Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery
Abstract
:1. Introduction
2. The Endocannabinoid System (ECS)
2.1. The History of Cannabis
2.2. The Disovery of the Endocannabinoid System
2.3. Cannabinoid Receptors
2.3.1. CB1-R
2.3.2. CB2-R
2.4. Endocannabinoid Signaling Pathways
2.4.1. CB1-R
2.4.2. CB2-R

2.5. Synthesis and Degradation of Endocannabinoids
2.6. Cannabinoids
| Cannabinoid | Receptor Affinity | Reference |
|---|---|---|
| Phytocannabinoids | ||
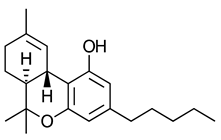 --THC) | CB1-R and CB2-R agonist | [150] |
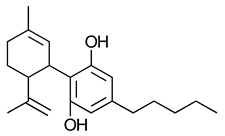 (-)-Cannabidiol (CBD) | No activity at CB1-R or CB2-R | [150] |
| Endogenous cannabinoids | ||
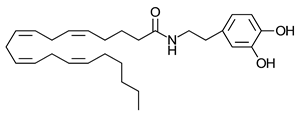
 Anandamide | Greater CB1-R than CB2-R agonist TRPV1 agonist | [104] |
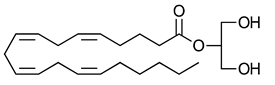 2-Arachidonoyl glycerol | CB1-R and CB2-R agonist | [70] |
 Virodhamine | Greater CB1-R than CB2-R agonist | [150] |
 N-Arachidonoyl dopamine | Greater CB1-R than CB2-R agonist TRPV1 agonist | [150] |
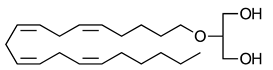 Noladin-ether | Greater CB1-R than CB2-R agonist | [150] |
| Synthetic cannabinoids | ||
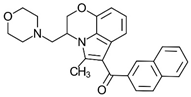 Aminoalkylindole WIN 55,212-2 | Highly selective CB2-R agonist. | [151] |
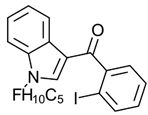 Benzoylindole AM694 | Potent CB receptor agonist. Highly selective for CB2 receptor. | [152] |
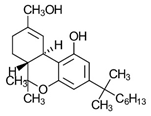 Dibenzopyran HU-210 | Highly potent CB1-R and CB2-R agonist. Preference for CB1-R receptors. | [153] |
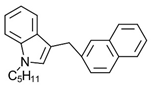 Naphthylmethylindole JWH-175 | Selective CB1-R agonist. | [154] |
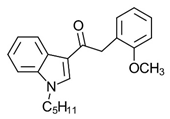 Phenylacetylindole JWH-250 | Potent CB agonist with greater affinity towards CB1-R. | [155] |
2.7. Selection of Cannabinoid Ligands for Specific Targeting
2.7.1. Allosteric Modulators
2.7.2. Positive Allosteric Modulators (PAM)
2.7.3. Negative Allosteric Modulators (NAM)
2.7.4. Functional Selectivity
2.7.5. Peripherally Acting Cannabinoids
3. Toolkit of Therapeutics of ECS
3.1. Enhanced Endocannabinoid Tone
3.1.1. The Inhibition of Endocannabinoid Degrading Enzymes
3.1.2. The Inhibition of Endocannabinoid Uptake and Intracellular Transport
3.2. Sites and Tissue Specificity of Endocannabinoid Receptor Distribution
3.3. G-Protein Subtype and β-Arrestin Specificity
3.4. Homology Modelling, Molecular Docking and Molecular Dynamics Simulation
4. Improving Drug Delivery through Use of Nanoprecision Tools
4.1. Drug Delivery Vehicles
4.1.1. Size and Surface Charge
4.1.2. Shape
4.1.3. Elasticity
4.1.4. Chemical Composition
4.1.5. Lipid-Based Drug Delivery Systems
4.1.6. Polymer-Based Drug Delivery Systems
4.2. Surface Modification
4.2.1. Ligand Density and Distribution Patterns
4.2.2. Dual, Asymmetric, and Clustered Ligands
5. Discussion and Future Perspectives
- The full characterization of signaling pathways is essential when considering candidates for targeting the ECS.
- Approaches to activate either G-protein signaling or β-arrestin signaling, include the use of endocytic lipid rafts, binding at specific extracellular loop motifs and plasticity of the receptor.
- Methods for using routes of biased signaling, include designing ligands as structural tools including allosteric modulators or ligands that exploit the plasticity of the receptor as G-protein biased ligands or β-arrestin biased ligands.
- High-resolution crvstal structures of cannabinoid receptors and molecular simulations effectively guide cannabinoid drug design.
- Nanoparticles can be engineered to provide site-specific delivery of cannabinoid ligands.
- Altering the density and surface pattern of ligands through clustering and patterns is a strategy to promote the use of one route of endocytosis over another.
- Overexpression of receptors in different pathological states can be used as potential targets for treatment of specific disorders.
- Receptor-mediated endocytosis can also be used to facilitate entry into specific sites through cannabinoid receptors.
- Molecular simulations can be used to predict the motion of every atom in a receptor and those in the molecules with which the receptor interacts.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| ECS | Endocannabinoid system |
| EPR | Enhanced permeation and retention |
| PEG | Polyethylene glycol |
| MAGL | Monoacylglycerol lipase |
| FAAH | Fatty acid amide hydrolase |
| Δ9-THC | (−)-Δ9-tetrahydrocannabinol |
| NAPE-PLD | N-acyl phosphatidylethanolamine-selective phospholipase D |
| DAGL | sn-1-selective diacylglycerol lipase |
| 2-AG | 2-arachidonoylglycerol |
| EMT | endocannabinoid membrane transporter |
| COX-1 | Cyclooxygenase type 1 |
| COX-2 | Cyclooxygenase type 2 |
| FABP5 | Fatty acid-binding proteins |
| DDV | Drug delivery vehicle |
References
- Śledziński, P.; Nowak-Terpiłowska, A.; Zeyland, J. Cannabinoids in Medicine: Cancer, Immunity, and Microbial Diseases. Int. J. Mol. Sci. 2020, 22, 263. [Google Scholar] [CrossRef]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef]
- Crocq, M.A. History of Cannabis and the Endocannabinoid System. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.P.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Soliman, N.; Rice, A.S.C. Cannabinoids, the Endocannabinoid System, and Pain: A Review of Preclinical Studies. Pain 2021, 162, S5–S25. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Liao, J.; Macleod, M.; Segelcke, D.; Sena, C.; Thomas, J.; Vollert, J.; et al. Systematic Review and Meta-Analysis of Cannabinoids, Cannabis-Based Medicines, and Endocannabinoid System Modulators Tested for Antinociceptive Effects in Animal Models of Injury-Related or Pathological Persistent Pain. Pain 2021, 162, S26–S44. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef]
- Kim, D.K.; Dobson, J. Nanomedicine for Targeted Drug Delivery. J. Mater. Chem. 2009, 19, 6294–6307. [Google Scholar] [CrossRef]
- Wakaskar, R.R. Promising Effects of Nanomedicine in Cancer Drug Delivery. J. Drug Target 2018, 26, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Pacardo, D.B.; Ligler, F.S.; Gu, Z. Programmable Nanomedicine Synergistic and Sequential Drug Delivery Systems. Nanoscale 2015, 7, 3381–3391. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Reddy, V.; Grogan, D.; Ahluwalia, M.; Salles, É.L.; Ahluwalia, P.; Khodadadi, H.; Alverson, K.; Nguyen, A.; Raju, S.P.; Gaur, P.; et al. Targeting the Endocannabinoid System: A Predictive, Preventive, and Personalized Medicine-Directed Approach to the Management of Brain Pathologies. EPMA J. 2020, 11, 217–250. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’sullivan, S.E. Towards Better Delivery of Cannabidiol (Cbd). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Nguyen, T.; Thomas, B.; Zhang, Y. Overcoming the Psychiatric Side Effects of the Cannabinoid CB1 Receptor Antagonists: Current Approaches for Therapeutics Development. Curr. Top Med. Chem. 2019, 19, 1418–1435. [Google Scholar] [CrossRef]
- Moreira, F.A.; Grieb, M.; Lutz, B. Central Side-Effects of Therapies Based on CB1 Cannabinoid Receptor Agonists and Antagonists: Focus on Anxiety and Depression. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 133–144. [Google Scholar] [CrossRef]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Canela, E.I. The Interplay between Cancer Biology and the Endocannabinoid System—Significance for Cancer Risk, Prognosis and Response to Treatment. Cancers 2020, 12, 3275. [Google Scholar] [CrossRef]
- Chen, D.J.; Gao, M.; Gao, F.F.; Su, Q.X.; Wu, J. Brain Cannabinoid Receptor 2: Expression, Function and Modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef] [Green Version]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kunos, G. Modulating the Endocannabinoid System in Human Health and Disease—Successes and Failures. FEBS J. 2013, 280, 1918–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasiulewicz, A.; Znajdek, K.; Grudzień, M.; Pawiński, T.; Sulkowska, J.I. A Guide to Targeting the Endocannabinoid System in Drug Design. Int. J. Mol. Sci. 2020, 21, 2778. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S. Exploration of Multiverse Activities of Endocannabinoids in Biological Systems. Int. J. Mol. Sci. 2022, 23, 5734. [Google Scholar] [CrossRef] [PubMed]
- Della Pietra, A.; Savinainen, J.; Giniatullin, R. Inhibiting Endocannabinoid Hydrolysis as Emerging Analgesic Strategy Targeting a Spectrum of Ion Channels Implicated in Migraine Pain. Int. J. Mol. Sci. 2022, 23, 4407. [Google Scholar] [CrossRef]
- Hua, T.; Li, X.; Wu, L.; Iliopoulos-Tsoutsouvas, C.; Wang, Y.; Wu, M.; Shen, L.; Brust, C.A.; Nikas, S.P.; Song, F.; et al. Activation and Signaling Mechanism Revealed by Cannabinoid Receptor-Gi Complex Structures. Cell 2020, 180, 655–665.e18. [Google Scholar] [CrossRef]
- Bassani, D.; Pavan, M.; Federico, S.; Spalluto, G.; Sturlese, M.; Moro, S. The Multifaceted Role of GPCRs in Amyotrophic Lateral Sclerosis: A New Therapeutic Perspective? Int. J. Mol. Sci. 2022, 23, 4504. [Google Scholar] [CrossRef]
- Insel, P.A.; Sriram, K.; Gorr, M.W.; Wiley, S.Z.; Michkov, A.; Salmerón, C.; Chinn, A.M. GPCRomics: An Approach to Discover GPCR Drug Targets. Trends Pharmacol. Sci. 2019, 40, 378–387. [Google Scholar] [CrossRef]
- Eglen, R.M.; Reisine, T. GPCRs Revisited: New Insights Lead to Novel Drugs. Pharmaceuticals 2011, 4, 244–272. [Google Scholar] [CrossRef] [Green Version]
- Parrill, A.; Bautista, D. GPCR Conformations: Implications for Rational Drug Design. Pharmaceuticals 2010, 4, 7–43. [Google Scholar] [CrossRef]
- Munk, C.; Mutt, E.; Isberg, V.; Nikolajsen, L.F.; Bibbe, J.M. Flock an Online Resource for GPCR Structure Determination and Analysis. Nat. Methods 2019, 16, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, S.; Calandra, B.; Delpech, M.; Dumont, X.; Kaghad, M.; le Fur, G.; Caput, D.; Ferrara, P. Structural Features of the Central Cannabinoid CB1 Receptor Involved in the Binding of the Specific CB1 Antagonist SR 141716A (∗). J. Biol. Chem. 1996, 271, 6941–6946. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Cao, Z.; Wang, W.; Zhou, N. New Insights in Cannabinoid Receptor Structure and Signaling. Curr. Mol. Pharmacol. 2019, 12, 239–248. [Google Scholar] [CrossRef]
- Kibret, B.G.; Ishiguro, H.; Horiuchi, Y.; Onaivi, E.S. New Insights and Potential Therapeutic Targeting of CB2 Cannabinoid Receptors in CNS Disorders. Int. J. Mol. Sci. 2022, 23, 975. [Google Scholar] [CrossRef]
- Fede, C.; Albertin, G.; Petrelli, L.; Sfriso, M.M. Expression of the Endocannabinoid Receptors in Human Fascial Tissue. Eur. J. Histochem. 2016, 2, 2643. [Google Scholar] [CrossRef] [Green Version]
- Mackie, K. Cannabinoid Receptors: Where They Are and What They Do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral Cannabinoid Receptor, CB2, Regulates Bone Mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Ashton, J.; Wright, J.; McPartland, J.; Tyndall, J. Cannabinoid CB1 and CB2 Receptor Ligand Specificity and the Development of CB2-Selective Agonists. Curr. Med. Chem. 2008, 15, 1428–1443. [Google Scholar] [CrossRef]
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res. 2017, 2, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V. Targeting the Endocannabinoid System: To Enhance or Reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Toczek, M.; Malinowska, B. Enhanced Endocannabinoid Tone as a Potential Target of Pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Guo, H.; Edman, M.; Hamm-Alvarez, S.F. Application of Advances in Endocytosis and Membrane Trafficking to Drug Delivery. Adv. Drug Deliv. Rev. 2020, 157, 118–141. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Hu, Q.; Ji, W.; Wright, G.; Gu, Z. Leveraging Physiology for Precision Drug Delivery. Physiol. Rev. 2017, 97, 189–225. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Zhu, L.; Weller, H.; Mews, A.; Parak, W.J.; Barz, M.; Feliu, N. Ligand Density on Nanoparticles: A Parameter with Critical Impact on Nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Claypool, S.; Liu, R. The Smart Targeting of Nanoparticles. Curr. Pharm. Des. 2013, 19, 6315–6329. [Google Scholar] [CrossRef] [Green Version]
- Green, B.; Kavanagh, D.; Young, R. Being Stoned: A Review of Self-Reported Cannabis Effects. Drug Alcohol. Rev. 2003, 22, 453–460. [Google Scholar] [CrossRef]
- Baudelaire, C.; De Quincey, T. Les Paradis Artificiels, Opium et Haschisch; Poulet-Malassis Et De Broise: Paris, France, 1860. [Google Scholar]
- Karche, T.; Singh, M.R. The Application of Hemp Cannabis Sativa L. for a Green Economy: A Review. Turk J. Botany 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Zuardi, A.W. History of Cannabis as a Medicine: A Review. Revista Brasileira de Psiquiatria 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Tubbs, S.R.; Riech, S.; Verma, K.; Chern, J.; Mortazavi, M.; Cohen-Gadol, A.A. China’s First Surgeon: Hua Tuo (c. 108–208 AD). Child’s Nerv. Syst. 2011, 27, 1357–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPartland, J.M.; Hillig, K.W. Early Iconography of Cannabis Sativa and Cannabis Indica. J. Ind. Hemp 2008, 13, 189–203. [Google Scholar] [CrossRef]
- Li, H.L. An Archaeological and Historical Account of Cannabis in China. Econ. Bot. 1973, 28, 437–448. [Google Scholar] [CrossRef]
- Dictionary, S.M. Pen Ts’ao. (n.d.). Available online: https://medical-dictionary.thefreedictionary.com/Pen+Ts%27ao (accessed on 27 June 2022).
- Hou, J.P. The Development of Chinese Herbal Medicine and the Pen-Ts’ao. Comp. Med. East West 1977, 5, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Wedman-St. Louis, B. Cannabis Use in Chinese Medicine, Ayurvedic Medicine, & Different Religions. In Cannabis as Medicine; CRC Press: Boca Raton, FL, USA, 2019; pp. 225–228. ISBN 9780429054723. [Google Scholar]
- Charitos, I.A.; Gagliano-Candela, R.; Santacroce, L.; Bottalico, L. The Cannabis Spread throughout the Continents and Its Therapeutic Use in History. Endocr. Metab. Immune Disord. Drug Targets 2020, 21, 407–417. [Google Scholar] [CrossRef]
- Small, E.; Cronquist, A. Cronquist A Practical and Natural Taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- Rätsch, C. Marijuana Medicine: A World Tour of the Healing and Visionary Powers of Cannabis; Inner Traditions/Bear & Co: Rochester, VT, USA, 2001; Volume 39. [Google Scholar]
- Russo, E.B. History of Cannabis and Its Preparations in Saga Science and Sobriquet, C. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Hazekamp, A. An Indian Perspective on Cannabis for Treatment of Pain. Int. J. Ayurveda Pharm. Chem. 2017, 7, 22–51. [Google Scholar]
- Mackworth, Y.W.; Kaplan, J. Marijuana: Report of the Indian Hemp Drugs Commission, 1893-1894; Thomas Jefferson Publishing Company: Simla, India, 1969. [Google Scholar]
- Fankhauser, M. History of Cannabis in Western Medicine. In Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential; Russo, E.B., Ed.; The Haworth Integrative Healing Press: New York, NY, USA, 2002; ISBN 9780789015082. [Google Scholar]
- Appendino, G. The Early History of Cannabinoid Research. Rendiconti Lincei 2020, 31, 919–929. [Google Scholar] [CrossRef]
- United Nations Commission on Narcotic Drugs. CND Votes on Recommendations for Cannabis and Cannabis-Related Substances; United Nations Commission on Narcotic Drugs: Vienna, Austria, 2020. [Google Scholar]
- Simiyu, D.; Jang, J.; Lee, O.R. Understanding Cannabis Sativa, L.: Current Status of Propagation, Use, Legalization, and Haploid-Inducer-Mediated Genetic Engineering. Plants 2022, 11, 1236. [Google Scholar] [CrossRef]
- ElSohly, M.; Mehmedic, Z.; Foster, S.; Gon, C.; Chandra, S.; Church, J. Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol. Psychiatry 2016, 79, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Leonard, C.M.; Ishiguro, H.; Zhang, P.W.; Lin, Z.; Akinshola, B.E.; Uhl, G.R. Endocannabinoids and Cannabinoid Receptor Genetics. Prog. Neurobiol. 2002, 66, 307–344. [Google Scholar] [CrossRef]
- Lu, H.C.; MacKie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Melck, D.; Bisogno, T.; Di Marzo, V. Endocannabinoids and Fatty Acid Amides in Cancer, Inflammation and Related Disorders. Chem. Phys. Lipids 2000, 108, 191–209. [Google Scholar] [CrossRef]
- Kuehl, F.A.; Jacob, T.A.; Ganley, O.H.; Ormond, R.E.; Meisinger, M.A.P. The Identification of N-(2-Hydroxyethyl)-Palmitamide as a Naturally Occurring Anti-Inflammatory Agent. J. Am. Chem. Soc. 1957, 79, 5577–5578. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Masaki, T.; Itoh, F.; Kondo, K.; Sudo, K. Isolation of Fatty Acid Amide as an Angiogenic Principle from Bovine Mesentery. Biochem. Biophys. Res. Commun. 1990, 168, 423–429. [Google Scholar] [CrossRef]
- Corey, E.J.; Cashman, J.R.; Kantner, S.S.; Wright, S.W. Rationally Designed, Potent Competitive Inhibitors of Leukotriene Biosynthesis. J. Am. Chem. Soc. 1984, 106, 1503–1504. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Di Marzo, V. An Introduction to the Endocannabinoid System: From the Early to the Latest Concepts. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 1–15. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Howlett, A.C. Cannabinoid Inhibition of Adenylate Cyclase. Biochemistry of the Response in Neuroblastoma Cell Membranes. Mol. Pharmacol. 1985, 27, 429–436. [Google Scholar] [PubMed]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting Receptor-Mediated Endocytotic Pathways with Nanoparticles: Rationale and Advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frielle, T.; Collins, S.; Daniel, K.W.; Caron, M.G.; Lefkowitz, R.J.; Kobilka, B.K. Cloning of the CDNA for the Human Β1-Adrenergic Receptor. Proc. Natl. Acad. Sci. USA 1987, 84, 7920–7924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, A.D.; McAllister, G.; Feighner, S.D.; Liu, Q.; Nargund, R.P.; Van der Ploeg, L.H.T.; Patchett, A.A. Orphan G-Protein-Coupled Receptors and Natural Ligand Discovery. Trends Pharmacol. Sci. 2001, 22, 132–140. [Google Scholar] [CrossRef]
- Klabunde, T.; Hessler, G. Drug Design Strategies for Targeting G-Protein-Coupled Receptors. ChemBioChem 2002, 3, 928–944. [Google Scholar] [CrossRef]
- Matsuda, L.A. Molecular Aspects of Cannabinoid Receptors. Crit. Rev. Neurobiol. 1997, 11, 143–166. [Google Scholar] [CrossRef]
- Svíženská, I.; Dubový, P.; Šulcová, A. Cannabinoid Receptors 1 and 2 (CB1 and CB2), Their Distribution, Ligands and Functional Involvement in Nervous System Structures—A Short Review. Pharmacol. Biochem. Behav. 2008, 90, 501–511. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762.e14. [Google Scholar] [CrossRef] [Green Version]
- Bramblett, R.D.; Panu, A.M.; Ballesteros, J.A.; Reggio, P.H. Construction of a 3D Model of the Cannabinoid Cb1 Receptor: Determination of Helix Ends and Helix Orientation. Life Sci. 1995, 56, 1971–1982. [Google Scholar] [CrossRef]
- Diaz-Laviada, I.; Ruiz-Llorente, L. Signal Transduction Activated by Cannabinoid Receptors. Mini-Rev. Med. Chem. 2005, 5, 619–630. [Google Scholar] [CrossRef]
- Manglik, A.; Kruse, A.C. Structural Basis for G Protein-Coupled Receptor Activation. Biochemistry 2017, 56, 5628–5634. [Google Scholar] [CrossRef] [PubMed]
- Latorraca, N.R.; Venkatakrishnan, A.J.; Dror, R.O. GPCR Dynamics: Structures in Motion. Chem. Rev. 2017, 117, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.H.; et al. Crystal Structures of Agonist-Bound Human Cannabinoid Receptor CB 1. Nature 2017, 547, 468–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, C.; Zhuang, Y.; Xu, T.H.; Feng, Z.; Zhou, X.E.; Chen, M.; Wang, L.; Meng, X.; Xue, Y.; Wang, J.; et al. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-Gi Signaling Complex. Cell 2020, 180, 645–654.e13. [Google Scholar] [CrossRef]
- Ji, B.; Liu, S.; He, X.; Man, V.H.; Xie, X.Q.; Wang, J. Prediction of the Binding Affinities and Selectivity for CB1 and CB2 Ligands Using Homology Modeling, Molecular Docking, Molecular Dynamics Simulations, and MM-PBSA Binding Free Energy Calculations. ACS Chem. Neurosci. 2020, 11, 1139–1158. [Google Scholar] [CrossRef]
- Chen, X.; Yang, W.; Fan, Y.; Luo, J.; Hong, K.; Wang, Z.; Yan, J.; Chen, X.; Lu, J.; Benovic, J.; et al. Structural Determinants in the Second Intracellular Loop of the Human Cannabinoid CB 1 Receptor Mediate Selective Coupling to G s and G i. Br. J. Pharmacol. 2010, 161, 1817–1834. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Chen, L.; Chen, X.; He, X.; Yang, J.; Shi, Y.; Zhou, N. The Second Intracellular Loop of the Human Cannabinoid CB2 Receptor Governs G Protein Coupling in Coordination with the Carboxyl Terminal Domain. PLoS ONE 2013, 8, e63262. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and Cannabinoid Receptors: The Story so Far. iScience 2020, 23, 101301. [Google Scholar] [CrossRef]
- Westlake, T.M.; Howlett, A.C.; Bonner, T.I.; Matsuda, L.A.; Herkenham, M. Cannabinoid Receptor Binding and Messenger RNA Expression in Human Brain: An in Vitro Receptor Autoradiography and in Situ Hybridization Histochemistry Study of Normal Aged and Alzheimer’s Brains. Neuroscience 1994, 63, 637–652. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; De Costa, B.R.; Rice, K.C. Cannabinoid Receptor Localization in Brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef] [Green Version]
- Scallet, A.C. Neurotoxicology of Cannabis and THC: A Review of Chronic Exposure Studies in Animals. Pharmacol. Biochem. Behav. 1991, 40, 671–676. [Google Scholar] [CrossRef]
- Sañudo-Peña, M.C.; Patrick, S.L.; Khen, S.; Patrick, R.L.; Tsou, K.; Walker, J.M. Cannabinoid Effects in Basal Ganglia in a Rat Model of Parkinson’s Disease. Neurosci. Lett. 1998, 248, 171–174. [Google Scholar] [CrossRef]
- Glass, M.; Faull, R.L.M.; Dragunow, M. Loss of Cannabinoid Receptors in the Substantia Nigra in Huntington’s Disease. Neuroscience 1993, 56, 523–527. [Google Scholar] [CrossRef]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S.; et al. The Endogenous Cennabinoid System Affects Energy Balance via Central Orexigenic Drive and Peripheral Lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Robledo, P.; Berrendero, F.; Ozaita, A.; Maldonado, R. Advances in the Field of Cannabinoid-Opioid Cross-Talk. Addict. Biol. 2008, 13, 213–224. [Google Scholar] [CrossRef]
- Strangman, N.M.; Patrick, S.L.; Hohmann, A.G.; Tsou, K.; Walker, J.M. Evidence for a Role of Endogenous Cannabinoids in the Modulation of Acute and Tonic Pain Sensitivity. Brain Res. 1998, 813, 323–328. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Mackie, K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. Handb. Exp. Pharmacol. 2005, 168, 299–325. [Google Scholar] [CrossRef]
- Wagner, J.A.; Járai, Z.; Bátkai, S.; Kunos, G. Hemodynamic Effects of Cannabinoids: Coronary and Cerebral Vasodilation Mediated by Cannabinoid CB1 Receptors. Eur. J. Pharmacol. 2001, 423, 203–210. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of Cannabinoid CB1 and CB2 Receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef]
- Rodríguez De Fonseca, F.; Del Arco, I.; Martín-Calderón, J.L.; Gorriti, M.A.; Navarro, M. Role of the Endogenous Cannabinoid System in the Regulation of Motor Activity. Neurobiol. Dis. 1998, 5, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Hoehe, M.R.; Caenazzo, L.; Martinez, M.M.; Hsieh, W.T.; Modi, W.S.; Gershon, E.S.; Bonner, T.I. Genetic and Physical Mapping of the Human Cannabinoid Receptor Gene to Chromosome 6q14-Q15. New Biol. 1991, 3, 880–885. [Google Scholar]
- Schacht, J.P.; Hutchison, K.E.; Filbey, F.M. Associations between Cannabinoid Receptor-1 (CNR1) Variation and Hippocampus and Amygdala Volumes in Heavy Cannabis Users. Neuropsychopharmacology 2012, 37, 2368–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, C.A.; Hopfer, C.J.; Haberstick, B.; Rhee, S.H.; Crowley, T.J.; Corley, R.P.; Hewitt, J.K.; Ehringer, M.A. The Association between Cannabinoid Receptor 1 Gene (CNR1) and Cannabis Dependence Symptoms in Adolescents and Young Adults. Drug Alcohol Depend. 2009, 104, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, A.; Lynskey, M.T. Candidate Genes for Cannabis Use Disorders: Findings, Challenges and Directions. Addiction 2009, 104, 518–532. [Google Scholar] [CrossRef] [Green Version]
- Mendizabal-Zubiaga, J.; Melser, S.; Bénard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; de Fonseca, F.R.; et al. Cannabinoid CB1 Receptors Are Localized in Striated Muscle Mitochondria and Regulate Mitochondrial Respiration. Front. Physiol. 2016, 7, 476. [Google Scholar] [CrossRef] [Green Version]
- Farquhar-Smith, W.P.; Egertová, M.; Bradbury, E.J.; McMahon, S.B.; Rice, A.S.C.; Elphick, M.R. Cannabinoid CB1 Receptor Expression in Rat Spinal Cord. Mol. Cell Neurosci. 2000, 15, 510–521. [Google Scholar] [CrossRef]
- Brusco, A.; Tagliaferro, P.; Saez, T.; Onaivi, E.S. Postsynaptic Localization of CB2 Cannabinoid Receptors in the Rat Hippocampus. Synapse 2008, 62, 944–949. [Google Scholar] [CrossRef]
- Brusco, A.; Tagliaferro, P.A.; Saez, T.; Onaivi, E.S. Ultrastructural Localization of Neuronal Brain CB2 Cannabinoid Receptors. Ann. N. Y. Acad. Sci. 2008, 1139, 450–457. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Johnson, M.R.; Melvin, L.S.; De Costa, B.R.; Rice, K.C. Characterization and Localization of Cannabinoid Receptors in Rat Brain: A Quantitative in Vitro Autoradiographic Study. J. Neurosci. 1991, 11, 563–583. [Google Scholar] [CrossRef] [Green Version]
- Freundt-Revilla, J.; Kegler, K.; Baumgärtner, W.; Tipold, A. Spatial Distribution of Cannabinoid Receptor Type 1 (CB1) in Normal Canine Central and Peripheral Nervous System. PLoS ONE 2017, 12, e0181064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raitio, K.; Salo, O.; Nevalainen, T.; Poso, A.; Jarvinen, T. Targeting the Cannabinoid CB2 Receptor: Mutations, Modeling and Development of CB2 Selective Ligands. Curr. Med. Chem. 2005, 12, 1217–1237. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the Presence and Functional Expression of Cannabinoid CB2 Receptors in Brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef] [PubMed]
- Bab, I.; Zimmer, A. Cannabinoid Receptors and the Regulation of Bone Mass. Br. J. Pharmacol. 2008, 153, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M. CB 2 Receptors in Reproduction. Br. J. Pharmacol. 2008, 153, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. Cannabinoid Receptors and Pain. Prog. Neurobiol. 2001, 63, 569–611. [Google Scholar] [CrossRef]
- Ameri, A. The Effects of Cannabinoids on the Brain. Prog. Neurobiol. 1999, 58, 315–348. [Google Scholar] [CrossRef]
- Gong, J.P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 Receptors: Immunohistochemical Localization in Rat Brain. Brain. Res 2006, 1071, 10–23. [Google Scholar] [CrossRef]
- Peters, K.Z.; Cheer, J.F.; Tonini, R. Modulating the Neuromodulators: Dopamine, Serotonin, and the Endocannabinoid System. Trends Neurosci. 2021, 44, 464–477. [Google Scholar] [CrossRef]
- Kaur, I.; Behl, T.; Bungau, S.; Zengin, G.; Kumar, A.; El-Esawi, M.A.; Khullar, G.; Venkatachalam, T.; Arora, S. The Endocannabinoid Signaling Pathway as an Emerging Target in Pharmacotherapy, Earmarking Mitigation of Destructive Events in Rheumatoid Arthritis. Life Sci. 2020, 257, 118109. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Alger, B.E. Endocannabinoids at the Synapse a Decade after the Dies Mirabilis (29 March 2001): What We Still Do Not Know. J. Physiol. 2012, 590, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Mukhopadhyay, S. Cellular Signal Transduction by Anandamide and 2-Arachidonoylglycerol. Chem. Phys. Lipids 2000, 108, 53–70. [Google Scholar] [CrossRef]
- Condie, R.; Herring, A.; Koh, W.S.; Lee, M.; Kaminski, N.E. Cannabinoid Inhibition of Adenylate Cyclase-Mediated Signal Transduction and Interleukin 2 (IL-2) Expression in the Murine T-Cell Line, EL4.IL-2. J. Biol. Chem. 1996, 271, 13175–13183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunton, L.; Parker, K.; Blumenthal, D.; Buxton, I. Goodman & Gilman’s: Manual of Pharmacology and Therapeutics; McGraw-Hill Companies: New York, NY, USA, 2008; Volume 95. [Google Scholar]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned CDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Walsh, K.B.; Andersen, H.K. Molecular Pharmacology of Synthetic Cannabinoids: Delineating Cb1 Receptor-Mediated Cell Signaling. Int. J. Mol. Sci. 2020, 21, 6115. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. Endocannabinoids and Their Pharmacological Actions. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2015; Volume 231, pp. 1–37. [Google Scholar]
- Wang, J.; Ueda, N. Biology of Endocannabinoid Synthesis System. Prostaglandins Other Lipid Mediat. 2009, 89, 112–119. [Google Scholar] [CrossRef]
- Ren, S.Y.; Wang, Z.-z.; Zhang, Y.; Chen, N. hong Potential Application of Endocannabinoid System Agents in Neuropsychiatric and Neurodegenerative Diseases—Focusing on FAAH/MAGL Inhibitors. Acta Pharmacol. Sin. 2020, 41, 1263–1271. [Google Scholar] [CrossRef]
- Zhu, D.; Gao, F.; Chen, C. Endocannabinoid Metabolism and Traumatic Brain Injury. Cells 2021, 10, 2979. [Google Scholar] [CrossRef]
- Zelasko, S.; Arnold, W.R.; Das, A. Endocannabinoid Metabolism by Cytochrome P450 Monooxygenases. Prostaglandins Other Lipid Mediat 2015, 116–117, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Endocannabinoids: Synthesis and Degradation. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–24. [Google Scholar] [CrossRef]
- Nikan, M.; Nabavi, S.M.; Manayi, A. Ligands for Cannabinoid Receptors, Promising Anticancer Agents. Life Sci. 2016, 146, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef] [PubMed]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Adams, R.; Hunt, M. Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Riquelme-Sandoval, A.; de Sá-Ferreira, C.O.; Miyakoshi, L.M.; Hedin-Pereira, C. New Insights into Peptide Cannabinoids: Structure, Biosynthesis and Signaling. Front. Pharmacol. 2020, 11, 2. [Google Scholar] [CrossRef]
- Kinghorn, A.; Falk; Gibbons, S.; Kobayashi, J.I. Phytocannabinoids; Springer International: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Vago, R.; Fiorio, F.; Trevisani, F.; Salonia, A.; Montorsi, F.; Bettiga, A. The Mediterranean Diet as a Source of Bioactive Molecules with Cannabinomimetic Activity in Prevention and Therapy Strategy. Nutrients 2022, 14, 468. [Google Scholar] [CrossRef]
- Maffei, M.E. Plant Natural Sources of the Endocannabinoid (E)-β-Caryophyllene: A Systematic Quantitative Analysis of Published Literature. Int. J. Mol. Sci. 2020, 21, 6540. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.L.; Thakur, G.A.; Makriyannis, A. Cannabinergic Ligands. Chem. Phys. Lipids 2002, 121, 3–19. [Google Scholar] [CrossRef]
- Makriyannis, A.; Hongfeng, D. Cannabimimetic Indole Derivatives. US Patent WO2001028557B1; 1999–2001, 7 June 2001. [Google Scholar]
- Robinson, L.; Goonawardena, A.V.; Pertwee, R.G.; Hampson, R.E.; Riedel, G. The Synthetic Cannabinoid HU210 Induces Spatial Memory Deficits and Suppresses Hippocampal Firing Rate in Rats. Br. J. Pharmacol. 2007, 151, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.W.; Mabon, R.; Wu, M.J.; Lu, J.; Hart, R.; Hurst, D.P.; Reggio, P.H.; Wiley, J.L.; Martin, B.R. 3-Indolyl-1-Naphthylmethanes: New Cannabimimetic Indoles Provide Evidence for Aromatic Stacking Interactions with the CB1 Cannabinoid Receptor. Bioorg. Med. Chem. 2002, 11, 539–549. [Google Scholar] [CrossRef]
- Huffman, J.W.; Szklennik, P.V.; Almond, A.; Bushell, K.; Selley, D.E.; He, H.; Cassidy, M.P.; Wiley, J.L.; Martin, B.R. 1-Pentyl-3-Phenylacetylindoles, a New Class of Cannabimimetic Indoles. Bioorg. Med. Chem. Lett. 2005, 15, 4110–4113. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, L.; Hua, T.; Liu, Z.J. Structural and Functional Insights into Cannabinoid Receptors. Trends Pharmacol. Sci. 2020, 41, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Padgett, L.W. Recent Developments in Cannabinoid Ligands. Life Sci. 2005, 77, 1767–1798. [Google Scholar] [CrossRef]
- Alaverdashvili, M.; Laprairie, R.B. The Future of Type 1 Cannabinoid Receptor Allosteric Ligands. Drug Metab. Rev. 2018, 50, 14–25. [Google Scholar] [CrossRef]
- Dopart, R.; Lu, D.; Lichtman, A.H.; Kendall, D.A. Allosteric Modulators of Cannabinoid Receptor 1: Developing Compounds for Improved Specificity. Drug Metab. Rev. 2018, 50, 3–13. [Google Scholar] [CrossRef]
- Hryhorowicz, S.; Kaczmarek-Ryś, M.; Andrzejewska, A.; Staszak, K.; Hryhorowicz, M.; Korcz, A.; Słomski, R. Allosteric Modulation of Cannabinoid Receptor 1—Current Challenges and Future Opportunities. Int. J. Mol. Sci. 2019, 20, 23. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Goya, P.; Jagerovic, N.; Hernandez-Folgado, L. Allosteric Modulators of the CB1 Cannabinoid Receptor: A Structural Update Review. Cannabis Cannabinoid Res. 2016, 1, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Khurana, L.; Fu, B.Q.; Duddupudi, A.L.; Liao, Y.H.; Immadi, S.S.; Kendall, D.A.; Lu, D. Pyrimidinyl Biphenylureas: Identification of New Lead Compounds as Allosteric Modulators of the Cannabinoid Receptor CB1. J. Med. Chem. 2017, 60, 1089–1104. [Google Scholar] [CrossRef] [Green Version]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P. The Concept of Allosteric Modulation: An Overview. Drug Discov. Today Technol. 2013, 10, e223–e228. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.R.; Sexton, P.M.; Christopoulos, A. Bridging the Gap: Bitopic Ligands of G-Protein-Coupled Receptors. Trends Pharmacol. Sci. 2013, 34, 59–66. [Google Scholar] [CrossRef]
- May, L.T.; Leach, K.; Sexton, P.M.; Christopoulos, A. Allosteric Modulation of G Protein-Coupled Receptors. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 1–51. [Google Scholar] [CrossRef]
- Pamplona, F.A.; Ferreira, J.; De Lima, O.M.; Duarte, F.S.; Bento, A.F.; Forner, S.; Villarinho, J.G.; Bellochio, L.; Wotjak, C.T.; Lerner, R.; et al. Anti-Inflammatory Lipoxin A4 Is an Endogenous Allosteric Enhancer of CB1 Cannabinoid Receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 21134–21139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polini, B.; Cervetto, C.; Carpi, S.; Pelassa, S.; Gado, F.; Ferrisi, R.; Bertini, S.; Nieri, P.; Marcoli, M.; Manera, C. Positive Allosteric Modulation of CB1 and CB2 Cannabinoid Receptors Enhances the Neuroprotective Activity of a Dual CB1R/CB2R Orthosteric Agonist. Life 2020, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Kulkarni, P.M.; Deschamps, J.R.; Kelly, M.E.M.; Janero, D.R.; Cascio, M.G.; Stevenson, L.A.; Pertwee, R.G.; Kenakin, T.P.; Denovan-Wright, E.M.; et al. Enantiospecific Allosteric Modulation of Cannabinoid 1 Receptor. ACS Chem. Neurosci. 2017, 8, 1188–1203. [Google Scholar] [CrossRef]
- Gado, F.; Di Cesare Mannelli, L.; Lucarini, E.; Bertini, S.; Cappelli, E.; Digiacomo, M.; Stevenson, L.A.; Macchia, M.; Tuccinardi, T.; Ghelardini, C.; et al. Identification of the First Synthetic Allosteric Modulator of the CB2 Receptors and Evidence of Its Efficacy for Neuropathic Pain Relief. J. Med. Chem. 2019, 62, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Yan, W.; Chapman, K.; Ramesh, K.; Ferrell, A.J.; Yin, J.; Wang, X.; Xu, Q.; Rosenbaum, D.M. Structure of an Allosteric Modulator Bound to the CB1 Cannabinoid Receptor. Nat. Chem. Biol. 2019, 15, 1199–1205. [Google Scholar] [CrossRef]
- Fowler, C.J. The Endocannabinoid System—Current Implications for Drug Development. J. Intern. Med. 2021, 290, 2–26. [Google Scholar] [CrossRef]
- Ford, B.M.; Franks, L.N.; Tai, S.; Fantegrossi, W.E.; Stahl, E.L.; Berquist, M.D.; Cabanlong, C.V.; Wilson, C.D.; Penthala, N.R.; Crooks, P.A.; et al. Characterization of Structurally Novel G Protein Biased CB1 Agonists: Implications for Drug Development. Pharmacol. Res. 2017, 125, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Daigle, T.L.; Kwok, M.L.; Mackie, K. Regulation of CB1 Cannabinoid Receptor Internalization by a Promiscuous Phosphorylation-Dependent Mechanism. J. Neurochem. 2008, 106, 70–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Dhopeshwarkar, A.S.; Huibregtse, M.; MacKie, K.; Hohmann, A.G. Slowly Signaling G Protein-Biased CB2 Cannabinoid Receptor Agonist LY2828360 Suppresses Neuropathic Pain with Sustained Efficacy and Attenuates Morphine Tolerance and Dependence. Mol. Pharmacol. 2018, 93, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Kalliomäki, J.; Segerdahl, M.; Webster, L.; Reimfelt, A.; Huizar, K.; Annas, P.; Karlsten, R.; Quiding, H. Evaluation of the Analgesic Efficacy of AZD1940, a Novel Cannabinoid Agonist, on Post-Operative Pain after Lower Third Molar Surgical Removal. Scand. J. Pain 2013, 4, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, J.; Annas, P.; Huizar, K.; Clarke, C.; Zettergren, A.; Karlsten, R.; Segerdahl, M. Evaluation of the Analgesic Efficacy and Psychoactive Effects of AZD1940, a Novel Peripherally Acting Cannabinoid Agonist, in Human Capsaicin-Induced Pain and Hyperalgesia. Clin. Exp. Pharmacol. Physiol. 2013, 40, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Cao, C.Q.; Martino, G.; Puma, C.; Morinville, A.; St-Onge, S.; Lessard, É.; Perkins, M.N.; Laird, J.M.A. A Peripherally Restricted Cannabinoid Receptor Agonist Produces Robust Anti-Nociceptive Effects in Rodent Models of Inflammatory and Neuropathic Pain. Pain 2010, 151, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The Endocannabinoid System and Its Therapeutic Exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Servier Medical Art Anatomy and The Human Body. Available online: https://smart.servier.com/smart_image/osteoporosis-7/ (accessed on 22 February 2022).
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the Endocannabinoid System: Future Therapeutic Strategies. Drug Discov. Today 2017, 22, 105–110. [Google Scholar] [CrossRef]
- Di Marzo, V. The Endocannabinoid System: Its General Strategy of Action, Tools for Its Pharmacological Manipulation and Potential Therapeutic Exploitation. Pharmacol. Res. 2009, 60, 77–84. [Google Scholar] [CrossRef]
- Pertwee, R.G. The Therapeutic Potential of Drugs That Target Cannabinoid Receptors or Modulate the Tissue Levels or Actions of Endocannabinoids. In Drug Addiction: From Basic Research to Therapy; Springer: New York, NY, USA, 2008; pp. 637–686. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Rusconi, F.; Rubino, T.; Battaglioli, E. Endocannabinoid-Epigenetic Cross-Talk: A Bridge toward Stress Coping. Int. J. Mol. Sci. 2020, 21, 6252. [Google Scholar] [CrossRef] [PubMed]
- Mouslech, Z.; Valla, V. Endocannabinoid System: An Overview of Its Potential in Current Medical Practice. Neuroendocrinol. Lett. 2009, 30, 153–179. [Google Scholar] [PubMed]
- Pertwee, R.G. Elevating Endocannabinoid Levels: Pharmacological Strategies and Potential Therapeutic Applications. Proc. Nutr. Soc. 2014, 73, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic Activity of Cannabinoids. J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the Use of Cannabinoids as Antitumour Agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.; Sánchez, C.; Galve-Roperh, I. Control of the Cell Survival/Death Decision by Cannabinoids. J. Mol. Med. 2001, 78, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; Romero, J.; Velasco, G.; Tolón, R.M.; Ramos, J.A.; Guzmán, M. Cannabinoid CB2 Receptor: A New Target for Controlling Neural Cell Survival? Trends Pharmacol. Sci. 2007, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Farrimond, J.A.; Mercier, M.S.; Whalley, B.J.; Williams, C.M. Cannabis Sativa and the Endogenous Cannabinoid System: Therapeutic Potential for Appetite Regulation. Phytother. Res. 2011, 25, 170–188. [Google Scholar] [CrossRef] [Green Version]
- Kola, B.; Farkas, I.; Christ-Crain, M.; Wittmann, G.; Lolli, F.; Amin, F.; Harvey-White, J.; Liposits, Z.; Kunos, G.; Grossman, A.B.; et al. The Orexigenic Effect of Ghrelin Is Mediated through Central Activation of the Endogenous Cannabinoid System. PLoS ONE 2008, 3, e1797. [Google Scholar] [CrossRef]
- Cecconi, S.; Rossi, G.; Oddi, S.; Di Nisio, V.; Maccarrone, M. Role of Major Endocannabinoid-Binding Receptors during Mouse Oocyte Maturation. Int. J. Mol. Sci. 2019, 20, 2866. [Google Scholar] [CrossRef]
- Walker, O.L.S.; Holloway, A.C.; Raha, S. The Role of the Endocannabinoid System in Female Reproductive Tissues. J. Ovarian Res. 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Beatka, M.; Sarvaideo, J. Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr. Physiol. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bovolin, P.; Cottone, E.; Pomatto, V.; Fasano, S.; Pierantoni, R.; Cobellis, G.; Meccariello, R. Endocannabinoids Are Involved in Male Vertebrate Reproduction: Regulatory Mechanisms at Central and Gonadal Level. Front. Endocrinol. 2014, 5, 54. [Google Scholar] [CrossRef]
- Meccariello, R.; Battista, N.; Bradshaw, H.B.; Wang, H. Updates in Reproduction Coming from the Endocannabinoid System. Int. J. Endocrinol. 2014, 2014, 412354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raux, P.; Vallée, M. Cross-talk between Neurosteroid and Endocannabinoid Systems in Cannabis Addiction. J. Neuroendocrinol. 2022, e13191. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, B.M.; Costa, M.A.; Almada, M.; Correia-Da-Silva, G.; Teixeira, N.A. Endogenous Cannabinoids Revisited: A Biochemistry Perspective. Prostaglandins Other Lipid Mediat. 2013, 102–103, 13–30. [Google Scholar] [CrossRef]
- Lambert, D.M.; Di Marzo, V. The Palmitoylethanolamide and Oleamide Enigmas: Are These Two Fatty Acid Amides Cannabimimetic? Curr. Med. Chem. 2022, 6, 757–773. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An Entourage Effect: Inactive Endogenous Fatty Acid Glycerol Esters Enhance 2-Arachidonoyl-Glycerol Cannabinoid Activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Piscitelli, F.; Di Marzo, V. “Redundancy” of Endocannabinoid Inactivation: New Challenges and Opportunities for Pain Control. ACS Chem. Neurosci. 2012, 3, 356–363. [Google Scholar] [CrossRef]
- Tripathi, R.K.P. A Perspective Review on Fatty Acid Amide Hydrolase (FAAH) Inhibitors as Potential Therapeutic Agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef] [PubMed]
- Granchi, C.; Caligiuri, I.; Minutolo, F.; Rizzolio, F.; Tuccinardi, T. A Patent Review of Monoacylglycerol Lipase (MAGL) Inhibitors (2013–2017). Expert Opin. Ther. Pat. 2017, 27, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Kaczocha, M.; Haj-Dahmane, S. Mechanisms of Endocannabinoid Transport in the Brain. Br. J. Pharmacol. 2022, 179, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Kaczocha, M.; Vivieca, S.; Sun, J.; Glaser, S.T.; Deutsch, D.G. Fatty Acid-Binding Proteins Transport N-Acylethanolamines to Nuclear Receptors and Are Targets of Endocannabinoid Transport Inhibitors. J. Biol. Chem. 2012, 287, 3415–3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsicano, G.; Chaouloff, F. Moving Bliss: A New Anandamide Transporter. Nat. Neurosci. 2012, 15, 5–6. [Google Scholar] [CrossRef]
- Nicolussi, S.; Gertsch, J. Endocannabinoid Transport Revisited. Vitam. Horm. 2015, 98, 441–485. [Google Scholar] [CrossRef]
- Hillard, C.J.; Huang, H.; Vogt, C.D.; Rodrigues, B.E.; Neumann, T.S.; Sem, D.S.; Schroeder, F.; Cunningham, C.W. Endocannabinoid Transport Proteins: Discovery of Tools to Study Sterol Carrier Protein-2. Methods Enzymol. 2017, 593, 99–121. [Google Scholar] [CrossRef]
- Meccariello, R.; Santoro, A.; D’Angelo, S.; Morrone, R.; Fasano, S.; Viggiano, A.; Pierantoni, R. The Epigenetics of the Endocannabinoid System. Int. J. Mol. Sci. 2020, 21, 1113. [Google Scholar] [CrossRef]
- Pucci, M.; Micioni Di Bonaventura, M.V.; Zaplatic, E.; Bellia, F.; Maccarrone, M.; Cifani, C.; D’Addario, C. Transcriptional Regulation of the Endocannabinoid System in a Rat Model of Binge-Eating Behavior Reveals a Selective Modulation of the Hypothalamic Fatty Acid Amide Hydrolase Gene. Int. J. Eat. Disord. 2019, 52, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Subbanna, S.; Nagre, N.N.; Umapathy, N.S.; Pace, B.S.; Basavarajappa, B.S. Ethanol Exposure Induces Neonatal Neurodegeneration by Enhancing CB1R Exon1 Histone H4K8 Acetylation and Up-Regulating CB1R Function Causing Neurobehavioral Abnormalities in Adult Mice. Int. J. Neuropsychopharmacol. 2015, 18, pyu028. [Google Scholar] [CrossRef] [Green Version]
- Proto, M.C.; Gazzerro, P.; Di Croce, L.; Santoro, A.; Malfitano, A.M.; Pisanti, S.; Laezza, C.; Bifulco, M. Interaction of Endocannabinoid System and Steroid Hormones in the Control of Colon Cancer Cell Growth. J. Cell Physiol. 2012, 227, 250–258. [Google Scholar] [CrossRef] [PubMed]
- D’Addario, C.; Di Francesco, A.; Arosio, B.; Gussago, C.; Dell’Osso, B.; Bari, M.; Galimberti, D.; Scarpini, E.; Altamura, A.C.; Mari, D.; et al. Epigenetic Regulation of Fatty Acid Amide Hydrolase in Alzheimer Disease. PLoS ONE 2012, 7, e39186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etcheverry, A.; Aubry, M.; de Tayrac, M.; Vauleon, E.; Boniface, R.; Guenot, F.; Saikali, S.; Hamlat, A.; Riffaud, L.; Menei, P.; et al. DNA Methylation in Glioblastoma: Impact on Gene Expression and Clinical Outcome. BMC Genomics 2010, 11, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasenoehrl, C.; Feuersinger, D.; Sturm, E.M.; Bärnthaler, T.; Heitzer, E.; Graf, R.; Grill, M.; Pichler, M.; Beck, S.; Butcher, L.; et al. G Protein-Coupled Receptor GPR55 Promotes Colorectal Cancer and Has Opposing Effects to Cannabinoid Receptor 1. Int. J. Cancer 2018, 142, 121–132. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Ehrlich, S.; Walton, E.; White, T.; Perrone-Bizzozero, N.; Bustillo, J.; Turner, J.A.; Calhoun, V.D. Methylation Patterns in Whole Blood Correlate with Symptoms in Schizophrenia Patients. Schizophr. Bull. 2014, 40, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Schrott, R.; Acharya, K.; Itchon-Ramos, N.; Hawkey, A.B.; Pippen, E.; Mitchell, J.T.; Kollins, S.H.; Levin, E.D.; Murphy, S.K. Cannabis Use Is Associated with Potentially Heritable Widespread Changes in Autism Candidate Gene DLGAP2 DNA Methylation in Sperm. Epigenetics 2020, 15, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Aguado, T.; Carracedo, A.; Julien, B.; Velasco, G.; Milman, G.; Mechoulamluis, R.; Alvarez, L.; Guzmán, M.; Galve-Roperh, I. Cannabinoids Induce Glioma Stem-like Cell Differentiation and Inhibit Gliomagenesis. J. Biol. Chem. 2007, 282, 6854–6862. [Google Scholar] [CrossRef] [Green Version]
- Večeřa, J.; Bártová, E.; Krejčí, J.; Legartová, S.; Komůrková, D.; Rudá-Kučerová, J.; Štark, T.; Dražanová, E.; Kašpárek, T.; Šulcová, A.; et al. HDAC1 and HDAC3 Underlie Dynamic H3K9 Acetylation during Embryonic Neurogenesis and in Schizophrenia-like Animals. J. Cell. Physiol. 2018, 233, 530–548. [Google Scholar] [CrossRef]
- Mukherjee, S.; Adams, M.; Whiteaker, K.; Daza, A.; Kage, K.; Cassar, S.; Meyer, M.; Yao, B.B. Species Comparison and Pharmacological Characterization of Rat and Human CB 2 Cannabinoid Receptors. Eur. J. Pharmacol. 2004, 505, 1–9. [Google Scholar] [CrossRef]
- Straiker, A.; Wager-Miller, J.; Hutchens, J.; Mackie, K. Differential Signalling in Human Cannabinoid CB 1 Receptors and Their Splice Variants in Autaptic Hippocampal Neurones. Br. J. Pharmacol. 2012, 165, 2660–2671. [Google Scholar] [CrossRef]
- Bingham, B.; Jones, P.G.; Uveges, A.J.; Kotnis, S.; Lu, P.; Smith, V.A.; Sun, S.C.; Resnick, L.; Chlenov, M.; He, Y.; et al. Species-Specific in Vitro Pharmacological Effects of the Cannabinoid Receptor 2 (CB2) Selective Ligand AM1241 and Its Resolved Enantiomers. Br. J. Pharmacol. 2007, 151, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Mallet, C.; Dubray, C.; Dualé, C. FAAH Inhibitors in the Limelight, but Regrettably. Int. J. Clin. Pharmacol. Ther. 2016, 54, 498–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, R.; Sidhu, P.; Singh, S. What Failed BIA 10-2474 Phase I Clinical Trial? Global Speculations and Recommendations for Future Phase I Trials. J. Pharmacol. Pharmacother. 2016, 7, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Kaczocha, M.; Glaser, S.T.; Deutsch, D.G. Identification of Intracellular Carriers for the Endocannabinoid Anandamide. Proc. Natl. Acad. Sci. USA 2009, 106, 6375–6380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukanowska, M.; Howl, J.; Jones, S. Bioportides: Bioactive Cell-Penetrating Peptides That Modulate Cellular Dynamics. Biotechnol. J. 2013, 8, 918–930. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Denovan-Wright, E.M. Cannabinoid Receptor Ligand Bias: Implications in the Central Nervous System. Curr. Opin. Pharmacol. 2017, 32, 32–43. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Howlett, A.C. Chemically Distinct Ligands Promote Differential CB1 Cannabinoid Receptor-Gi Protein Interactions. Mol. Pharmacol. 2005, 67, 2016–2024. [Google Scholar] [CrossRef]
- Bonhaus, D.W.; Chang, L.K.; Kwan, J.; Martin, G.R. Dual Activation and Inhibition of Adenylyl Cyclase by Cannabinoid Receptor Agonists: Evidence for Agonist-Specific Trafficking of Intracellular Responses. J. Pharmacol. Exp. Ther. 1998, 287, 884–888. [Google Scholar]
- Polit, A.; Mystek, P.; Błasiak, E. Every Detail Matters. That Is, How the Interaction between Gα Proteins and Membrane Affects Their Function. Membranes 2021, 11, 222. [Google Scholar] [CrossRef]
- Nobles, K.N.; Xiao, K.; Ahn, S.; Shukla, A.K.; Lam, C.M.; Rajagopal, S.; Strachan, R.T.; Huang, T.Y.; Bressler, E.A.; Hara, M.R.; et al. Distinct Phosphorylation Sites on the β 2-Adrenergic Receptor Establish a Barcode That Encodes Differential Functions of β-Arrestin. Sci. Signal. 2011, 4, ra51. [Google Scholar] [CrossRef] [Green Version]
- Liggett, S.B. Phosphorylation Barcoding as a Mechanism of Directing GPCR Signaling. Sci. Signal. 2011, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Nogueras-Ortiz, C.; Yudowski, G.A. The Multiple Waves of Cannabinoid 1 Receptor Signaling. Mol. Pharmacol. 2016, 90, 620–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciaramella, V.; Meccariello, R.; Chioccarelli, T.; Sirleto, M.; Fasano, S.; Pierantoni, R.; Chianese, R. Anandamide Acts via Kisspeptin in the Regulation of Testicular Activity of the Frog, Pelophylax Esculentus. Mol. Cell. Endocrinol. 2016, 420, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-K.; Hou, Z.-S.; Tao, Y.-X. Biased Signaling in Naturally Occurring Mutations of G Protein-Coupled Receptors Associated with Diverse Human Diseases. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 165973. [Google Scholar] [CrossRef]
- Ahow, M.; Min, L.; Pampillo, M.; Nash, C.; Wen, J.; Soltis, K.; Carroll, R.S.; Glidewell-Kenney, C.A.; Mellon, P.L.; Bhattacharya, M.; et al. KISS1R Signals Independently of Gαq/11 and Triggers LH Secretion via the β-Arrestin Pathway in the Male Mouse. Endocrinology 2014, 155, 4433–4446. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; Fasano, S.; Pierantoni, R. Kisspeptins, New Local Modulators of Male Reproduction: A Comparative Overview. Gen. Comp. Endocrinol. 2020, 299, 113618. [Google Scholar] [CrossRef]
- Meccariello, R.; Chianese, R.; Fasano, S.; Pierantoni, R. Endocannabinoids and Kisspeptins: Two Modulators in Fight for the Regulation of GnRH Activity. In Gonadotropin; IntechOpen: London, UK, 2013; pp. 57–88. [Google Scholar] [CrossRef] [Green Version]
- Chianese, R.; Cobellis, G.; Chioccarelli, T.; Ciaramella, V.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptins, Estrogens and Male Fertility. Curr. Med. Chem. 2016, 23, 4070–4091. [Google Scholar] [CrossRef]
- Santoro, A.; Mele, E.; Marino, M.; Viggiano, A.; Nori, S.L.; Meccariello, R. The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. Int. J. Mol. Sci. 2021, 22, 972. [Google Scholar] [CrossRef]
- Wilheim, T.; Nagy, K.; Mohanraj, M.; Ziarniak, K.; Watanabe, M.; Sliwowska, J.; Kalló, I. Expression of Type One Cannabinoid Receptor in Different Subpopulation of Kisspeptin Neurons and Kisspeptin Afferents to GnRH Neurons in Female Mice. Brain Struct. Funct. 2021, 226, 2387–2399. [Google Scholar] [CrossRef]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. In Recent Advances in Cannabinoid Physiology and Pathology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1162, pp. 1–12. [Google Scholar] [CrossRef]
- Manglik, A.; Kobilka, B.K.; Steyaert, J. Nanobodies to Study G Protein-Coupled Receptor Structure and Function. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Tikhonova, I.; Costanzi, S. Unraveling the Structure and Function of G Protein-Coupled Receptors Through NMR Spectroscopy. Curr. Pharm. Des. 2009, 15, 4003–4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacker, D.; Stevens, R.C.; Roth, B.L. How Ligands Illuminate GPCR Molecular Pharmacology. Cell 2017, 170, 414–427. [Google Scholar] [CrossRef] [Green Version]
- Adcock, S.A.; McCammon, J.A. Molecular Dynamics: Survey of Methods for Simulating the Activity of Proteins. Chem. Rev. 2006, 106, 1589–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Yang, H.; Zhou, Y.F.; Hu, B. Dual and Multi-Targeted Nanoparticles for Site-Specific Brain Drug Delivery. J. Control. Release 2020, 317, 195–215. [Google Scholar] [CrossRef]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-Based Systems for Targeted and Site Specific Therapeutic Delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef]
- Mishra, B.; Patel, R.R. Nanotheranostics: Implications of Nanotechnology in Simultaneous Diagnosis and Therapy. In Nanobiotechnology; CRC Press: Boca Raton, FL, USA, 2018; pp. 35–68. [Google Scholar] [CrossRef]
- Li, W.; Wei, H.; Li, H.; Gao, J.; Feng, S.S.; Guo, Y. Cancer Nanoimmunotherapy Using Advanced Pharmaceutical Nanotechnology. Nanomedicine 2014, 9, 2587–2605. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat Nanotechnol 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Mishra, V.; Thakur, S.; Riyaz, B.; Kaur, A.; Khursheed, R.; Patil, K.; Sathe, B. Nanotechnology Derived Nanotools in Biomedical Perspectives: An Update. Curr. Nanosci. 2018, 15, 137–146. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liu, X.; Pan, W.; Li, N.; Tang, B. Designing and Engineering of Nanocarriers for Bioapplication in Cancer Immunotherapy. ACS Appl. Bio. Mater. 2020, 3, 8321–8337. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, Á.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the Surface Modification, Size, and Shape on Cellular Uptake of Nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.J. Nanomedicines for Overcoming Biological Barriers. Biomed. Pharmacother. 2004, 58, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Moros, M.; Mitchell, S.; Grazu, V.; de Fuente, J.M. The Fate of Nanocarriers as Nanomedicines In Vivo: Important Considerations and Biological Barriers to Overcome. Curr. Med. Chem. 2013, 20, 2759–2778. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gou, K.; Guo, X.; Ke, J.; Li, S.; Li, H. Advances in Regulating Physicochemical Properties of Mesoporous Silica Nanocarriers to Overcome Biological Barriers. Acta Biomater. 2021, 123, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Ma, C.; Li, C.; Wang, T.; Tang, Y.; Wang, H.; Mou, X.; Chen, Z.; He, N. Influence of Nanoparticle Shape, Size, and Surface Functionalization on Cellular Uptake. J. Nanosci. Nanotechnol. 2013, 13, 6485–6498. [Google Scholar] [CrossRef]
- Rabanel, J.M.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-Loaded Nanocarriers: Passive Targeting and Crossing of Biological Barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef]
- Wilson, D.B. Cellular Transport Mechanisms. Annu. Rev. Biochem. 1978, 47, 933–965. [Google Scholar] [CrossRef]
- Devarajan, P.V.; Dandekar, P.; D’souza, A.A. Targeted Intracellular Drug Delivery by Receptor Mediated Endocytosis; AAPS Advances in the Pharmaceutical Sciences Series; Springer: Berlin/Heidelberg, Germany, 2019; Volume 39. [Google Scholar]
- Vyas, S.P.; Singh, A.; Sihorkar, V. Ligand-Receptor-Mediated Drug Delivery: An Emerging Paradigm in Cellular Drug Targeting. Crit. Rev. Ther. Drug Carrier. Syst. 2001, 18, 1–76. [Google Scholar] [CrossRef]
- Wang, S.; Meng, Y.; Li, C.; Qian, M.; Huang, R. Receptor-Mediated Drug Delivery Systems Targeting to Glioma. Nanomaterials 2015, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent Progress in Drug Delivery. Acta. Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [Green Version]
- Galliford CV, L.P. Receptor-Mediated Drug Delivery. In Drug Delivery: Principles and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 451, pp. 451–474. ISBN 1118833236. [Google Scholar]
- Balogh, L.P. Nano-Enabled Medical Applications; Jenny Stanford Publishing: Dubai, UAE, 2020; ISBN 9780429399039. [Google Scholar]
- Shen, Z.; Nieh, M.P.; Li, Y. Decorating Nanoparticle Surface for Targeted Drug Delivery: Opportunities and Challenges. Polymers 2016, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Balmert, S.C.; Little, S.R. Biomimetic Delivery with Micro- and Nanoparticles. Adv. Mater. 2012, 24, 3757–3778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano. 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; He, Q. The Interaction of Nanoparticles with Plasma Proteins and the Consequent Influence on Nanoparticles Behavior. Expert. Opin. Drug Deliv. 2014, 11, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Panyam, J.; Dali, M.M.; Sahoo, S.K.; Ma, W.; Chakravarthi, S.S.; Amidon, G.L.; Levy, R.J.; Labhasetwar, V. Polymer Degradation and in Vitro Release of a Model Protein from Poly(D,L-Lactide-Co-Glycolide) Nano- and Microparticles. J. Control Release 2003, 92, 173–187. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Gao, Y.; Shi, J.; Li, Y. Intracellular Localization and Cytotoxicity of Spherical Mesoporous Silica Nano-and Microparticles. Small 2009, 5, 2722–2729. [Google Scholar] [CrossRef]
- Choi, J.; Zhang, Q.; Reipa, V.; Wang, N.S.; Stratmeyer, M.E.; Hitchins, V.M.; Goering, P.L. Comparison of Cytotoxic and Inflammatory Responses of Photoluminescent Silicon Nanoparticles with Silicon Micron-Sized Particles in RAW 264.7 Macrophages. J. Appl. Toxicol. 2009, 29, 52–60. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Au, L.; Zhang, Q.; Xia, Y. The Effects of Size, Shape, and Surface Functional Group of Gold Nanostructures on Their Adsorption and Internalization by Cells. Small 2010, 6, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and Surface Characteristics of Gold Nanoparticles Influence Their Biodistribution and Uptake by Macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millstone, J.E.; Hurst, S.J.; Métraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal Gold and Silver Triangular Nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, A.; Novikov, S.M.; Solís, D.M.; Taboada, J.M.; Obelleiro, F.; Liz-Marzán, L.M. Plasmon Modes and Hot Spots in Gold Nanostar-Satellite Clusters. J. Phys. Chem. C 2015, 119, 10836–10843. [Google Scholar] [CrossRef] [Green Version]
- Sau, T.K.; Murphy, C.J. Room Temperature, High-Yield Synthesis of Multiple Shapes of Gold Nanoparticles in Aqueous Solution. J. Am. Chem. Soc. 2004, 126, 8648–8649. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Heo, J.; Kim, M.; Lee, Y.W.; Han, S.W. Size-Controlled Synthesis of Monodisperse Gold Nanooctahedrons and Their Surface-Enhanced Raman Scattering Properties. Chem. Phys. Lett. 2009, 468, 245–248. [Google Scholar] [CrossRef]
- Millstone, J.E.; Park, S.; Shuford, K.L.; Qin, L.; Schatz, G.C.; Mirkin, C.A. Observation of a Quadrupole Plasmon Mode for a Colloidal Solution of Gold Nanoprisms. J. Am. Chem. Soc. 2005, 127, 5312–5313. [Google Scholar] [CrossRef]
- Agarwal, R.; Singh, V.; Jurney, P.; Shi, L.; Sreenivasan, S.V.; Roy, K. Mammalian Cells Preferentially Internalize Hydrogel Nanodiscs over Nanorods and Use Shape-Specific Uptake Mechanisms. Proc. Natl. Acad. Sci. USA 2013, 110, 17247–17252. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, S.; Auth, T.; Gompper, G. Shape and Orientation Matter for the Cellular Uptake of Nonspherical Particles. Nano Lett. 2014, 14, 687–693. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping Cancer Nanomedicine: The Effect of Particle Shape on the in Vivo Journey of Nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of Shape on Cellular Uptake of Gold Nanoparticles in the Forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, K.; Fedosov, D.A.; Gompper, G. Margination of Micro- and Nano-Particles in Blood Flow and Its Effect on Drug Delivery. Sci. Rep. 2014, 4, 4871. [Google Scholar] [CrossRef] [Green Version]
- Jurney, P.; Agarwal, R.; Singh, V.; Roy, K.; Sreenivasan, S.V.; Shi, L. Size-Dependent Nanoparticle Margination and Adhesion Propensity in a Microchannel. J. Nanotechnol. Eng. Med. 2013, 4, 31002. [Google Scholar] [CrossRef]
- Yi, X.; Gao, H. Kinetics of Receptor-Mediated Endocytosis of Elastic Nanoparticles. Nanoscale 2017, 9, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Dagastine, R.R.; Manica, R.; Carnie, S.L.; Chan, D.Y.C.; Stevens, G.W.; Grieser, F. Dynamic Forces between Two Deformable Oil Droplets in Water. Science 2006, 313, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano. 2015, 9, 3169–3177. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Wang, J.; Feng, Q.; Liu, D.; Yin, Q.; Xu, D.; Wei, Y.; Ding, B.; Shi, X.; et al. Tunable Rigidity of (Polymeric Core)-(Lipid Shell) Nanoparticles for Regulated Cellular Uptake. Adv. Mater. 2015, 27, 1402–1407. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Liu, D.; Subramanyam, K.; Wang, B.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Nanoparticle Elasticity Directs Tumor Uptake. Nat. Commun. 2018, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Ribeiro, A.M. Biomimetic Nanoparticles: Preparation, Characterization and Biomedical Applications. Int. J. Nanomed. 2010, 5, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef] [PubMed]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef] [Green Version]
- Cascio, M.G.; Marini, P. Biosynthesis and Fate of Endocannabinoids. Handb Exp. Pharmacol. 2015, 231, 39–58. [Google Scholar] [CrossRef]
- Thors, L.; Fowler, C.J. Is There a Temperature-Dependent Uptake of Anandamide into Cells? Br. J. Pharmacol. 2006, 149, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theato, P.; Sumerlin, B.S.; O’reilly, R.K.; Epp, T.H. Stimuli Responsive Materials. Chem. Soc. Rev. 2013, 42, 7055–7056. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid Classification, Structures and Tools. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Kohli, A.G.; Kierstead, P.H.; Venditto, V.J.; Walsh, C.L.; Szoka, F.C. Designer Lipids for Drug Delivery: From Heads to Tails. J. Control. Release 2014, 190, 274–287. [Google Scholar] [CrossRef]
- Astruc-Diaz, F.; Mcdaniel, S.W.; Xu, J.J.; Parola, S.; Brown, D.L.; Naguib, M.; Diaz, P. In Vivo Efficacy of Enabling Formulations Based on Hydroxypropyl-β-Cyclodextrins, Micellar Preparation, and Liposomes for the Lipophilic Cannabinoid CB2 Agonist, MDA7. J. Pharm. Sci. 2013, 102, 352–364. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, H.; Gorain, B.; Pandey, M.; Khurana, R.K.; Kesharwani, P. Strategizing Biodegradable Polymeric Nanoparticles to Cross the Biological Barriers for Cancer Targeting. Int. J. Pharm. 2019, 565, 509–522. [Google Scholar] [CrossRef]
- Dmour, I.; Taha, M.O. Natural and Semisynthetic Polymers in Pharmaceutical Nanotechnology. In Organic Materials as Smart Nanocarriers for Drug Delivery; William Andrew: Norwich, NY, USA, 2018; pp. 35–100. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric Micelles: Basic Research to Clinical Practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Xian, S.; Parayath, N.N.; Nehoff, H.; Giles, N.M.; Greish, K. The Use of Styrene Maleic Acid Nanomicelles Encapsulating the Synthetic Cannabinoid Analog WIN55,212-2 for the Treatment of Cancer. Anticancer Res. 2015, 35, 4707–4712. [Google Scholar] [PubMed]
- Martín-Banderas, L.; Muñoz-Rubio, I.; Álvarez-Fuentes, J.; Durán-Lobato, M.; Arias, J.L.; Holgado, M.Á.; Fernández-Arévalo, M. Engineering of Δ9-Tetrahydrocannabinol Delivery Systems Based on Surface Modified-PLGA Nanoplatforms. Colloids Surf. B Biointerfaces 2014, 123, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hernan Perez De La Ossa, D.; Ligresti, A.; Gil-Alegre, M.E.; Aberturas, M.R.; Molpeceres, J.; Di Marzo, V.; Torres Suárez, A.I. Poly-ε-Caprolactone Microspheres as a Drug Delivery System for Cannabinoid Administration: Development, Characterization and in Vitro Evaluation of Their Antitumoral Efficacy. J. Control Release 2012, 161, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Durán-Lobato, M.; Martín-Banderas, L.; Gonçalves, L.M.D.; Fernández-Arévalo, M.; Almeida, A.J. Comparative Study of Chitosan- and PEG-Coated Lipid and PLGA Nanoparticles as Oral Delivery Systems for Cannabinoids. J. Nanoparticle Res. 2015, 17, 61. [Google Scholar] [CrossRef]
- Barnett-Norris, J.; Lynch, D.; Reggio, P.H. Lipids, Lipid Rafts and Caveolae: Their Importance for GPCR Signaling and Their Centrality to the Endocannabinoid System. Life Sci. 2005, 77, 1625–1639. [Google Scholar] [CrossRef]
- Thomas, C.M.; Smart, E.J. Caveolae Structure and Function: Caveolae Review Series. J. Cell Mol. Med. 2008, 12, 796–809. [Google Scholar] [CrossRef]
- Keren, O.; Sarne, Y. Multiple Mechanisms of CB1 Cannabinoid Receptors Regulation. Brain Res. 2003, 980, 197–205. [Google Scholar] [CrossRef]
- Galbiati, F.; Volonté, D.; Meani, D.; Milligan, G.; Lublin, D.M.; Lisanti, M.P.; Parenti, M. The Dually Acylated NH2-Terminal Domain of G(I1α) Is Sufficient to Target a Green Fluorescent Protein Reporter to Caveolin-Enriched Plasma Membrane Domains: Palmitoylation of Caveolin-1 Is Required for the Recognition of Dually Acylated G-Protein α Subuni. J. Biol. Chem. 1999, 274, 5843–5850. [Google Scholar] [CrossRef] [Green Version]
- Couet, J.; Li, S.; Okamoto, T.; Ikezu, T.; Lisanti, M.P. Identification of Peptide and Protein Ligands for the Caveolin- Scaffolding Domain. Implications for the Interaction of Caveolin with Caveolae-Associated Proteins. J. Biol. Chem. 1997, 272, 6525–6533. [Google Scholar] [CrossRef] [Green Version]
- Izzo, A.A.; Fezza, F.; Capasso, R.; Bisogno, T.; Pinto, L.; Iuvone, T.; Esposito, G.; Mascolo, N.; Di Marzo, V.; Capasso, F. Cannabinoid CB1-Receptor Mediated Regulation of Gastrointestinal Motility in Mice in a Model of Intestinal Inflammation. Br. J. Pharmacol. 2001, 134, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Sorkin, A.; Von Zastrow, M. Signal Transduction and Endocytosis: Close Encounters of Many Kinds. Nat. Rev. Mol. Cell Biol. 2002, 3, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Tsao, P.; Cao, T.; Von Zastrow, M. Role of Endocytosis in Mediating Downregulation of G-Protein-Coupled Receptors. Trends Pharmacol. Sci. 2001, 22, 91–96. [Google Scholar] [CrossRef]
- Moradi, E.; Vllasaliu, D.; Garnett, M.; Falcone, F.; Stolnik, S. Ligand Density and Clustering Effects on Endocytosis of Folate Modified Nanoparticles. RSC Adv. 2012, 2, 3025–3033. [Google Scholar] [CrossRef]
- Large, D.E.; Soucy, J.R.; Hebert, J.; Auguste, D.T. Advances in Receptor-Mediated, Tumor-Targeted Drug Delivery. Adv. Ther. 2019, 2, 1800091. [Google Scholar] [CrossRef] [Green Version]
- Flores-Otero, J.; Ahn, K.H.; Delgado-Peraza, F.; Mackie, K.; Kendall, D.A.; Yudowski, G.A. Ligand-Specific Endocytic Dwell Times Control Functional Selectivity of the Cannabinoid Receptor 1. Nat. Commun. 2014, 5, 4589. [Google Scholar] [CrossRef] [Green Version]
- Barak, L.S.; Oakley, R.H.; Laporte, S.A.; Caron, M.G. Constitutive Arrestin-Mediated Desensitization of a Human Vasopressin Receptor Mutant Associated with Nephrogenic Diabetes Insipidus. Proc. Natl. Acad. Sci. USA 2001, 98, 93–98. [Google Scholar] [CrossRef]
- Poon, Z.; Lee, J.A.; Huang, S.; Prevost, R.J.; Hammond, P.T. Highly Stable, Ligand-Clustered “Patchy” Micelle Nanocarriers for Systemic Tumor Targeting. Nanomedicine 2011, 7, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Poon, Z.; Chen, S.; Engler, A.C.; Lee, H.I.; Atas, E.; Von Maltzahn, G.; Bhatia, S.N.; Hammond, P.T. Ligand-Clustered “Patchy” Nanoparticles for Modulated Cellular Uptake and in Vivo Tumor Targeting. Angew. Chem.-Int. Ed. 2010, 49, 7266–7270. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Peng, B.; Sohrabi, S.; Liu, Y. The Configuration of Copolymer Ligands on Nanoparticles Affects Adhesion and Uptake. Langmuir 2016, 32, 10136–10143. [Google Scholar] [CrossRef]

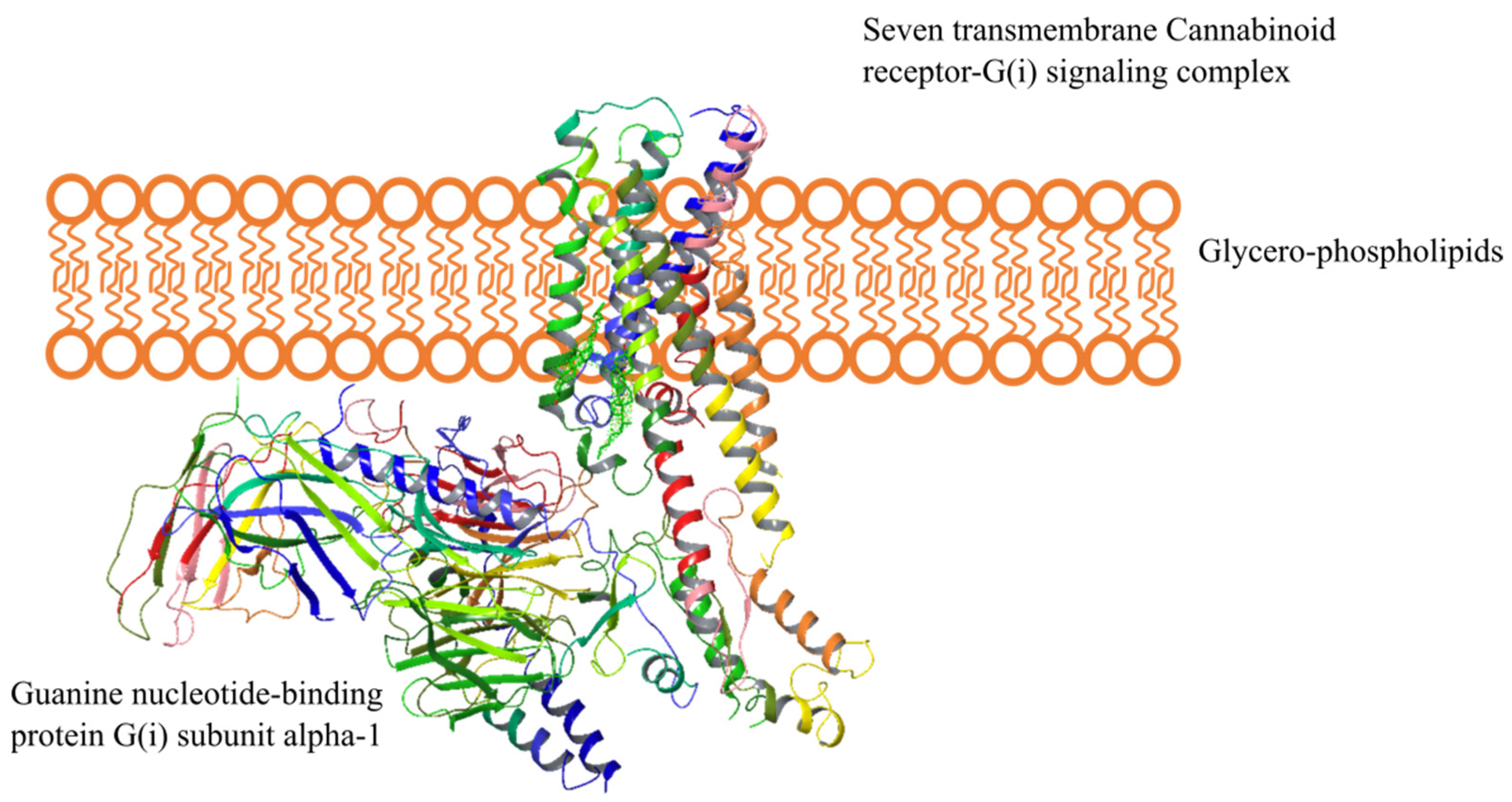
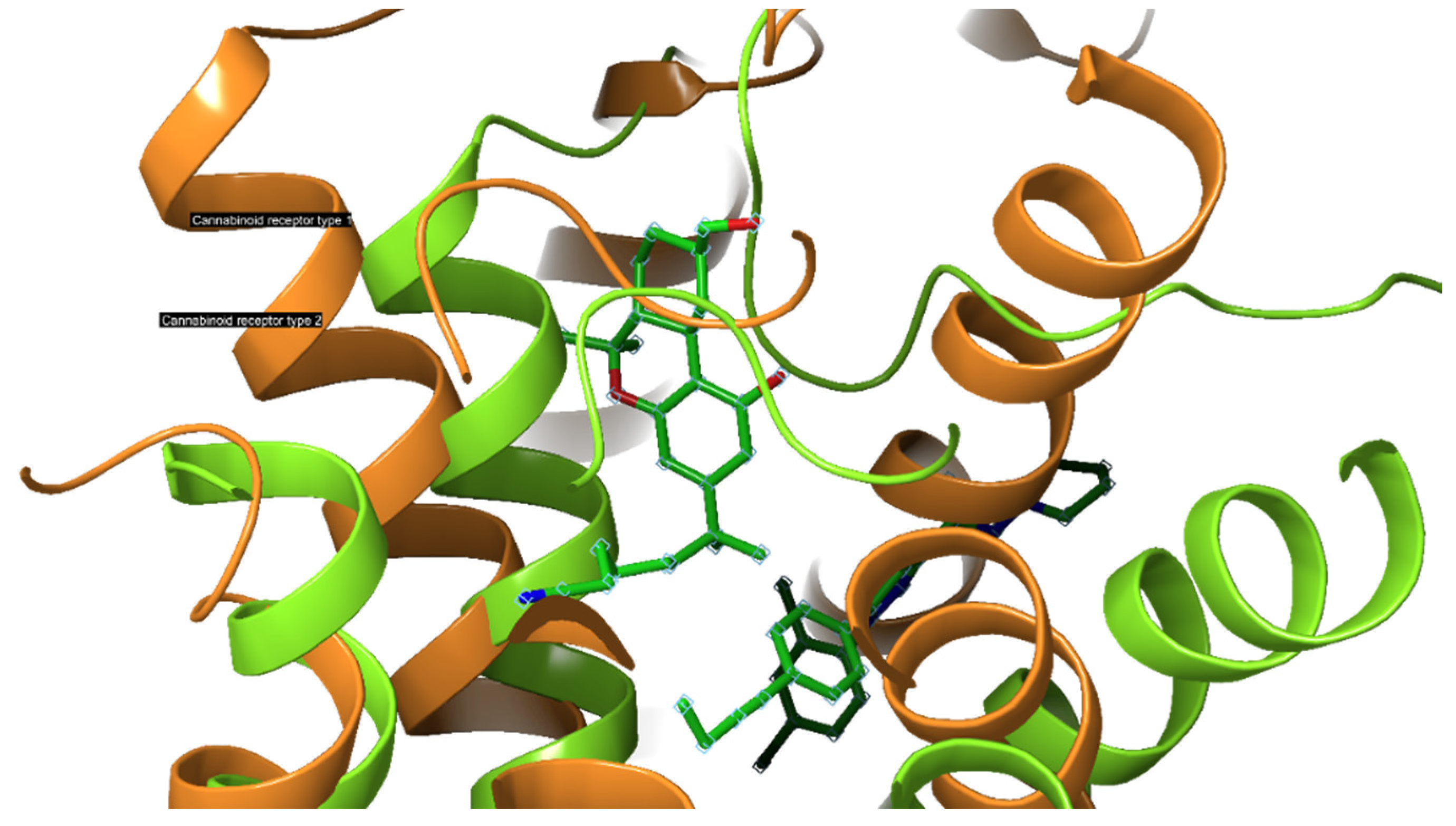
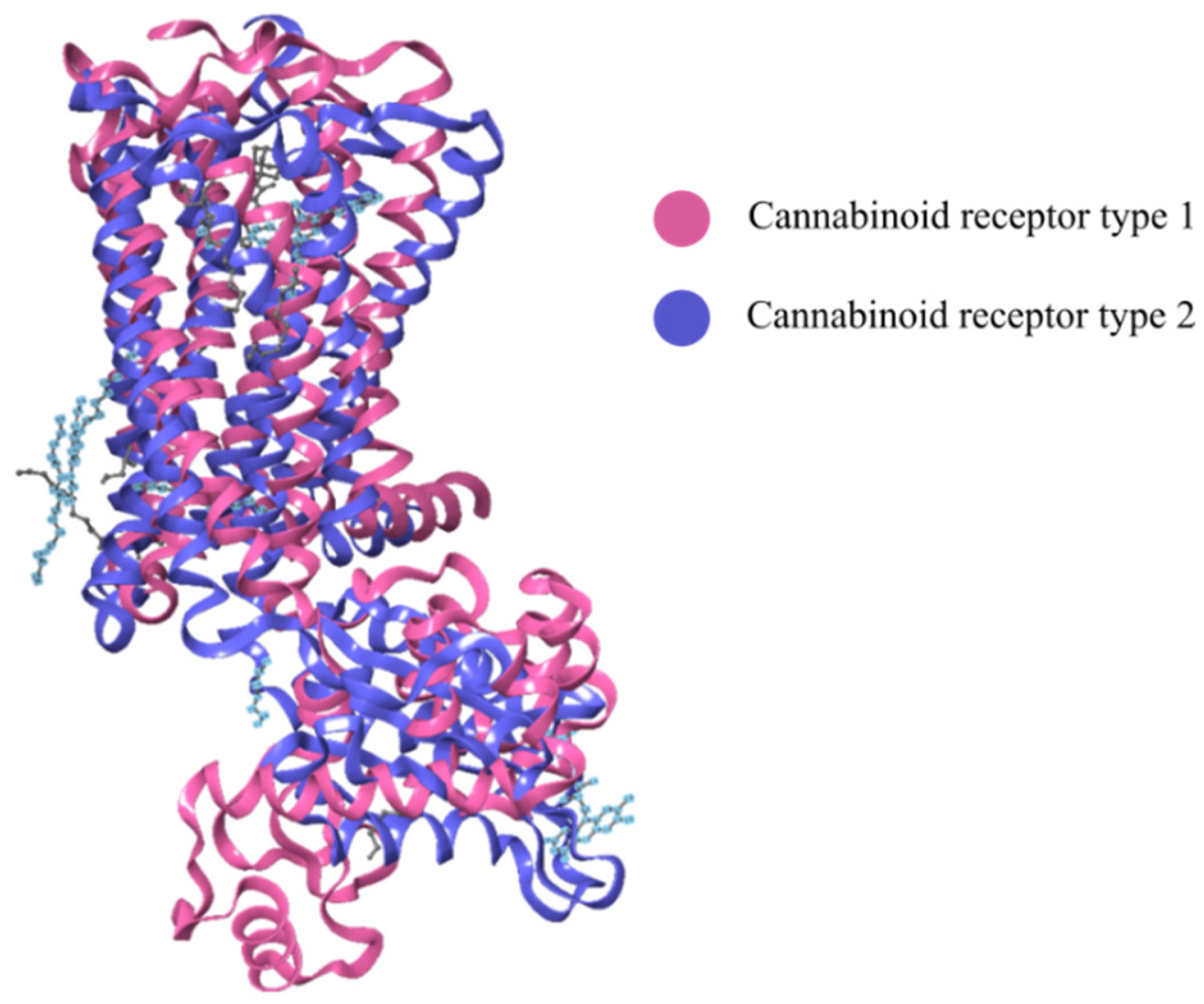
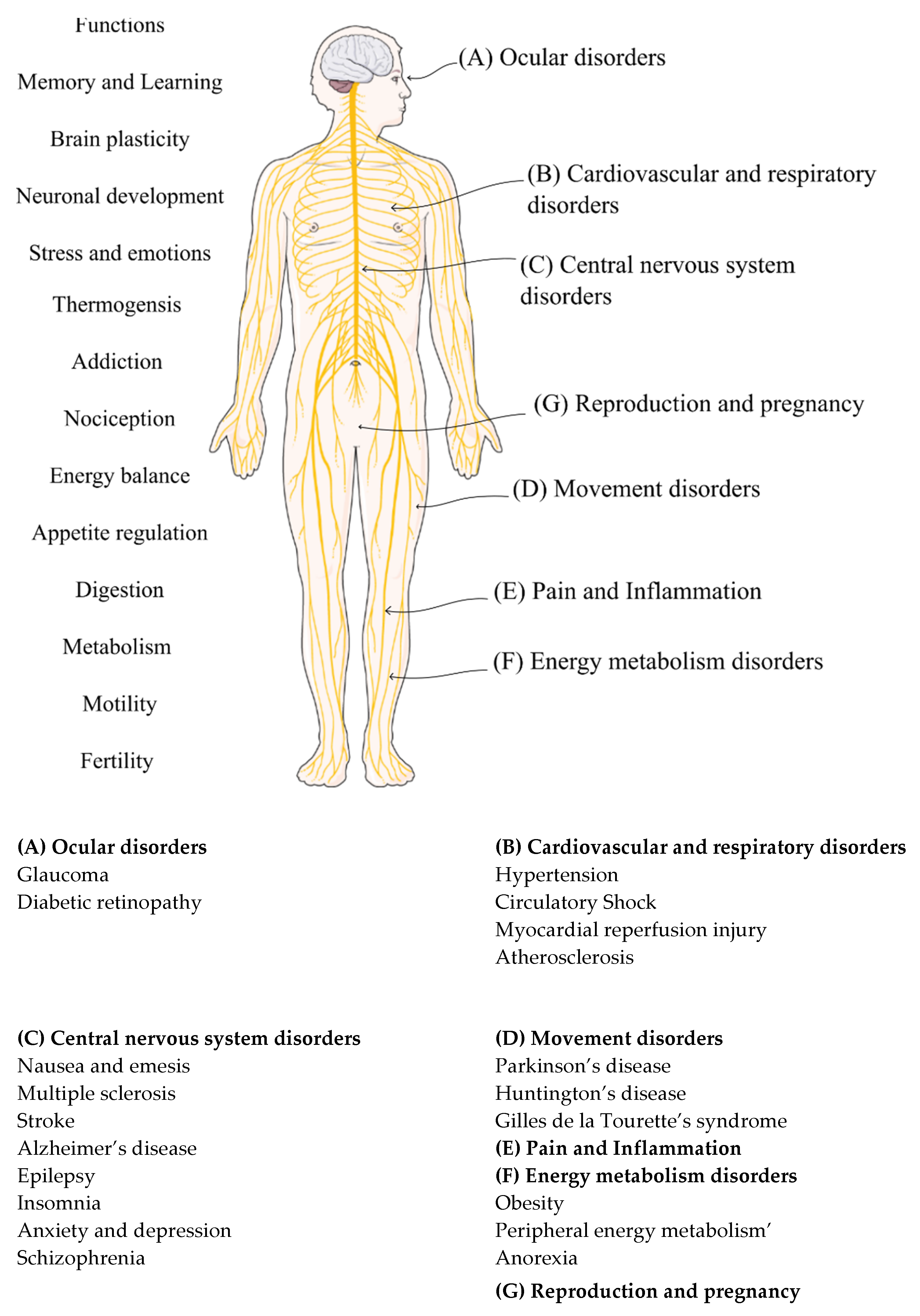

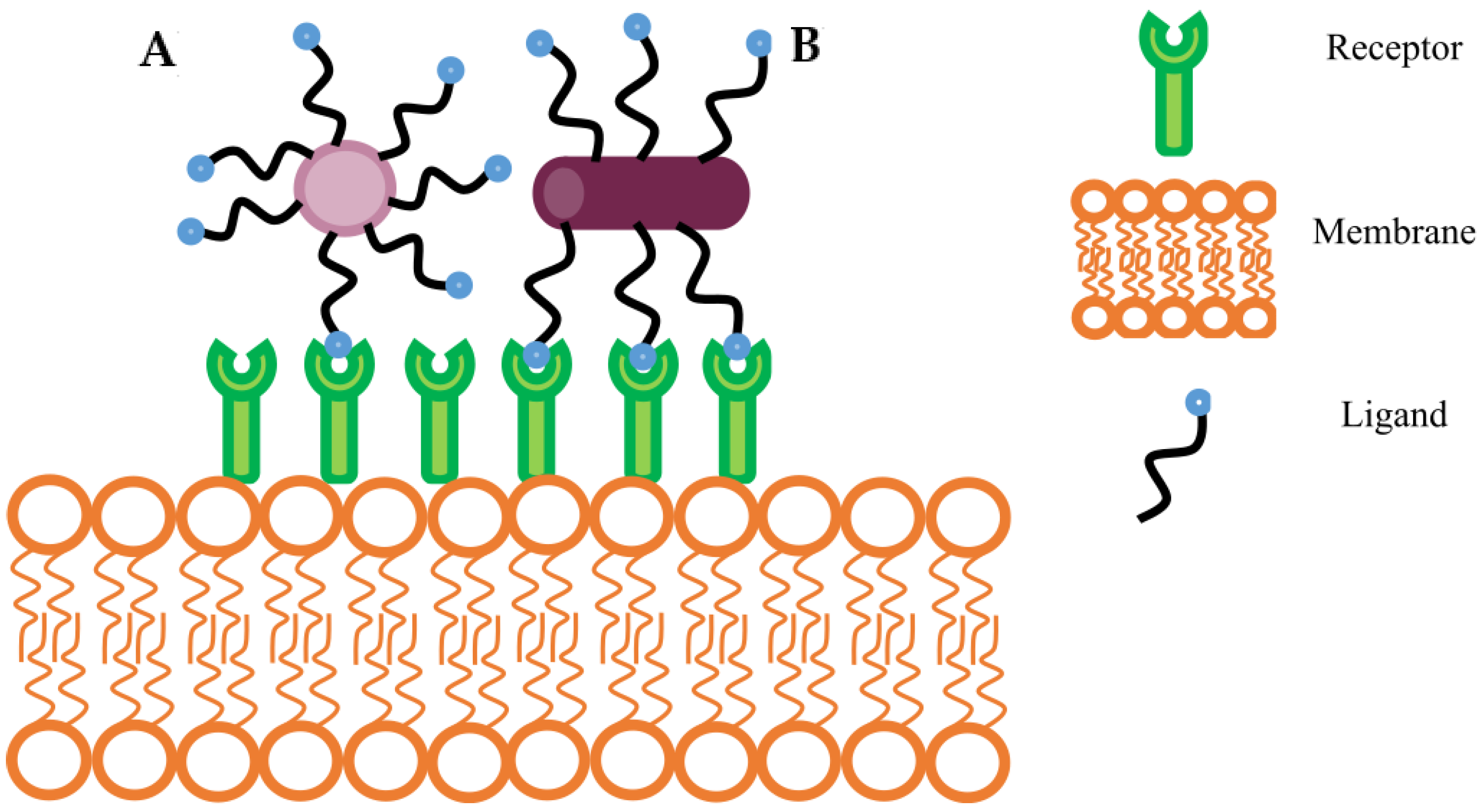
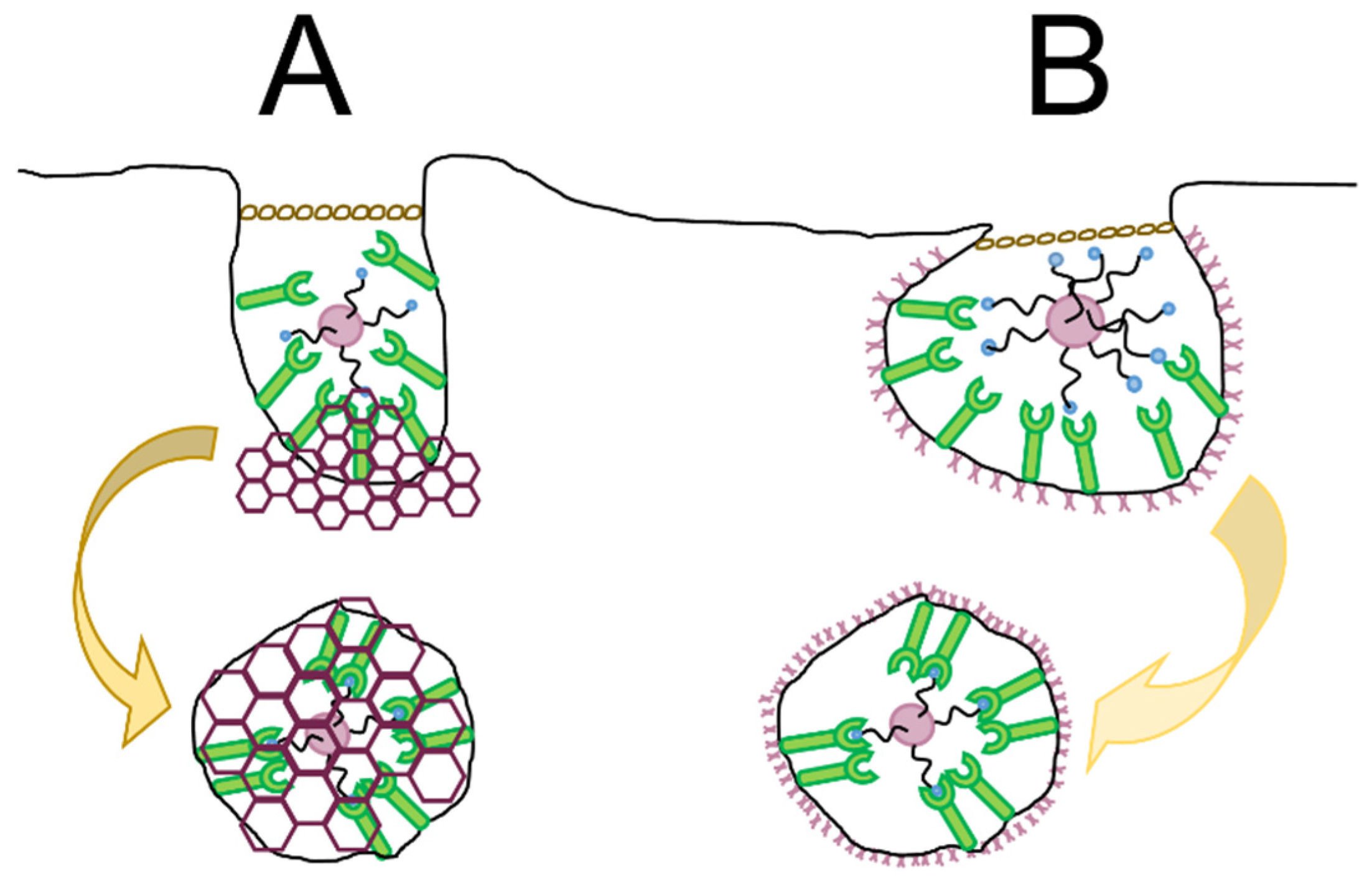
| Intensity | Location | Reference |
|---|---|---|
| Brainstem cardiorespiratory centers | [10] | |
| Low | ||
| Moderate | Spinal cord | [114] |
| Dense | Hippocampus | [115] |
| Basal Ganglia (globus pallidus, substantia nigra) | [116] | |
| Cerebral cortex (cingulate gyrus and prefrontal cortex) | [117] | |
| Amygdala | ||
| Cerebellum | ||
| Ventral horn | [118] | |
| Hypothalamus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasram, M.H.; Walker, R.B.; Khamanga, S.M. Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 13223. https://doi.org/10.3390/ijms232113223
Dasram MH, Walker RB, Khamanga SM. Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery. International Journal of Molecular Sciences. 2022; 23(21):13223. https://doi.org/10.3390/ijms232113223
Chicago/Turabian StyleDasram, Mendhi Henna, Roderick B. Walker, and Sandile M. Khamanga. 2022. "Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery" International Journal of Molecular Sciences 23, no. 21: 13223. https://doi.org/10.3390/ijms232113223
APA StyleDasram, M. H., Walker, R. B., & Khamanga, S. M. (2022). Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery. International Journal of Molecular Sciences, 23(21), 13223. https://doi.org/10.3390/ijms232113223








