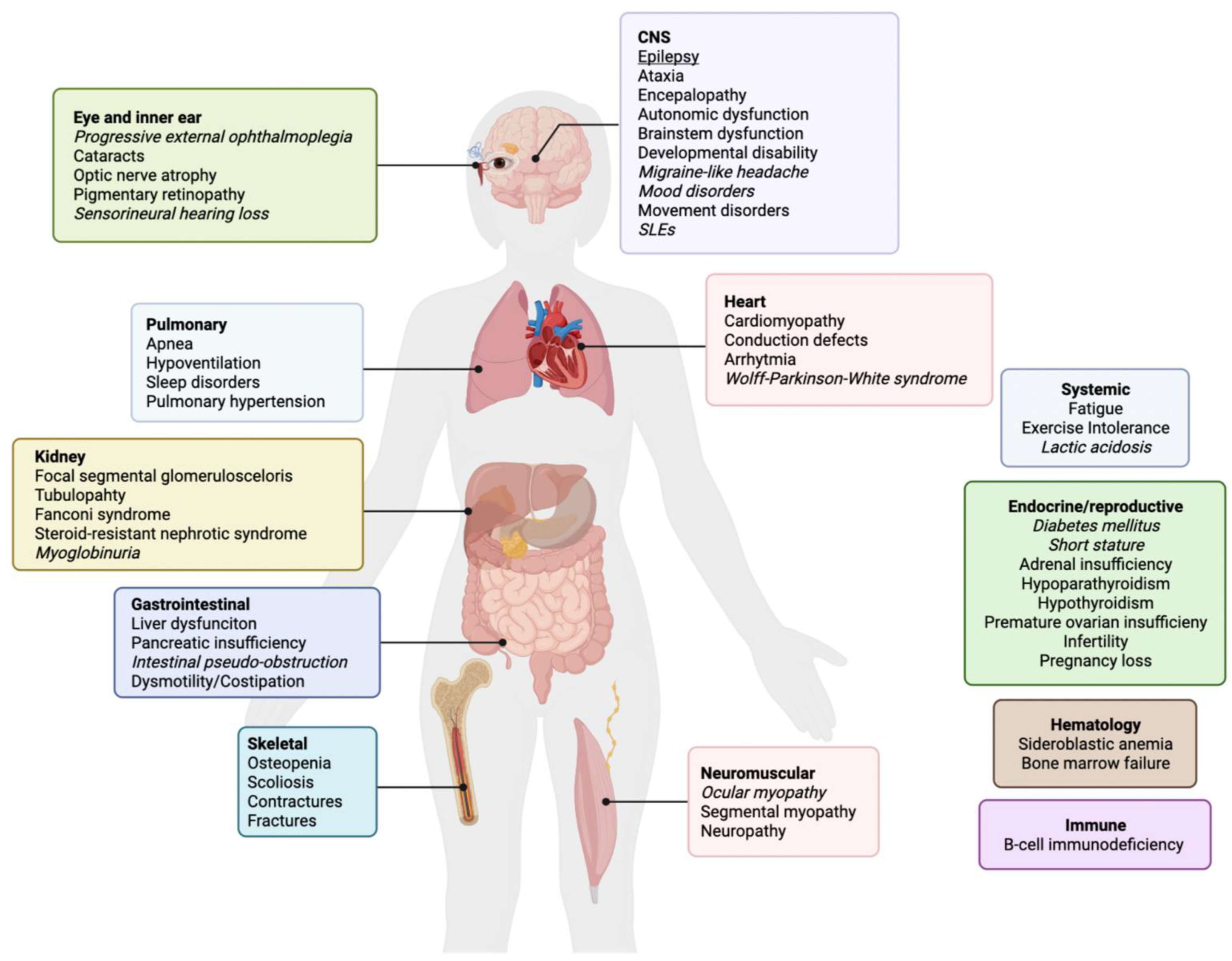
2. Epilepsy in Primary Mitochondrial Disease
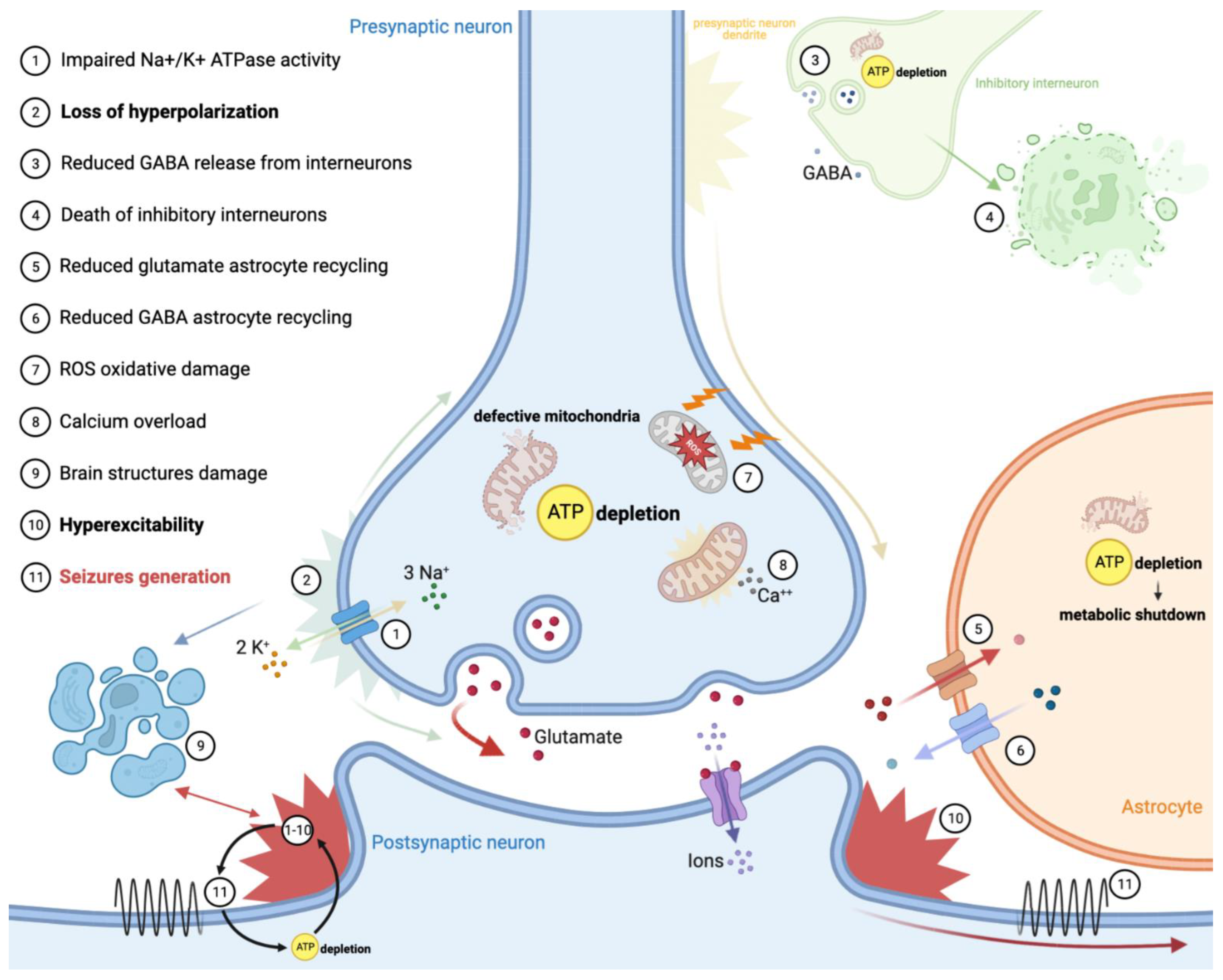
3. Pathomechanism
4. Mitochondrial Epilepsy Phenotypes
4.1. Mitochondrial Encephalopathy, Lactic Acidosis and Stroke-like Episodes—MELAS
4.2. Myoclonic Epilepsy with Ragged Red Fibers—MERRF
4.3. POLG-Related Disorders
4.4. Leigh Syndrome—LS
5. Management
5.1. Pharmacological Treatment
5.2. Non-Pharmacological Treatment
5.3. Stroke like Episodes and Status Epilepticus
5.4. New Therapeutic Approaches
6. How to Recognize a Mitochondrial Epilepsy?
7. Key Points
- -
- Seizures and status epilepticus represent one of the most frequent symptoms of mitochondrial diseases; approximately 20–50% of mitochondrial patients will have seizures during their disease course.
- -
- Pathomechanism of mitochondrial epilepsy is not totally understood, but the role of mitochondrial defects in the development of seizures is well recognized.
- -
- Patients with specific genotypes are more at risk of showing seizures in their lives than others, and they need to be followed up on to prevent these events (m.3243A > G, m.8344A > G, POLG).
- -
- The management and treatment of mitochondrial epilepsy need to be personalized for each patient, due to the variability of phenotypes that mitochondrial patients may present.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bornstein, R.; Gonzalez, B.; Johnson, S.C. Mitochondrial pathways in human health and aging. Mitochondrion 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Gorman, G.S.; Schaefer, A.M.; Ng, Y.; Gomez, N.; Blakely, E.L.; Alston, C.L.; Feeney, C.; Horvath, R.; Yu-Wai-Man, P.; Chinnery, P.F.; et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 2015, 77, 753. [Google Scholar] [CrossRef] [PubMed]
- Schlieben, L.D.; Prokisch, H. The Dimensions of Primary Mitochondrial Disorders. Front. Cell Dev. Biol. 2020, 8, 600079. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.S.; Bindoff, L.A.; Gorman, G.S.; Klopstock, T.; Kornblum, C.; Mancuso, M.; McFarland, R.; Sue, C.M.; Suomalainen, A.; Taylor, R.W.; et al. Mitochondrial disease in adults: Recent advances and future promise. Lancet Neurol. 2021, 20, 573–584. [Google Scholar] [CrossRef]
- Bindoff, L.A.; Engelsen, B.A. Mitochondrial diseases and epilepsy. Epilepsia 2012, 53 (Suppl. 4), 92–97. [Google Scholar] [CrossRef] [PubMed]
- Rahamn, S. Mitochondrial disease and epilepsy. Dev. Med. Child Neurol. 2012, 54, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.G.; Devine, H.E. Epilepsy in adults with mitochondrial disease. Ann. Neurol. 2015, 78, 949–957. [Google Scholar] [CrossRef]
- Ticci, C.; Sicca, F.; Ardissone, A.; Bertini, E.; Carelli, V.; Diodato, D.; Di Vito, L.; Filosto, M.; La Morgia, C.; Lamperti, C.; et al. Mitochondrial epilepsy: A cross-sectional nationwide Italian survey. Neurogenetics 2020, 21, 87–96. [Google Scholar] [CrossRef]
- Rahman, S. Mitochondrial diseases and status epilepticus. Epilepsia 2018, 59, 70–77. [Google Scholar] [CrossRef]
- Khurana, D.S.; Salganicoff, L.; Melvin, J.J.; Hobdell, E.F.; Valencia, I.; Hardison, H.H.; Marks, H.G.; Grover, W.D.; Legido, A. Epilepsy and respiratory chain defects in children with mitochondrial encephalopathies. Neuropediatrics 2008, 39, 8–13. [Google Scholar] [CrossRef]
- Fine, A.L.; Liebo, G.; Gavrilova, R.H.; Britton, J.W. Seizure Semiology, EEG, and Imaging Findings in Epilepsy Secondary to Mitochondrial Disease. Front. Neurol. 2021, 12, 779052. [Google Scholar] [CrossRef] [PubMed]
- Orsucci, D.; Caldarazzo Ienco, E.; Rossi, A.; Siciliano, G.; Mancuso, M. Mitochondrial syndromes revisited. J. Clin. Med. 2021, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Pickett, S.J.; Grady, J.P.; Ng, Y.S.; Gorman, G.S.; Schaefer, A.M.; Wilson, I.J.; Cordell, H.J.; Turnbull, D.M.; Taylor, R.W.; McFarland, R. Phenotypic heterogeneity in m.3243A>G mitochondrial disease: The role of nuclear factors. Ann. Clin. Transl. Neurol. 2018, 5, 333–345. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W.; Adesina, A.M.; Jones, J.; Scaglia, F. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol. Genet. Metab. 2015, 116, 4–12. [Google Scholar] [CrossRef]
- Mancuso, M.; Orsucci, D.; Angelini, C.; Bertini, E.; Carelli, V.; Comi, G.P.; Minetti, C.; Moggio, M.; Mongini, T.; Servidei, S.; et al. Phenotypic heterogeneity of the 8344A>G mtDNA “MERRF” mutation. Neurology 2013, 80, 2049–2054. [Google Scholar] [CrossRef]
- Lamperti, C.; Zeviani, M. Myoclonus epilepsy in mitochondrial disorders. Epileptic Disord. 2016, 18, S94–S102. [Google Scholar] [CrossRef]
- Rahman, S.; Copeland, W.C. POLG-related disorders and their neurological manifestations. Nat. Rev. Neurol. 2019, 15, 40. [Google Scholar] [CrossRef]
- Hikmat, O.; Naess, K.; Engvall, M.; Klingenberg, C.; Rasmussen, M.; Tallaksen, C.M.; Brodtkorb, E.; Ostergaard, E.; de Coo, I.F.M.; Pias-Peleteiro, L.; et al. Simplifying the clinical classification of polymerase gamma (POLG) disease based on age of onset; studies using a cohort of 155 cases. J. Inherit. Metab. Dis. 2020, 43, 726–736. [Google Scholar] [CrossRef]
- Schubert, M.B.; Vilarinho, L. Molecular basis of Leigh syndrome: A current look. Orphanet J. Rare Dis. 2020, 15, 31. [Google Scholar] [CrossRef]
- Bakare, A.B.; Lesnefsky, E.J.; Iyer, S. Leigh Syndrome: A Tale of Two Genomes. Front. Physiol. 2021, 12, 1286. [Google Scholar] [CrossRef]
- Patel, K.P.; O’Brien, T.W.; Subramony, S.H.; Shuster, J.; Stacpoole, P.W. The Spectrum of Pyruvate Dehydrogenase Complex Deficiency: Clinical, Biochemical and Genetic Features in 371 Patients. Mol. Genet. Metab. 2012, 105, 34. [Google Scholar] [CrossRef] [PubMed]
- Angebault, C.; Charif, M.; Guegen, N.; Piro-Megy, C.; Mousson de Camaret, B.; Procaccio, V.; Guichet, P.-O.; Hebrard, M.; Manes, G.; Leboucq, N.; et al. Mutation in NDUFA13/GRIM19 leads to early onset hypotonia, dyskinesia and sensorial deficiencies, and mitochondrial complex I instability. Hum. Mol. Genet. 2015, 24, 3948–3955. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, A.I.; Renkema, G.H.; Bras, M.; van den Heuvel, L.P.; Hoischen, A.; Gilissen, C.; Nabuurs, S.B.; Huynen, M.A.; de Vries, M.C.; Smeitink, J.A.; et al. A complex V ATP5A1 defect causes fatal neonatal mitochondrial encephalopathy. Brain 2013, 136 Pt 5, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Kashiki, T.; Kido, J.; Momosaki, K.; Kusunoki, S.; Ozasa, S.; Nomura, K.; Imai-Okazaki, A.; Tsuruoka, T.; Murayama, K.; Koga, Y.; et al. Mitochondrial DNA depletion syndrome with a mutation in SLC25A4 developing epileptic encephalopathy: A case report. Brain Dev. 2022, 44, 56–62. [Google Scholar] [CrossRef]
- Pacitti, D.; Levene, M.; Garone, C.; Nirmalananthan, N.; Bax, B.E. Mitochondrial Neurogastrointestinal Encephalomyopathy: Into the Fourth Decade, What We Have Learned So Far. Front. Genet. 2018, 9, 669. [Google Scholar] [CrossRef]
- Elpeleg, O.; Miller, C.; Hershkovitz, E.; Bitner-Glindzicz, M.; Bondi-Rubinstein, G.; Rahman, S.; Pagnamenta, A.; Eshhar, S.; Saada, A. Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am. J. Hum. Genet. 2005, 76, 1081–1086. [Google Scholar] [CrossRef]
- Mandel, H.; Szargel, R.; Labay, V.; Elpeleg, O.; Saada, A.; Shalata, A.; Anbinder, Y.; Berkowitz, D.; Hartman, C.; Barak, M.; et al. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat. Genet. 2001, 29, 337–341. [Google Scholar] [CrossRef]
- Bourdon, A.; Minai, L.; Serre, V.; Jais, J.P.; Sarzi, E.; Aubert, S.; Chrétien, D.; de Lonlay, P.; Paquis-Flucklinger, V.; Arakawa, H.; et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat. Genet. 2007, 39, 776–780. [Google Scholar] [CrossRef]
- Bonnen, P.E.; Yarham, J.W.; Besse, A.; Wu, P.; Faqeih, E.A.; Al-Asmari, A.M.; Saleh, M.A.; Eyaid, W.; Hadeel, A.; He, L.; et al. Mutations in FBXL4 cause mitochondrial encephalopathy and a disorder of mitochondrial DNA maintenance. Am. J. Hum. Genet. 2013, 93, 471–481. [Google Scholar] [CrossRef]
- Stiles, A.R.; Simon, M.T.; Stover, A.; Eftekharian, S.; Khanlou, N.; Wang, H.L.; Magaki, S.; Lee, H.; Partynski, K.; Dorrani, N.; et al. Mutations in TFAM, encoding mitochondrial transcription factor A, cause neonatal liver failure associated with mtDNA depletion. Mol. Genet. Metab. 2016, 119, 91–99. [Google Scholar] [CrossRef]
- Hallmann, K.; Zsurka, G.; Moskau-Hartmann, S.; Kirschner, J.; Korinthenberg, R.; Ruppert, A.K.; Ozdemir, O.; Weber, Y.; Becker, F.; Lerche, H.; et al. A homozygous splice-site mutation in CARS2 is associated with progressive myoclonic epilepsy. Neurology 2014, 83, 2183–2187. [Google Scholar] [CrossRef] [PubMed]
- Ngo, J.; Prokop, J.W.; Umfleet, J.; Seaver, L.H. Perinatal Manifestations of DARS2-Associated Leukoencephalopathy with Brainstem and Spinal Cord Involvement and Lactate Elevation (LBSL). Child Neurol. Open 2021, 8, 2329048X2110191. [Google Scholar] [CrossRef] [PubMed]
- Elo, J.M.; Yadavalli, S.S.; Euro, L.; Isohanni, P.; Götz, A.; Carroll, C.J.; Valanne, L.; Alkuraya, F.S.; Uusimaa, J.; Paetau, A.; et al. Mitochondrial phenylalanyl-tRNA synthetase mutations underlie fatal infantile Alpers encephalopathy. Hum. Mol. Genet. 2012, 21, 4521–4529. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, I.; Kennaway, N.G.; Shoubridge, E.A. A molecular chaperone for mitochondrial complex I assembly is mutated in a progressive encephalopathy. J. Clin. Investig. 2005, 115, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Sofou, K.; Kollberg, G.; Holmström, M.; Dávila, M.; Darin, N.; Gustafsson, C.M.; Holme, E.; Oldfors, A.; Tulinius, M.; Asin-Cayuela, J. Whole exome sequencing reveals mutations in NARS2 and PARS2, encoding the mitochondrial asparaginyl-tRNA synthetase and prolyl-tRNA synthetase, in patients with Alpers syndrome. Mol. Genet. Genom. Med. 2015, 3, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Edvardson, S.; Shaag, A.; Kolesnikova, O.; Gomori, J.M.; Tarassov, I.; Einbinder, T.; Saada, A.; Elpeleg, O. Deleterious Mutation in the Mitochondrial Arginyl–Transfer RNA Synthetase Gene Is Associated with Pontocerebellar Hypoplasia. Am. J. Hum. Genet. 2007, 81, 857. [Google Scholar] [CrossRef]
- Diodato, D.; Melchionda, L.; Haack, T.B.; Dallabona, C.; Baruffini, E.; Donnini, C.; Granata, T.; Ragona, F.; Balestri, P.; Margollicci, M.; et al. VARS2 and TARS2 Mutations in Patients with Mitochondrial Encephalomyopathies. Hum. Mutat. 2014, 35, 983–989. [Google Scholar] [CrossRef]
- Smeitink, J.A.; Elpeleg, O.; Antonicka, H.; Diepstra, H.; Saada, A.; Smits, P.; Sasarman, F.; Vriend, G.; Jacob-Hirsch, J.; Shaag, A.; et al. Distinct Clinical Phenotypes Associated with a Mutation in the Mitochondrial Translation Elongation Factor EFTs. Am. J. Hum. Genet. 2006, 79, 869. [Google Scholar] [CrossRef]
- Kopajtich, R.; Nicholls, T.J.; Rorbach, J.; Metodiev, M.D.; Freisinger, P.; Mandel, H.; Vanlander, A.; Ghezzi, D.; Carrozzo, R.; Taylor, R.W.; et al. Mutations in GTPBP3 Cause a Mitochondrial Translation Defect Associated with Hypertrophic Cardiomyopathy, Lactic Acidosis, and Encephalopathy. Am. J. Hum. Genet. 2014, 95, 708. [Google Scholar] [CrossRef]
- Janer, A.; Antonicka, H.; Lalonde, E.; Nishimura, T.; Sasarman, F.; Brown, G.K.; Brown, R.M.; Majewski, J.; Shoubridge, E.A. An RMND1 Mutation Causes Encephalopathy Associated with Multiple Oxidative Phosphorylation Complex Deficiencies and a Mitochondrial Translation Defect. Am. J. Hum. Genet. 2012, 91, 737. [Google Scholar] [CrossRef]
- Serre, V.; Rozanska, A.; Beinat, M.; Chretien, D.; Boddaert, N.; Munnich, A.; Rötig, A.; Chrzanowska-Lightowlers, Z.M. Mutations in mitochondrial ribosomal protein MRPL12 leads to growth retardation, neurological deterioration and mitochondrial translation deficiency. Biochim. Biophys. Acta 2013, 1832, 1304. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.; Naini, A.; Salviati, L.; Trevisson, E.; Navas, P.; Dimauro, S.; Hirano, M. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am. J. Hum. Genet. 2006, 78, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Malicdan, M.C.V.; Vilboux, T.; Ben-Zeev, B.; Guo, J.; Eliyahu, A.; Pode-Shakked, B.; Dori, A.; Kakani, S.; Chandrasekharappa, S.C.; Ferreira, C.R.; et al. A novel inborn error of the coenzyme Q10 biosynthesis pathway: Cerebellar ataxia and static encephalomyopathy due to COQ5 C-methyltransferase deficiency. Hum. Mutat. 2018, 39, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.J.; Bitner-Glindzicz, M.; Meunier, B.; Costello, H.; Hargreaves, I.P.; López, L.C.; Hirano, M.; Quinzii, C.M.; Sadowski, M.I.; Hardy, J.; et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: A potentially treatable form of mitochondrial disease. Am. J. Hum. Genet. 2009, 84, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Saada, A.; Vogel, R.O.; Hoefs, S.J.; van den Brand, M.A.; Wessels, H.J.; Willems, P.H.; Venselaar, H.; Shaag, A.; Barghuti, F.; Reish, O.; et al. Mutations in NDUFAF3 (C3ORF60), encoding an NDUFAF4 (C6ORF66)-interacting complex I assembly protein, cause fatal neonatal mitochondrial disease. Am. J. Hum. Genet. 2009, 84, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, M.; Tiranti, V.; Zeviani, M. Ethylmalonic encephalopathy: Clinical course and therapy response in an uncommon mild case with a severe ETHE1 mutation. Mol. Genet. Metab. Rep. 2020, 25, 100641. [Google Scholar] [CrossRef]
- Hikmat, O.; Tzoulis, C.; Knappskog, P.M.; Johansson, S.; Boman, H.; Sztromwasser, P.; Lien, E.; Brodtkorb, E.; Ghezzi, D.; Bindoff, L.A. ADCK3 mutations with epilepsy, stroke-like episodes and ataxia: A POLG mimic? Eur. J. Neurol. 2016, 23, 1188–1194. [Google Scholar] [CrossRef]
- Hanes, I.; McMillan, H.J.; Ito, Y.; Kernohan, K.D.; Lazier, J.; Lines, M.A.; Dyment, D.A. A splice variant in ATAD3A expands the clinical and genetic spectrum of Harel-Yoon syndrome. Neurol. Genet. 2020, 6, e452. [Google Scholar] [CrossRef]
- Molinari, F.; Kaminska, A.; Fiermonte, G.; Boddaert, N.; Raas-Rothschild, A.; Plouin, P.; Palmieri, L.; Brunelle, F.; Palmieri, F.; Dulac, O.; et al. Mutations in the mitochondrial glutamate carrier SLC25A22 in neonatal epileptic encephalopathy with suppression bursts. Clin. Genet. 2009, 76, 188–194. [Google Scholar] [CrossRef]
- Morton, S.U.; Prabhu, S.P.; Lidov, H.G.W.; Shi, J.; Anselm, I.; Brownstein, C.A.; Bainbridge, M.N.; Beggs, A.H.; Vargas, S.O.; Agrawal, P.B. AIFM1 mutation presenting with fatal encephalomyopathy and mitochondrial disease in an infant. Cold Spring Harb. Mol. Case Stud. 2017, 3, a001560. [Google Scholar] [CrossRef]
- Saada, A.; Edvardson, S.; Rapoport, M.; Shaag, A.; Amry, K.; Miller, C.; Lorberboum-Galski, H.; Elpeleg, O. C6ORF66 is an assembly factor of mitochondrial complex I. Am. J. Hum. Genet. 2008, 82, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, L.C.; Sue, C.M.; Davidson, M.M.; Tanji, K.; Nishino, I.; Sadlock, J.E.; Krishna, S.; Walker, W.; Selby, J.; Glerum, D.M.; et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 1999, 23, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, D.; Saada, A.; D’Adamo, P.; Fernandez-Vizarra, E.; Gasparini, P.; Tiranti, V.; Elpeleg, O.; Zeviani, M. FASTKD2 nonsense mutation in an infantile mitochondrial encephalomyopathy associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 2008, 83, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Antonicka, H.; Leary, S.C.; Guercin, G.H.; Agar, J.N.; Horvath, R.; Kennaway, N.G.; Harding, C.O.; Jaksch, M.; Shoubridge, E.A. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 2003, 12, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Oquendo, C.E.; Antonicka, H.; Shoubridge, E.A.; Reardon, W.; Brown, G.K. Functional and genetic studies demonstrate that mutation in the COX15 gene can cause Leigh syndrome. J. Med. Genet. 2004, 41, 540–544. [Google Scholar] [CrossRef]
- Cízková, A.; Stránecký, V.; Mayr, J.A.; Tesarová, M.; Havlícková, V.; Paul, J.; Ivánek, R.; Kuss, A.W.; Hansíková, H.; Kaplanová, V.; et al. TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy. Nat. Genet. 2008, 40, 1288–1290. [Google Scholar] [CrossRef]
- MELAS|MedLink Neurology. Available online: https://www.medlink.com/articles/melas (accessed on 26 September 2022).
- Mancuso, M.; Orsucci, D.; Angelini, C.; Bertini, E.; Carelli, V.; Comi, G.P.; Donati, A.; Minetti, C.; Moggio, M.; Mongini, T.; et al. The m. 3243A > G mitochondrial DNA mutation and related phenotypes. A matter of gender? J. Neurol. 2014, 261, 504–510. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Cui, Z.; Li, Z.; Jiang, T.; Meng, H. Epilepsy Associated with Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes. Front. Neurol. 2021, 12, 675816. [Google Scholar] [CrossRef]
- Lee, H.N.; Eom, S.; Kim, S.H.; Kang, H.C.; Lee, J.S.; Kim, H.D.; Lee, Y.M. Epilepsy Characteristics and Clinical Outcome in Patients with Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes (MELAS). Pediatr. Neurol. 2016, 64, 59–65. [Google Scholar] [CrossRef]
- Kaufman, K.R.; Zuber, N.; Rueda-Lara, M.A.; Tobia, A. MELAS with recurrent complex partial seizures, nonconvulsive status epilepticus, psychosis, and behavioral disturbances: Case analysis with literature review. Epilepsy Behav. 2010, 18, 494–497. [Google Scholar] [CrossRef]
- Rahman, S. Epilepsy & Behavior Advances in the treatment of mitochondrial epilepsies. Epilepsy Behav. 2019, 101 Pt B, 106546. [Google Scholar]
- Cheng, W.; Zhang, Y.; He, L. MRI Features of Stroke-like Episodes in Mitochondrial Encephalomyopathy with Lactic Acidosis and Stroke-like Episodes. Front. Neurol. 2022, 13, 843386. [Google Scholar] [CrossRef] [PubMed]
- Hikmat, O.; Eichele, T.; Tzoulis, C.; Bindoff, L.A. Understanding the Epilepsy in POLG Related Disease. Int. J. Mol. Sci. 2017, 18, 1845. [Google Scholar] [CrossRef]
- Hikmat, O.; Naess, K.; Engvall, M.; Klingenberg, C.; Rasmussen, M.; Tallaksen, C.M.E.; Brodtkorb, E.; Fiskerstrand, T.; Isohanni, P.; Uusimaa, J.; et al. Elevated cerebrospinal fluid protein in POLG-related epilepsy: Diagnostic and prognostic implications. Epilepsia 2018, 59, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Thomas, R.H. The mitochondrial epilepsies. Eur. J. Paediatr. Neurol. 2020, 24, 47–52. [Google Scholar] [CrossRef] [PubMed]
- POLG-Related Disorders. GeneReviews®. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26471/ (accessed on 26 September 2022).
- Wes, D.; Rokicki, D. Epilepsy in Mitochondrial Diseases—Current State of Knowledge on Aetiology and Treatment. Children 2021, 8, 532. [Google Scholar]
- Lake, N.J.; Compton, A.G.; Rahman, S.; Thorburn, D.R. Leigh syndrome: One disorder, more than 75 monogenic causes. Ann. Neurol. 2016, 79, 190–203. [Google Scholar] [CrossRef]
- Parikh, S.; Goldstein, A.; Karaa, A.; Koenig, M.K.; Anselm, I.; Brunel-Guitton, C.; Christodoulou, J.; Cohen, B.H.; Dimmock, D.; Enns, G.M.; et al. Patient care standards for primary mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Genet. Med. 2017, 19, 1380. [Google Scholar] [CrossRef]
- De Vries, M.C.; Brown, D.A.; Allen, M.E.; Bindoff, L.; Gorman, G.S.; Karaa, A.; Keshavan, N.; Lamperti, C.; McFarland, R.; Ng, Y.S.; et al. Safety of drug use in patients with a primary mitochondrial disease: An international Delphi-based consensus. J. Inherit. Metab. Dis. 2020, 43, 800–818. [Google Scholar] [CrossRef]
- Primiano, G.; Vollono, C.; Dono, F.; Servidei, S. Drug-resistant epilepsy in MELAS: Safety and potential efficacy of lacosamide. Epilepsy Res. 2018, 139, 135–136. [Google Scholar] [CrossRef]
- Arthur, T.M.; Saneto, R.P.; de Menezes, M.S.; Devinsky, O.; Lajoie, J.; Murphy, P.J.; Cook, W.B.; Ojemann, J.G. Vagus nerve stimulation in children with mitochondrial electron transport chain deficiencies. Mitochondrion 2007, 7, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Na, J.H.; Kim, H.D.; Lee, Y.M. Effective application of corpus callosotomy in pediatric intractable epilepsy patients with mitochondrial dysfunction. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221092551. [Google Scholar] [CrossRef] [PubMed]
- Zweers, H.; Van Wegberg, A.M.; Janssen, M.C.H.; Wortmann, S.B. Ketogenic diet for mitochondrial disease: A systematic review on efficacy and safety. Orphanet J. Rare Dis. 2021, 295, 295. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, H.; Zhong, J.; Yang, L.; Chen, G.; Wang, D.; Zheng, G.; Han, H.; Han, X.; Long, Y.; et al. Efficacy and Safety of the Ketogenic Diet for Mitochondrial Disease with Epilepsy: A Prospective, Open-labeled, Controlled Study. Front. Neurol. 2022, 13, 880944. [Google Scholar] [CrossRef]
- Ng, Y.S.; Bindoff, L.A.; Gorman, G.S.; Horvath, R.; Klopstock, T.; Mancuso, M.; Martikainen, M.H.; Mcfarland, R.; Nesbitt, V.; Pitceathly, R.D.S.; et al. Consensus-based statements for the management of mitochondrial stroke-like episodes. Wellcome Open Res. 2019, 4, 201. [Google Scholar] [CrossRef]
- Santamarina, E.; Alpuente, A.; Maisterra, O.; Sueiras, M.; Sarria, S.; Guzman, L.; Abraira, L.; Salas-Puig, J.; Toledo, M. Perampanel: A therapeutic alternative in refractory status epilepticus associated with MELAS syndrome. Epilepsy Behav. Case Rep. 2019, 11, 92–95. [Google Scholar] [CrossRef]
- Almannai, M.; El-Hattab, A.W.; Ali, M.; Soler-Alfonso, C.; Scaglia, F. Clinical trials in mitochondrial disorders, an update. Mol. Genet. Metab. 2020, 131, 1–13. [Google Scholar] [CrossRef]
- Parikh, S.; Goldstein, A.; Koenig, M.K.; Scaglia, F.; Enns, G.M.; Saneto, R.; Anselm, I.; Cohen, B.H.; Falk, M.J.; Greene, C.; et al. Diagnosis and management of mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Genet. Med. 2015, 17, 689–701. [Google Scholar] [CrossRef]
| Phenotypes | Genes | References |
|---|---|---|
| MELAS | MT-TL1 (3243A > G, 3271T > C), MT-ND5 (m.13513G > A) | [14] |
| MERRF | MT-TK (m.8344A> G, 8356T > C, 8363G > A, 8361G > A) | [15,16] |
| POLG-related disorders spectrum (MCHS, AHS, MEMSA, ANS) | POLG (c.1399G > A, c.2243G > C, c.2542G > A) | [17,18] |
| Leigh syndrome | >75 nuclear-encoded genes | [19] |
| MILS | MT-ATP6 (8993T > G) | [19,20] |
| Pyruvate dehydrogenase complex deficiency | PDHA, PDHB, LIAS, LIPT1, DLD, PDH | [21] |
| Others (Leigh-like syndrome, AHS, MDS, NAS encephalopathy) | NDUFA13, ATP5A1, NDUFAF2, NDUFAF3, NDUFAF4, ACAD9, SCO2, FASTKD2, COX10, COX15, TMEM70, ANT1, TYMP, SUCLA2, DGUOK, RRM2B, FBXL4, TFAM, CARS2, DARS2, NARS2, PARS2, RARS2, VARS2, TARS2, TSFM, GTPBP3, RMND1, MRPL12, COQ2, COQ5, COQ8A, COQ9, ETHE1, ATAD3, SLC25A22, AIFM1 | [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopriore, P.; Gomes, F.; Montano, V.; Siciliano, G.; Mancuso, M. Mitochondrial Epilepsy, a Challenge for Neurologists. Int. J. Mol. Sci. 2022, 23, 13216. https://doi.org/10.3390/ijms232113216
Lopriore P, Gomes F, Montano V, Siciliano G, Mancuso M. Mitochondrial Epilepsy, a Challenge for Neurologists. International Journal of Molecular Sciences. 2022; 23(21):13216. https://doi.org/10.3390/ijms232113216
Chicago/Turabian StyleLopriore, Piervito, Fábio Gomes, Vincenzo Montano, Gabriele Siciliano, and Michelangelo Mancuso. 2022. "Mitochondrial Epilepsy, a Challenge for Neurologists" International Journal of Molecular Sciences 23, no. 21: 13216. https://doi.org/10.3390/ijms232113216
APA StyleLopriore, P., Gomes, F., Montano, V., Siciliano, G., & Mancuso, M. (2022). Mitochondrial Epilepsy, a Challenge for Neurologists. International Journal of Molecular Sciences, 23(21), 13216. https://doi.org/10.3390/ijms232113216








