CADMA-Chem: A Computational Protocol Based on Chemical Properties Aimed to Design Multifunctional Antioxidants
Abstract
:1. Introduction
2. Results and Discussion
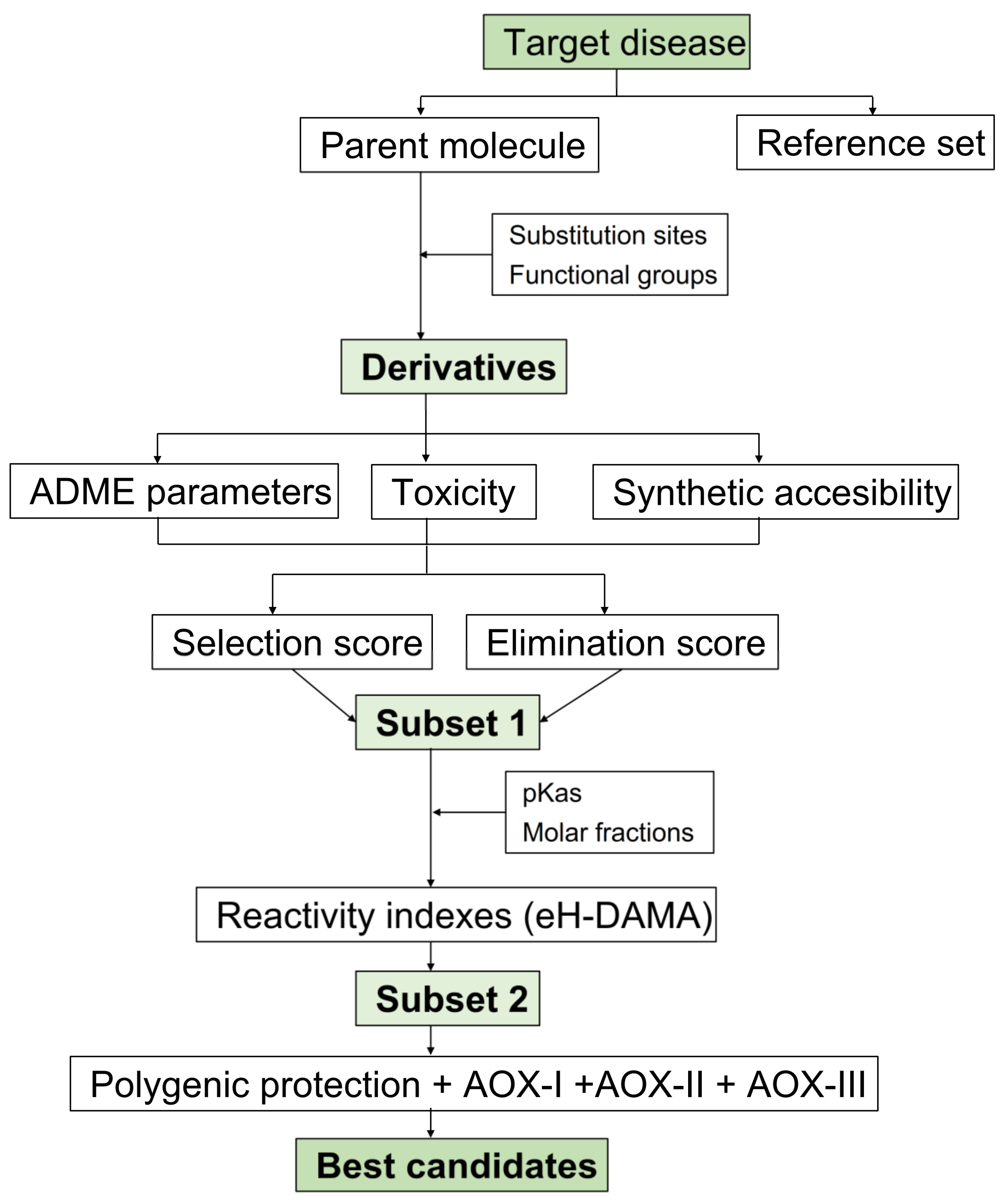
2.1. The CADMA-Chem Protocol
- Drug-like behavior (i.e., adequate permeation and bioavailability).
- Low toxicity.
- Easy manufacturability.
- Free radical scavenging capability.
- Metal chelation properties (●OH inactivating ligand behavior).
- Efficient for repairing oxidatively damaged biological targets (lipids, DNA and proteins).
- Polygenic neuroprotection, i.e., inhibitors of COMT, AChE and/or MAOB.
2.1.1. Building the Candidates
- i.
- They can influence the acid-base behavior, thus modulating the proportion of neutral species at specific pH values, which is important for drugs passing across lipid barriers via passive diffusion.
- ii.
- They may contribute to increased free radical scavenging activity (via H or electron donation.
- iii.
- They may contribute to increased metal chelating capability.
2.1.2. Sampling the Search Space
- -
- To consider that an effective drug molecule is subject to more objectives than the binding affinity.
- -
- To define positive design restricts, which are those properties that allow for identifying the chemical subspace with a higher probability of containing drug-like molecules.
- -
- To define negative design restricts, or ‘tabu zones’, which are characterized by adverse properties and/or unwanted structures.
- -
- To reformulate the multi-objective problem into a single objective using a weighted score function. In such a function, the individual objectives are summed and frequently multiplied by a weighting factor.
- -
- This map is meant to analyze SFR processes for free radicals that are natural targets of antioxidants, for example peroxyl radicals, i.e., not highly reactive ones. If it is used otherwise, it might be misleading. A typical case would be a radical, such as ●OH, that usually reacts with antioxidants (via electron transfer) in a highly exergonic way. Such a reaction would be in the inverted region of the Marcus parabola. Consequently, albeit thermochemically viable, it may be a very slow reaction, not significantly contributing to antioxidant activity.
- -
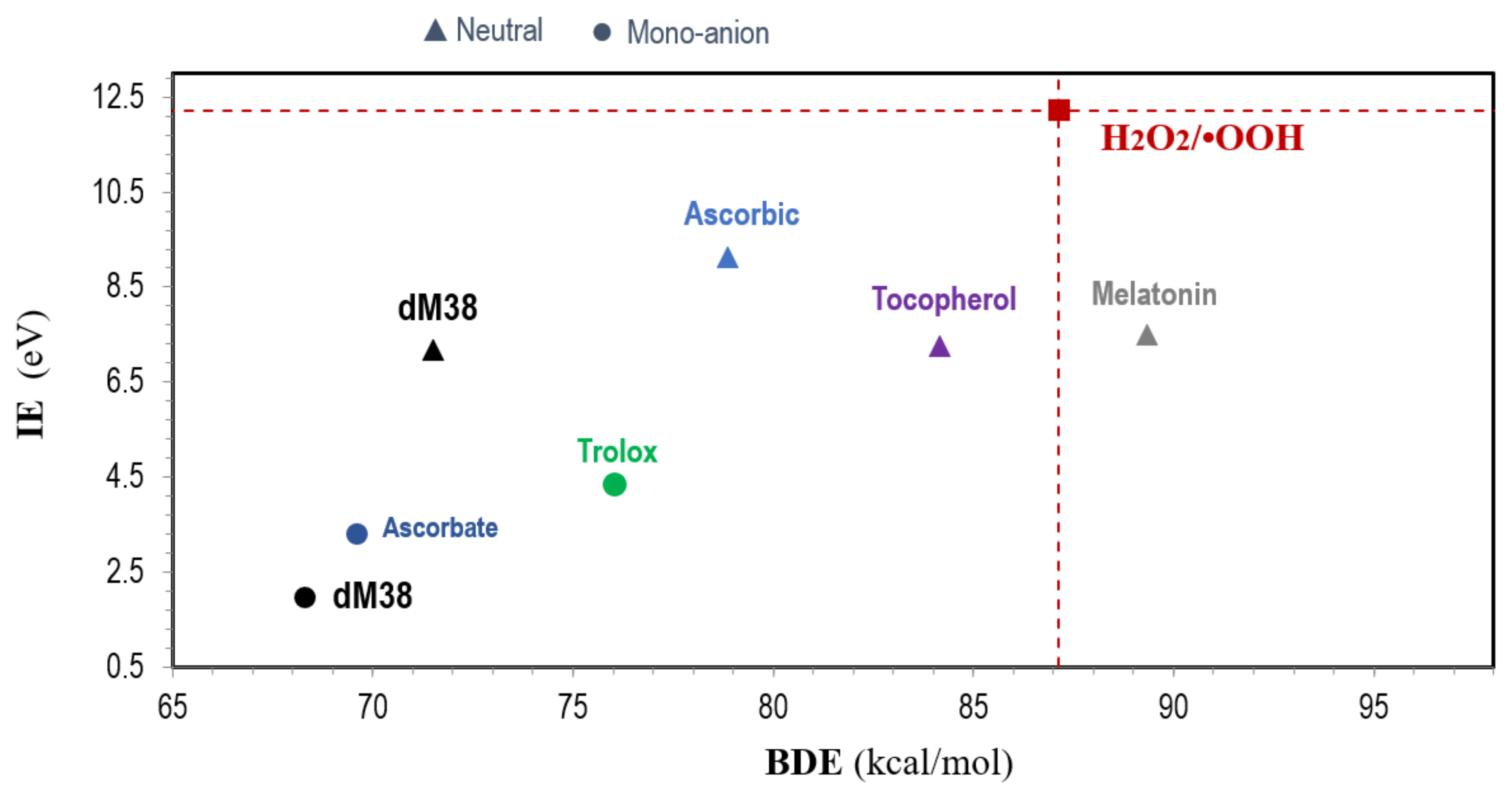
- It is useful to include the target radical in the map (for example: ●OOH), as well as some reference antioxidants (for example: Trolox, ascorbic acid, and/or α-tocopherol).
- -
- The species located at the bottom-left of the map are those expected to be the best free radical scavengers, via SET and f-HAT.
2.1.3. Evaluating Multifunctional Antioxidant Behavior
- -
- Free radical scavenging activity (AOX-I) accounts for AOX activity in the absence of redox metal ions.
- -
- ●OH inactivating ligand behavior (OIL, AOX-II) accounts for AOX in the presence of redox metal ions.
- -
- Repair of biological molecules (AOX-III).
2.1.3.1. Molar Fractions at Physiological pH
2.1.3.2. Free Radical Scavenging (AOX-I)
2.1.3.3. OIL Behavior (AOX-II)
2.1.3.4. Repairing Biological Molecules (AOX-III)
- -
- Lipids: A simplified model of linoleic acid (LM), with 2 allylic H atoms (the key chemical feature of easily oxidizable lipids), is used to represent unsaturated fatty acids [114].
- -
- Amino acid residues in proteins: Six residues, highly susceptible to OS [115,116,117,118,119,120,121], are considered, namely cysteine, histidine, leucine, methionine, tryptophan, and tyrosine. To represent them, the model known as the realistic model is used [120,122,123,124,125,126,127,128,129,130,131,132].
- -
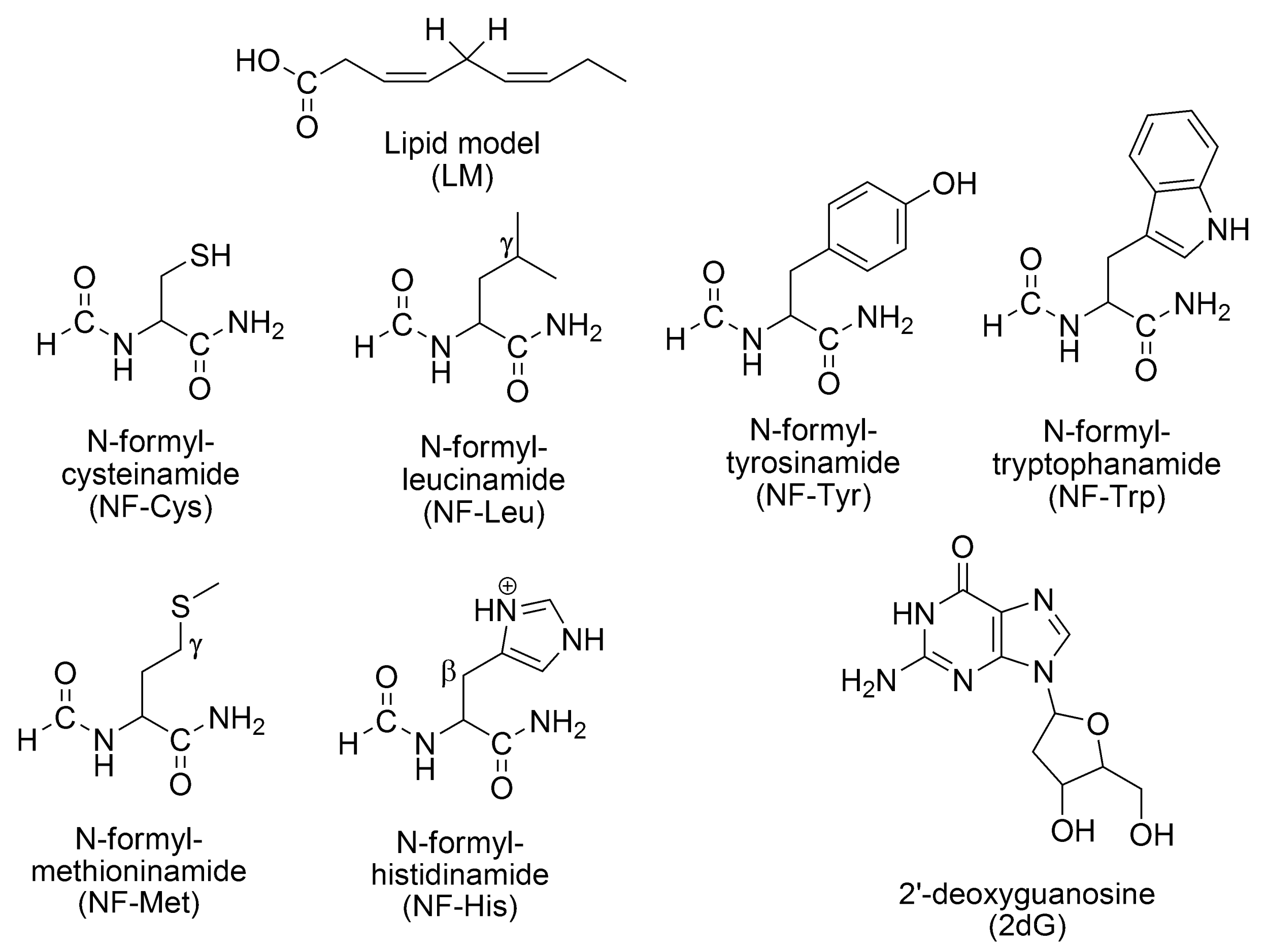
- -
- Lipids: f-HAT, involving the allylic hydrogens.
- -
- Amino acid residues in proteins: SET from Tyr and Trp. f-HAT from Cys, Tyr, Leu, Met, and His.
- -
- DNA: SET from 2dG sites (the nucleoside most easily oxidizable) [138], f-HAT from the deoxyribose unit (yielding C-centered radicals) [139,140,141,142], RAF yielding the 8-OH-dG adduct, the precursor of one of the most abundant lesions in DNA: 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) [143], which is a biomarker of OS [144,145].
- a.
- Repairing guanine-centered radical cations, via SET.
- b.
- Repairing C-centered radicals, in the deoxyribose unit, via f-HAT.
- c.
- Repairing 8-OH-dG lesions via sequential hydrogen atom transfer followed by dehydration (SHATD) [146].
2.1.4. Evaluating Polygenic Neuroprotection
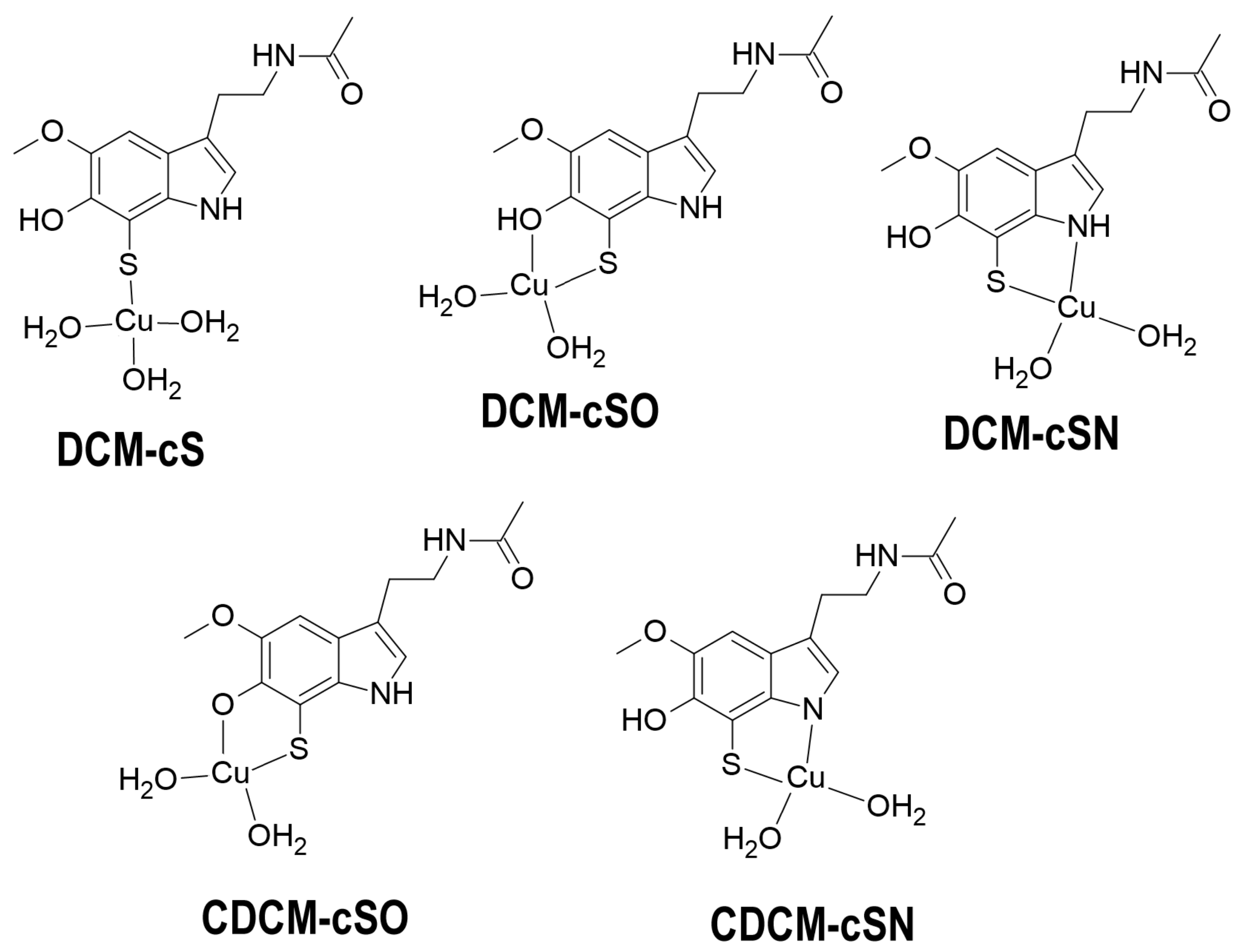
2.2. Study Case
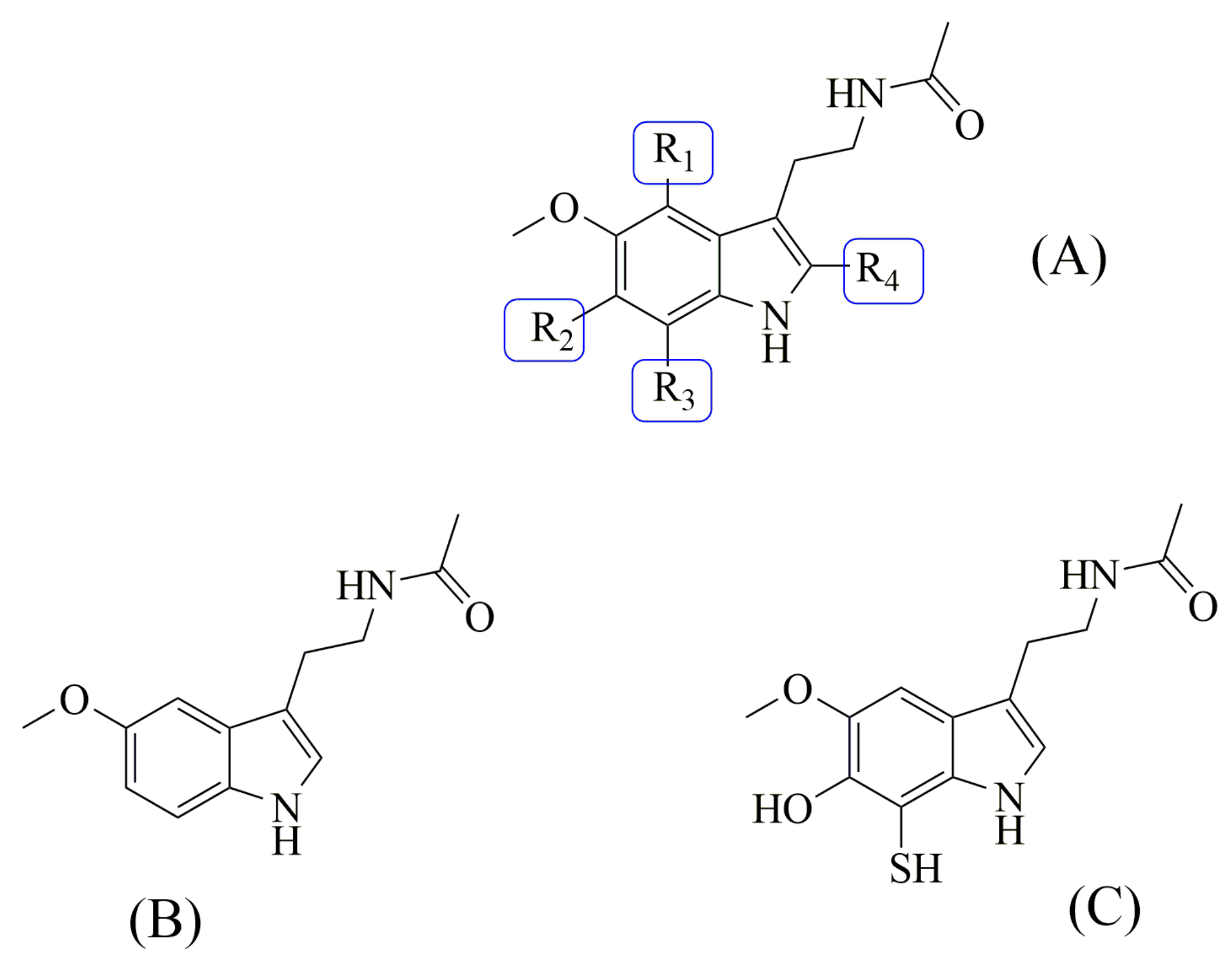
2.2.1. Building the Candidates
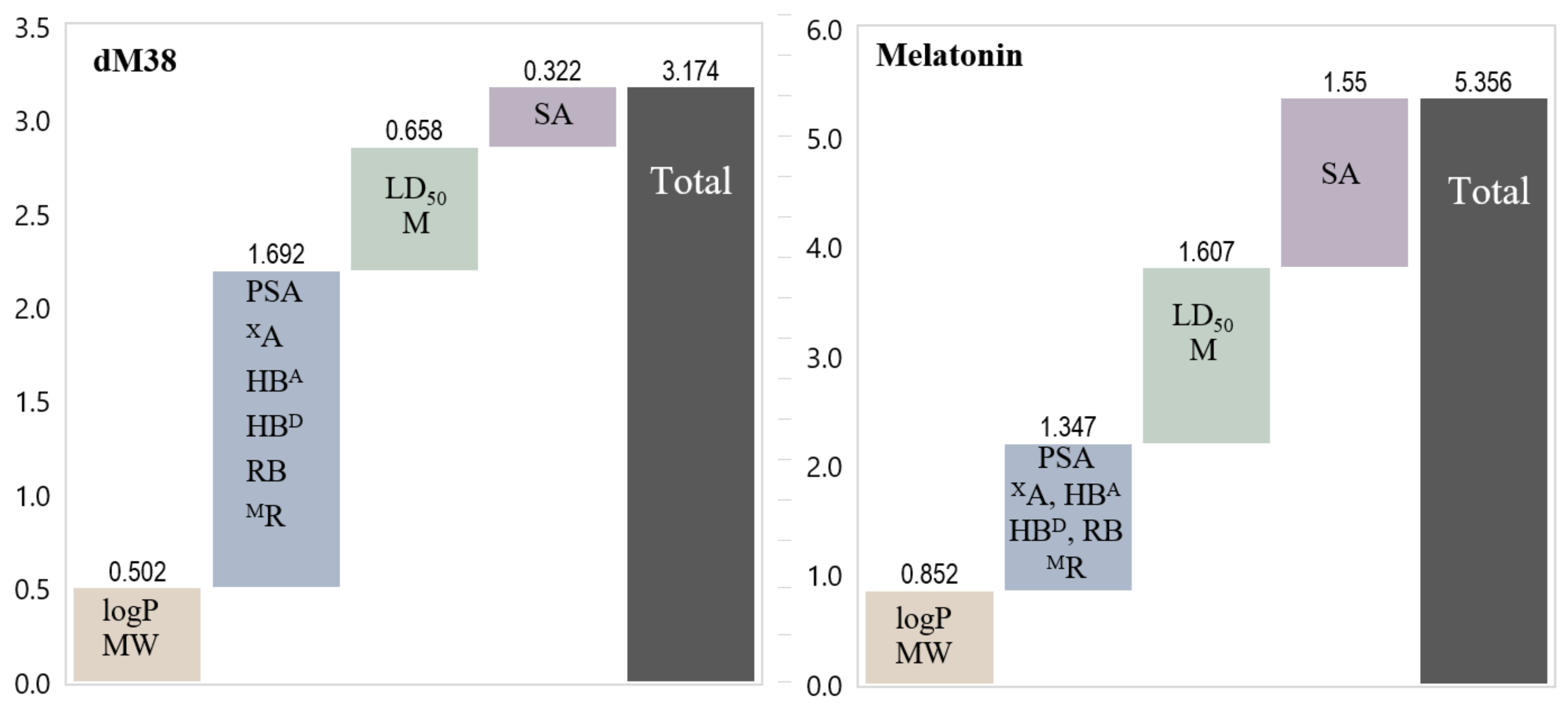
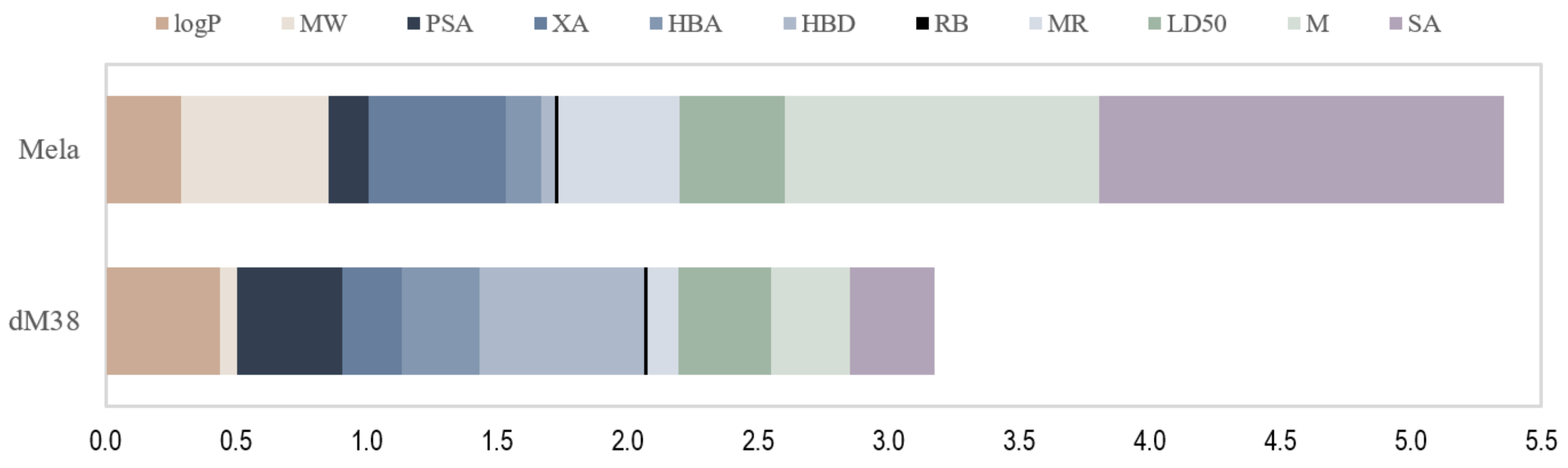
2.2.2. Sampling the Search Space
2.2.3. Evaluating Multifunctional Antioxidant Behavior
2.2.3.1. Molar Fractions at Physiological pH
2.2.3.2. Free Radical Scavenging (AOX-I)
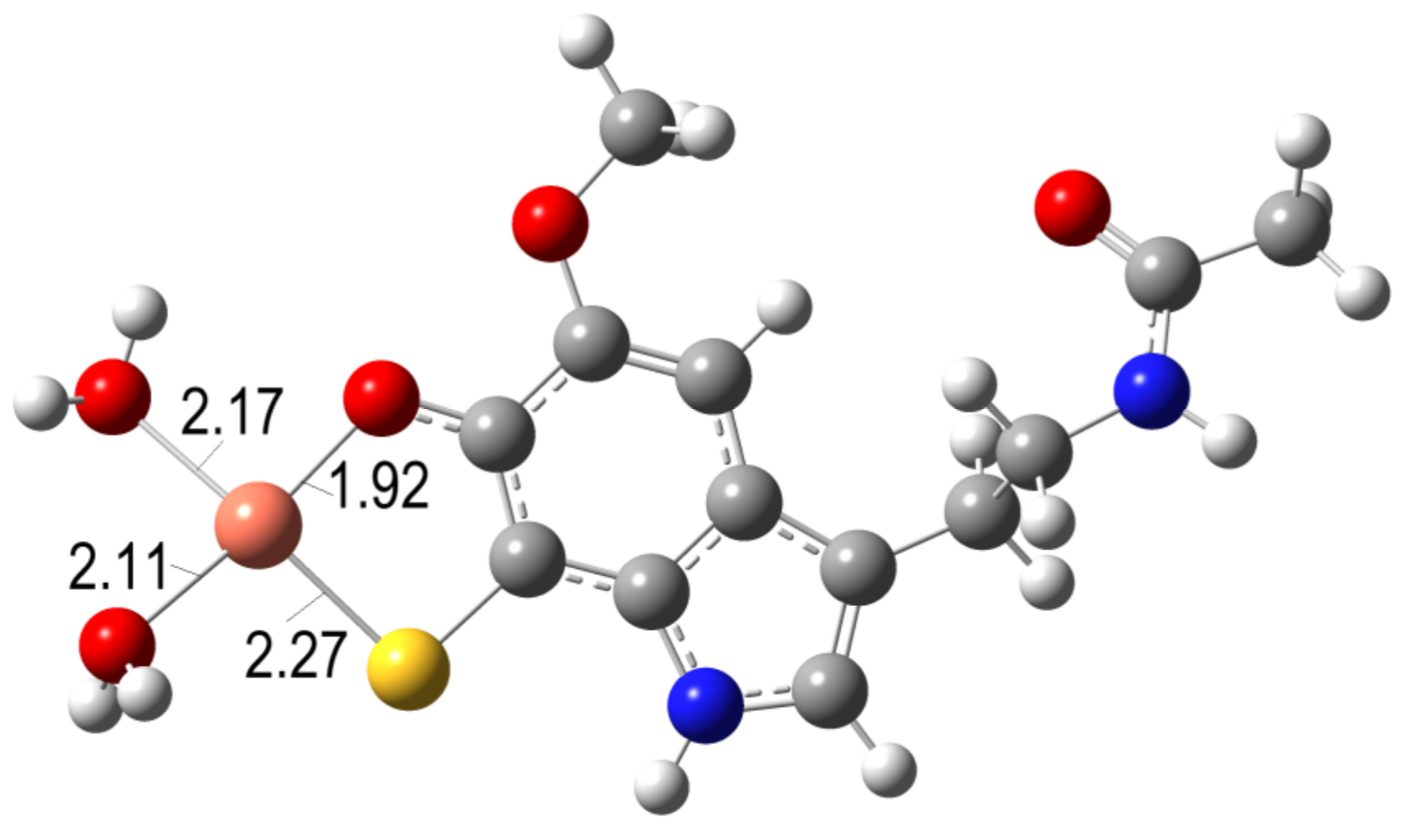
2.2.3.3. OIL Behavior (AOX-II)
- -
- The ascorbate anion: A moderate reductant, which is frequently used in experiments to induce oxidative conditions (mixed with copper).
- -
- The superoxide radical anion (O2•−): A very strong reductant, present in biological systems and involved in Fenton-like reactions.
2.2.3.4. Repairing Biological Damaged Molecules (AOX-III)
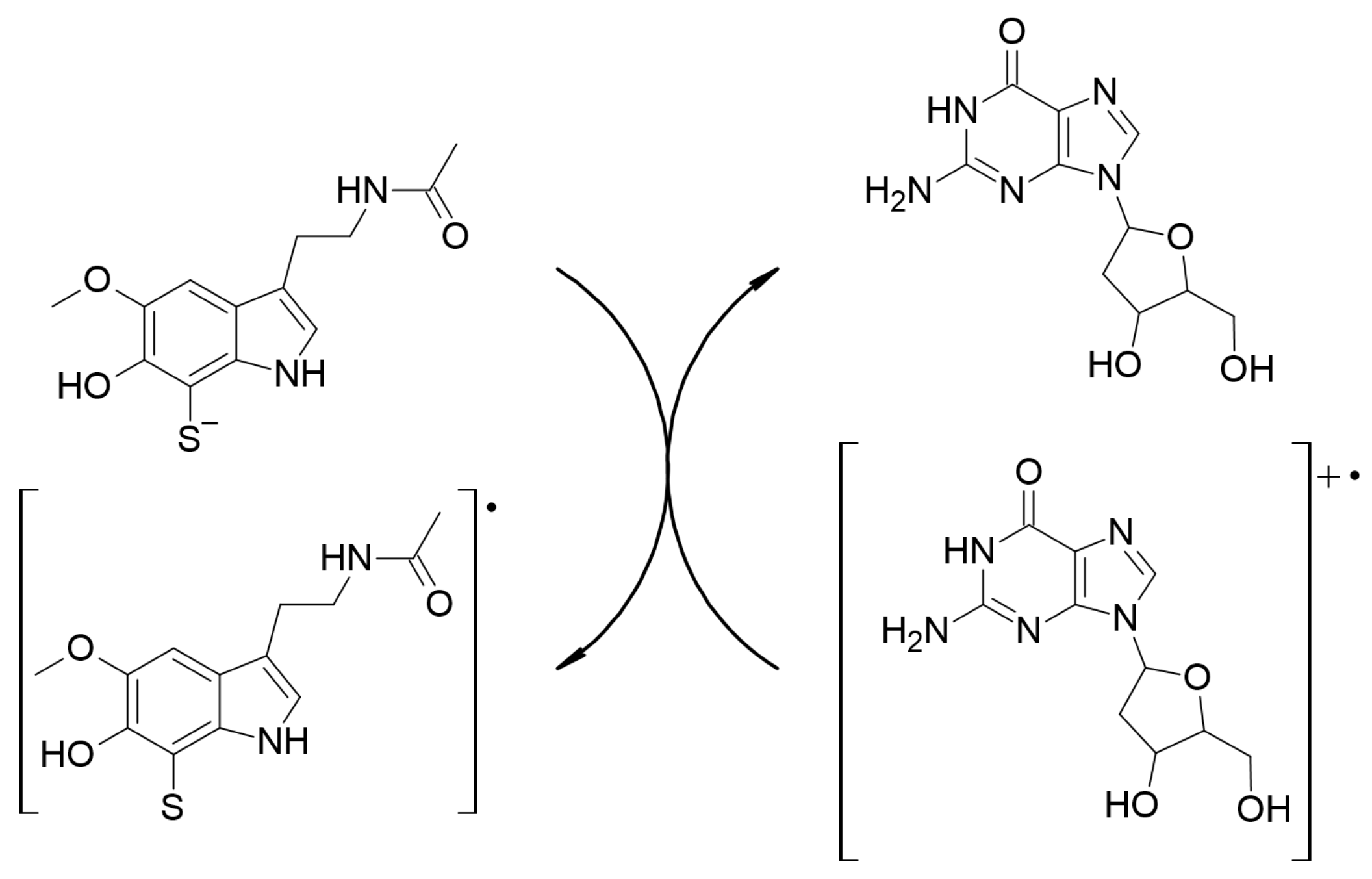
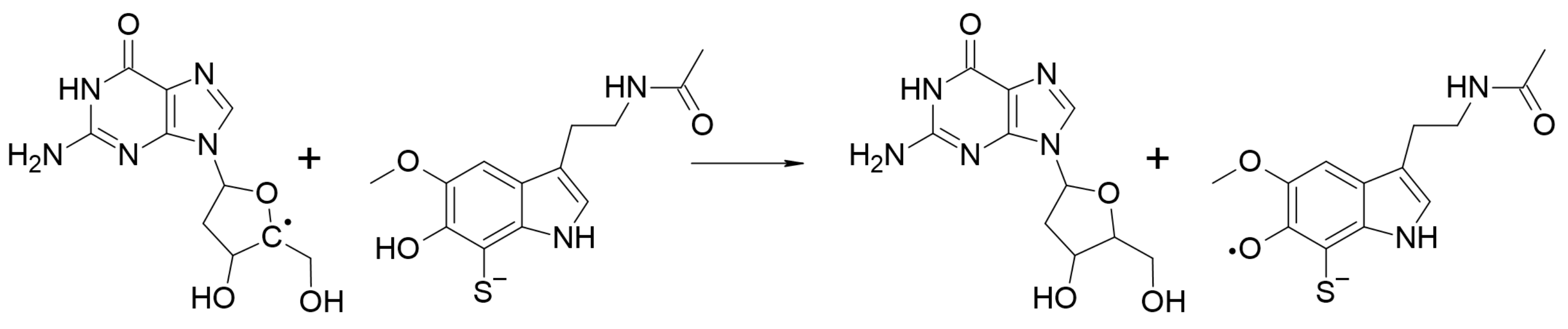
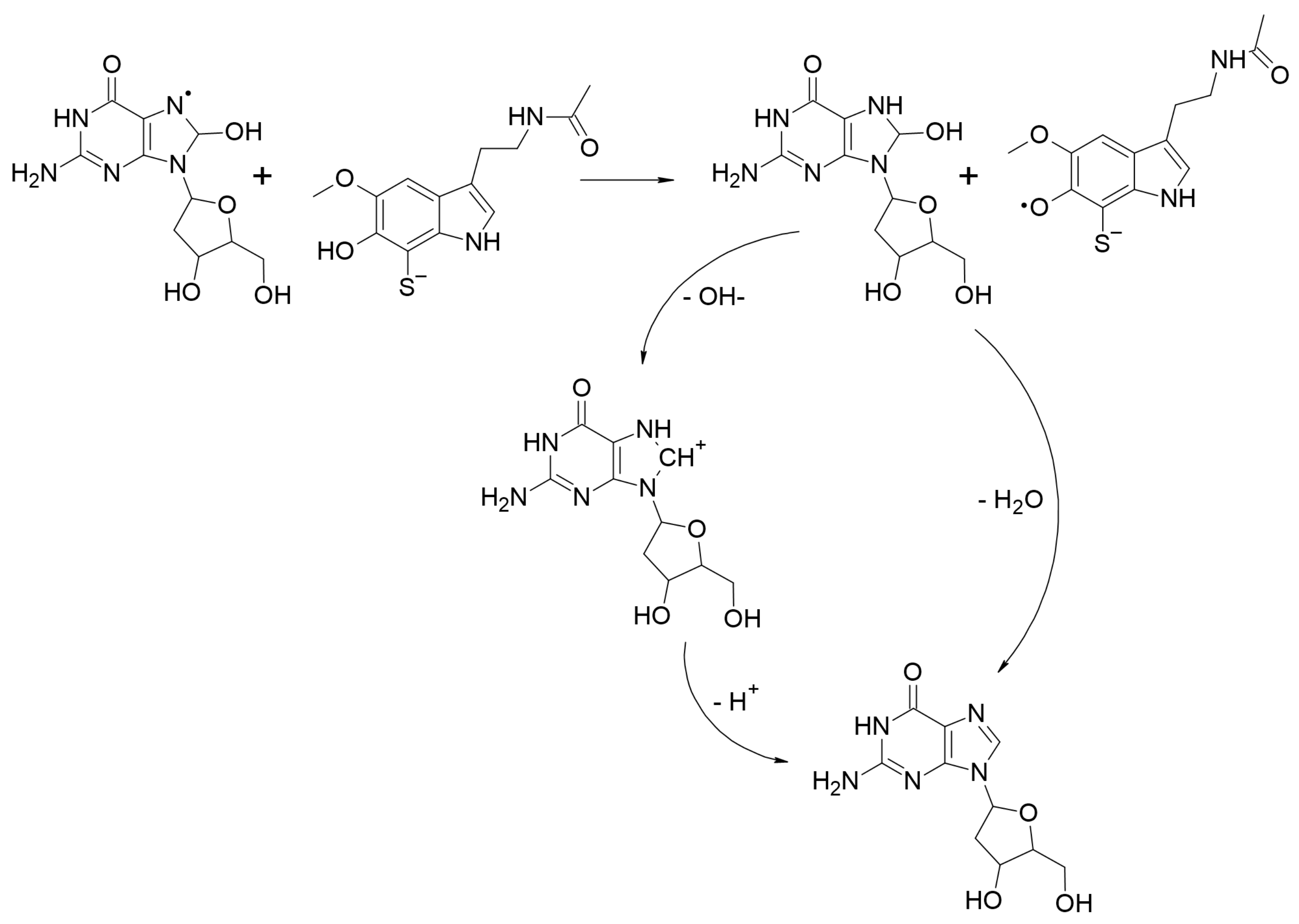
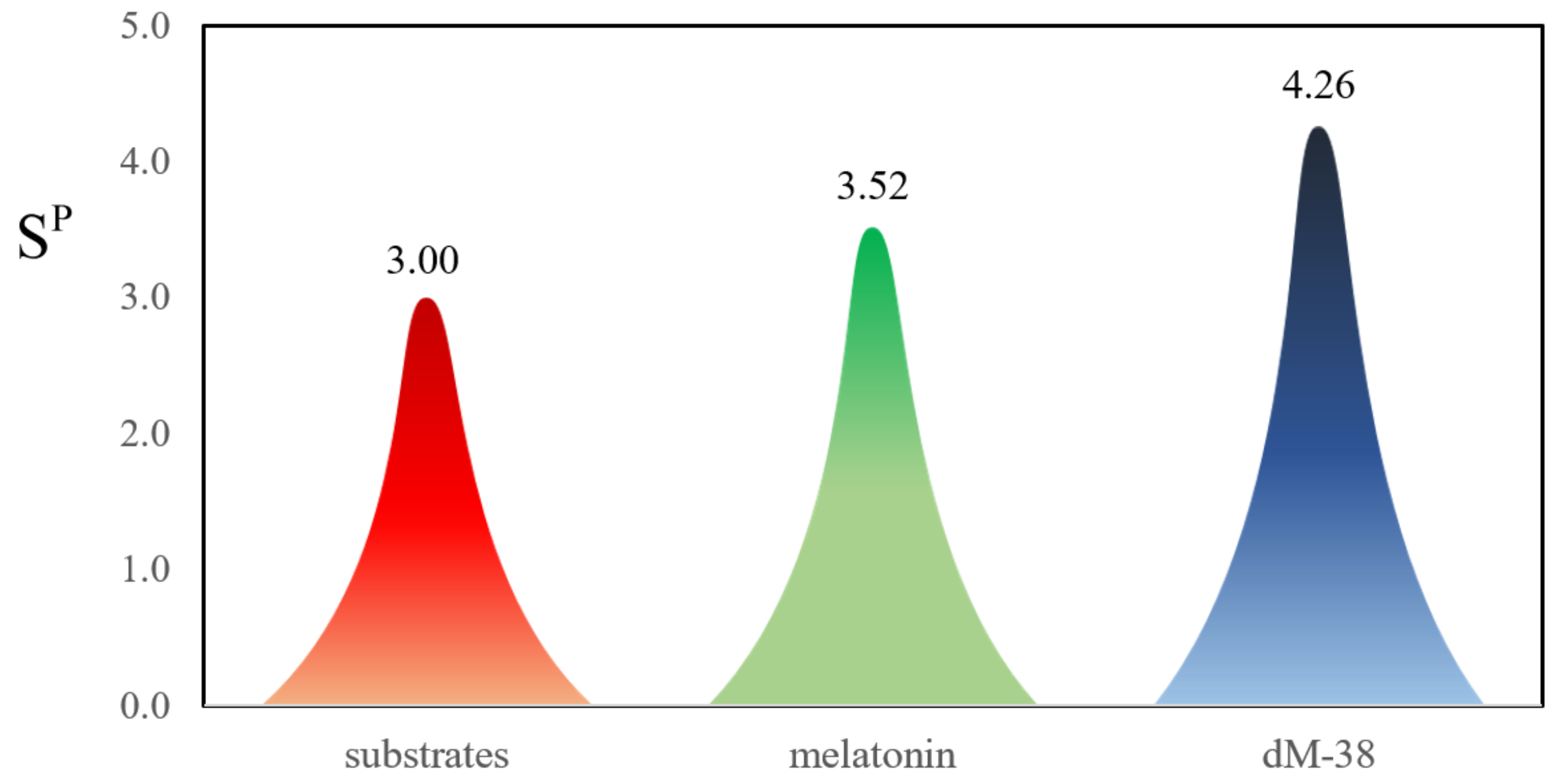
2.2.4. Evaluating Polygenic Neuroprotection
2.2.5. CADMA-Chem Flowchart
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crimmins, E.M. Lifespan and healthspan: Past, present, and promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations. World Population Prospects 2019: Highlights; United Nations: New York, NY, USA, 2019; p. 10. ISBN 978-92-1-148316-1. [Google Scholar]
- Vaupel, J.W.; Villavicencio, F.; Bergeron-Boucher, M.P. Demographic perspectives on the rise of longevity. Proc. Natl. Acad. Sci. USA 2021, 118, e2019536118. [Google Scholar] [CrossRef]
- Garmany, A.; Yamada, S.; Terzic, A. Longevity leap: Mind the healthspan gap. NPJ Regen. Med. 2021, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Tintut, Y. Interactive and Multifactorial Mechanisms of Calcific Vascular and Valvular Disease. Trends Endocrinol. Metab. 2019, 30, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.; Wang, Y.; Zeng, Z.; Deng, B.; Zhu, B.S.; Cao, T.; Li, Y.K.; Xiao, J.; Han, Q.; Wu, Q. Colorectal cancer (CRC) as a multifactorial disease and its causal correlations with multiple signaling pathways. Biosci. Rep. 2020, 40, BSR20200265. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.; Waters, E.A.; Hamilton, J.G.; Vu, M.; Gabriel, J.; Roberts, M.C. Multifactorial causal beliefs and colorectal cancer screening: A structural equation modeling investigation. J. Health Psychol. 2021, 27, 2463–2477. [Google Scholar] [CrossRef]
- Malik, S.S.; Batool, R.; Masood, N.; Yasmin, A. Risk factors for prostate cancer: A multifactorial case-control study. Curr. Probl. Cancer 2018, 42, 337–343. [Google Scholar] [CrossRef]
- Rajpert-De Meyts, E.; Skotheim, R.I. Complex Polygenic Nature of Testicular Germ Cell Cancer Suggests Multifactorial Aetiology. Eur. Urol. 2018, 73, 832–833. [Google Scholar] [CrossRef]
- Zabaleta, J. Multifactorial etiology of gastric cancer. Methods Mol. Biol. 2012, 863, 411–435. [Google Scholar]
- Nazarian, A.; Arbeev, K.G.; Yashkin, A.P.; Kulminski, A.M. Genome-wide analysis of genetic predisposition to common polygenic cancers. J. Appl. Genet. 2022, 63, 315–325. [Google Scholar] [CrossRef]
- Guerra, J.V.S.; Dias, M.M.G.; Brilhante, A.J.V.C.; Terra, M.F.; García-Arévalo, M.; Figueira, A.C.M. Multifactorial basis and therapeutic strategies in metabolism-related diseases. Nutrients 2021, 13, 2830. [Google Scholar] [CrossRef]
- Kong, W.J.; Vernieri, C.; Foiani, M.; Jiang, J.D. Berberine in the treatment of metabolism-related chronic diseases: A drug cloud (dCloud) effect to target multifactorial disorders. Pharmacol. Ther. 2020, 209, 107496. [Google Scholar] [CrossRef]
- Pant, D.C.; Aguilera-Albesa, S.; Pujol, A. Ceramide signalling in inherited and multifactorial brain metabolic diseases. Neurobiol. Dis. 2020, 143, 105014. [Google Scholar] [CrossRef]
- Arrigoni, C.; Lopa, S.; Candrian, C.; Moretti, M. Organs-on-a-chip as model systems for multifactorial musculoskeletal diseases. Curr. Opin. Biotechnol. 2020, 63, 79–88. [Google Scholar] [CrossRef]
- Bashir, A.; Duseja, A.; De, A.; Mehta, M.; Tiwari, P. Non-alcoholic fatty liver disease development: A multifactorial pathogenic phenomena. Liver Res. 2022, 6, 72–83. [Google Scholar] [CrossRef]
- Brocardo, P.S.; Gil-Mohapel, J. Mechanisms underlying the neuropathology of huntington’s disease, a multifactorial neurodegenerative disorder. In Neuropathology: New Research; Nova Science Publishers Inc.: New York, NY, USA, 2012; pp. 55–74. [Google Scholar]
- Galea, E.; Launay, N.; Portero-Otin, M.; Ruiz, M.; Pamplona, R.; Aubourg, P.; Ferrer, I.; Pujol, A. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: A paradigm for multifactorial neurodegenerative diseases? Biochim. Biophys. Acta, Mol. Basis Dis. 2012, 1822, 1475–1488. [Google Scholar] [CrossRef] [Green Version]
- Shamsuzzama; Kumar, L.; Haque, R.; Nazir, A. Role of MicroRNA Let-7 in Modulating Multifactorial Aspect of Neurodegenerative Diseases: An Overview. Mol. Neurobiol. 2016, 53, 2787–2793. [Google Scholar] [CrossRef]
- Toprak, I.; Fenkci, S.M.; Fidan Yaylali, G.; Martin, C.; Yaylali, V. Early retinal neurodegeneration in preclinical diabetic retinopathy: A multifactorial investigation. Eye 2020, 34, 1100–1107. [Google Scholar] [CrossRef]
- Pavan, S.; Prabhu, A.N.; Prasad Gorthi, S.; Das, B.; Mutreja, A.; Shetty, V.; Ramamurthy, T.; Ballal, M. Exploring the multifactorial aspects of Gut Microbiome in Parkinson’s Disease. Folia Microbiol. 2022, 67, 693–706. [Google Scholar] [CrossRef]
- Albers, J.A.; Chand, P.; Anch, A.M. Multifactorial sleep disturbance in Parkinson’s disease. Sleep Med. 2017, 35, 41–48. [Google Scholar] [CrossRef]
- Kaur, R.; Mehan, S.; Singh, S. Understanding multifactorial architecture of Parkinson’s disease: Pathophysiology to management. Neurol. Sci. 2019, 40, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.M. Parkinson’s disease as multifactorial oxidative neurodegeneration: Implications for integrative management. Altern. Med. Rev. 2000, 5, 502–529. [Google Scholar] [PubMed]
- Riess, O.; Krüger, R. Parkinson’s disease—A multifactorial neurodegenerative disorder. In Diagnosis and Treatment of Parkinson’s Disease—State of the Art; Springer: Vienna, Austria, 1999; pp. 113–125. [Google Scholar]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ashraf, G.M.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Multi-Target Drug Candidates for Multifactorial Alzheimer’s Disease: AChE and NMDAR as Molecular Targets. Mol. Neurobiol. 2021, 58, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Mamun, A.A.; Rahman, M.A.; Behl, T.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Emerging proof of protein misfolding and interactions in multifactorial alzheimer’s disease. Curr. Top. Med. Chem. 2020, 20, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary polyphenols: A multifactorial strategy to target alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, C.X.; Liu, F.; Iqbal, K. Multifactorial Hypothesis and Multi-Targets for Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, S107–S117. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Hachinski, V.; Evans, A.C. The vascular facet of late-onset Alzheimer’s disease: An essential factor in a complex multifactorial disorder. Curr. Opin. Neurol. 2017, 30, 623–629. [Google Scholar] [CrossRef]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F.L.; Munteanu, M.A.; Brisc, M.C.; et al. Current trends in neurodegeneration: Cross talks between oxidative stress, cell death, and inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef]
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, e551–e567. [Google Scholar] [CrossRef]
- Ehrenberg, A.J.; Khatun, A.; Coomans, E.; Betts, M.J.; Capraro, F.; Thijssen, E.H.; Senkevich, K.; Bharucha, T.; Jafarpour, M.; Young, P.N.E.; et al. Relevance of biomarkers across different neurodegenerative. Alzheimer’s Res. Ther. 2020, 12, 56. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2022 Alzheimer’s Disease Facts and Figures; Alzheimer’s Association: Chicago, IL, USA, 2022; pp. 700–789. [Google Scholar]
- Abramov, A.Y.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V. Interaction of oxidative stress and misfolded proteins in the mechanism of neurodegeneration. Life 2020, 10, 101. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’Anca, M.; Galimberti, D.; Fenoglio, C.; Scarpini, E. Role of oxidative damage in alzheimer’s disease and neurodegeneration: From pathogenic mechanisms to biomarker discovery. Antioxidants 2021, 10, 1353. [Google Scholar] [CrossRef]
- Espinós, C.; Galindo, M.I.; García-Gimeno, M.A.; Ibáñez-Cabellos, J.S.; Martínez-Rubio, D.; Millán, J.M.; Rodrigo, R.; Sanz, P.; Seco-Cervera, M.; Sevilla, T.; et al. Oxidative stress, a crossroad between rare diseases and neurodegeneration. Antioxidants 2020, 9, 313. [Google Scholar] [CrossRef] [Green Version]
- Gkekas, I.; Gioran, A.; Boziki, M.K.; Grigoriadis, N.; Chondrogianni, N.; Petrakis, S. Oxidative stress and neurodegeneration: Interconnected processes in polyq diseases. Antioxidants 2021, 10, 1450. [Google Scholar] [CrossRef]
- Jantas, D.; Lasoń, W. Preclinical evidence for the interplay between oxidative stress and rip1-dependent cell death in neurodegeneration: State of the art and possible therapeutic implications. Antioxidants 2021, 10, 1518. [Google Scholar] [CrossRef]
- Jurcau, A. Insights into the pathogenesis of neurodegenerative diseases: Focus on mitochondrial dysfunction and oxidative stress. Int. J. Mol. Sci. 2021, 22, 11847. [Google Scholar] [CrossRef]
- Lee, Y.M.; He, W.; Liou, Y.C. The redox language in neurodegenerative diseases: Oxidative post-translational modifications by hydrogen peroxide. Cell Death Dis. 2021, 12, 58. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Mastroiacovo, F.; Polzella, M.; Lazzeri, G.; Fornai, F. Merging the multi-target effects of phytochemicals in neurodegeneration: From oxidative stress to protein aggregation and inflammation. Antioxidants 2020, 9, 1022. [Google Scholar] [CrossRef]
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimer’s Dis. 2021, 82, S109–S126. [Google Scholar] [CrossRef]
- Michalska, P.; León, R. When it comes to an end: Oxidative stress crosstalk with protein aggregation and neuroinflammation induce neurodegeneration. Antioxidants 2020, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, Oxidative Stress and Protein–Quinone Modifications in Parkinson’s and Other Neurodegenerative Diseases. Angew. Chem. Int. Ed. 2019, 58, 6512–6527. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Oh, C.K.; Zhang, X.; Lipton, S.A. Protein S-nitrosylation and oxidation contribute to protein misfolding in neurodegeneration. Free Rad. Biol. Med. 2021, 172, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial dysfunction, oxidative stress, and neuroinflammation: Intertwined roads to neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Gautam, P. A Review on Antioxidants as Therapeutic in Use of Oxidative Stress and Neurodegenerative Disease. Int. J. Pharm. Qual. Assur. 2022, 13, 77–82. [Google Scholar]
- Rivas, F.; Poblete-Aro, C.; Pando, M.E.; Allel, M.J.; Fernandez, V.; Soto, A.; Nova, P.; Garcia-Diaz, D. Effects of Polyphenols in Aging and Neurodegeneration Associated with Oxidative Stress. Curr. Med. Chem. 2022, 29, 1045–1060. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Kumar Jha, N.; Kumar Kesari, K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022, 38, 223–244. [Google Scholar] [CrossRef]
- Shi, X.; Li, P.; Liu, H.; Prokosch, V. Oxidative Stress, Vascular Endothelium, and the Pathology of Neurodegeneration in Retina. Antioxidants 2022, 11, 543. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. Ros generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Singh, E.; Devasahayam, G. Neurodegeneration by oxidative stress: A review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020, 47, 3133–3140. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ahmad, J.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020, 886, 173412. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [Green Version]
- Praticò, D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: Lights and shadows. Ann. N. Y. Acad. Sci. 2008, 1147, 70–78. [Google Scholar] [CrossRef]
- Schapira, A.H. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008, 7, 97–109. [Google Scholar] [CrossRef]
- Lev, N.; Ickowicz, D.; Melamed, E.; Offen, D. Oxidative insults induce DJ-1 upregulation and redistribution: Implications for neuroprotection. Neurotoxicology 2008, 29, 397–405. [Google Scholar] [CrossRef]
- Gandhi, S.; Wood-Kaczmar, A.; Yao, Z.; Plun-Favreau, H.; Deas, E.; Klupsch, K.; Downward, J.; Latchman, D.S.; Tabrizi, S.J.; Wood, N.W.; et al. PINK1-Associated Parkinson’s Disease Is Caused by Neuronal Vulnerability to Calcium-Induced Cell Death. Mol. Cell 2009, 33, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, A.; Bolognesi, M.L.; Mìnarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Chopade, P.; Chopade, N.; Zhao, Z.M.; Mitragotri, S.; Liao, R.; Suja, V.C. Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng. Transl. Med. 2022, e10367. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Moreta, M.P.G.; Burgos-Alonso, N.; Torrecilla, M.; Marco-Contelles, J.; Bruzos-Cidón, C. Efficacy of acetylcholinesterase inhibitors on cognitive function in alzheimer’s disease. Review of reviews. Biomedicines 2021, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Müller, T. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Drugs 2015, 75, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Nakamagoe, K.; Tsuji, H.; Ishii, K.; Tamaoka, A. Remarkable clinical responses of non-fluctuating Parkinson’s disease (PD) after alternating catechol O-methyltransferase inhibitors: Case series switching from entacapone 200~300 mg/day to opicapone 25 mg/day. Neurol. Sci. 2021, 42, 4813–4814. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Ferreira, J.J.; Rascol, O. COMT Inhibitors in the Management of Parkinson’s Disease. CNS Drugs 2022, 36, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Onge, E.S.; Vanderhoof, M.; Miller, S. Opicapone (Ongentys): A New COMT Inhibitor for the Treatment of Parkinson’s Disease. Ann. Pharmacother. 2021, 55, 1159–1166. [Google Scholar] [CrossRef]
- Finberg, J.P.M. Inhibitors of MAO-B and COMT: Their effects on brain dopamine levels and uses in Parkinson’s disease. J. Neural Transm. 2019, 126, 433–448. [Google Scholar] [CrossRef]
- Jost, W.H. A critical appraisal of MAO-B inhibitors in the treatment of Parkinson’s disease. J. Neural Transm. 2022, 129, 723–736. [Google Scholar] [CrossRef]
- Özdemir, Z.; Alagöz, M.A.; Bahçecioğlu, Ö.F.; Gök, S. Monoamine oxidase-B (MAO-B) inhibitors in the treatment of alzheimer’s and parkinson’s disease. Curr. Med. Chem. 2021, 28, 6045–6065. [Google Scholar] [CrossRef]
- Parambi, D.G.T. Treatment of parkinson’s disease by MAO-B inhibitors, new therapies and future challenges—A mini-review. Comb. Chem. High Throughput Screen. 2020, 23, 847–861. [Google Scholar] [CrossRef]
- Maan, G.; Sikdar, B.; Kumar, A.; Shukla, R.; Mishra, A. Role of flavonoids in neurodegenerative diseases: Limitations and future perspectives. Curr. Top. Med. Chem. 2020, 20, 1169–1194. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Nam, G.; Kang, J.; Lee, H.J.; Lee, M.; Lim, M.H. Development of multifunctional molecules as potential therapeutic candidates for Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis in the last decade. Chem. Rev. 2019, 119, 1221–1322. [Google Scholar] [CrossRef]
- Zięba, A.; Stępnicki, P.; Matosiuk, D.; Kaczor, A.A. What are the challenges with multi-targeted drug design for complex diseases? Expert Opin. Drug Discov. 2022, 17, 673–683. [Google Scholar] [CrossRef]
- Caruso, L.; Nadur, N.F.; da Fonseca, M.B.; Ferreira, L.A.P.; Lacerda, R.B.; Graebin, C.S.; Kümmerle, A.E. The Design of Multi-target Drugs to Treat Cardiovascular Diseases: Two (or more) Birds on One Stone. Curr. Top. Med. Chem. 2022, 22, 366–394. [Google Scholar] [CrossRef]
- Catto, M.; Trisciuzzi, D.; Alberga, D.; Mangiatordi, G.F.; Nicolotti, O. Multitarget drug design for neurodegenerative diseases. In Methods in Pharmacology and Toxicology; Springer Nature: Cham, Switzerland, 2019; pp. 93–105. [Google Scholar]
- De Freitas Silva, M.; Dias, K.S.T.; Gontijo, V.S.; Ortiz, C.J.C.; Viegas, C. Multi-target directed drugs as a modern approach for drug design towards Alzheimer’s disease: An update. Curr. Med. Chem. 2018, 25, 3491–3525. [Google Scholar] [CrossRef]
- Dias, K.S.T.; de Paula, C.T.; dos Santos, T.; Souza, I.N.O.; Boni, M.S.; Guimarães, M.J.R.; da Silva, F.M.R.; Castro, N.G.; Neves, G.A.; Veloso, C.C.; et al. Design, synthesis and evaluation of novel feruloyl-donepezil hybrids as potential multitarget drugs for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 130, 440–457. [Google Scholar] [CrossRef]
- Dias, K.S.T.; Viegas, C. Multi-Target directed drugs: A modern approach for design of new drugs for the treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2014, 12, 239–255. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Mu, Y.; Lei, H.; Wang, P.; Li, X.; Leng, Q.; Han, L.; Qu, X.; Wang, Z.; Huang, X. Design, synthesis and evaluation of novel tacrine-ferulic acid hybrids as multifunctional drug candidates against Alzheimer’s disease. Molecules 2016, 21, 1338. [Google Scholar] [CrossRef] [Green Version]
- Gontijo, V.S.; Dias Viegas, F.P.; Ortiz, C.J.C.; Silva, M.F.; Damasio, C.M.; Rosa, M.C.; Campos, T.G.; Couto, D.S.; Dias, K.S.T.; Viegas, C. Molecular hybridization as a tool in the design of multi-target directed drug candidates for neurodegenerative diseases. Curr. Neuropharmacol. 2020, 18, 348–407. [Google Scholar] [CrossRef]
- Halder, A.K.; Moura, A.S.; Cordeiro, M.N.D.S. Advanced chemometric modeling approaches for the design of multitarget drugs against neurodegenerative diseases. In Methods in Pharmacology and Toxicology; Springer Nature: Cham, Switzerland, 2019; pp. 155–186. [Google Scholar]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef] [PubMed]
- Katsamakas, S.; Hadjipavlou-Litina, D. Computational design of multitarget drugs against Alzheimer’s disease. In Methods in Pharmacology and Toxicology; Springer Nature: Cham, Switzerland, 2019; pp. 203–253. [Google Scholar]
- Katselou, M.G.; Matralis, A.N.; Kourounakis, A.P. Multi-target drug design approaches for multifactorial diseases: From neurodegenerative to cardiovascular applications. Curr. Med. Chem. 2014, 21, 2743–2787. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tiwari, A.; Sharma, A. Changing paradigm from one target one ligand towards multi-target directed ligand design for key drug targets of alzheimer disease: An important role of in silico methods in multi-target directed ligands design. Curr. Neuropharmacol. 2018, 16, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiang, X.; Luo, L.; Yang, X.; Xiao, G.; Zheng, Y.; Cao, Z.; Su, F.; Deng, Y.; Sang, Z. Multitarget drug design strategy against Alzheimer’s disease: Homoisoflavonoid Mannich base derivatives serve as acetylcholinesterase and monoamine oxidase B dual inhibitors with multifunctional properties. Bioorg. Med. Chem. 2017, 25, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.J.C.; de Freitas Silva, M.; Gontijo, V.S.; Viegas, F.P.D.; Dias, K.S.T.; Viegas, C. Design of multi-target directed ligands as a modern approach for the development of innovative drug candidates for Alzheimer’s disease. In Methods in Pharmacology and Toxicology; Springer Nature: Cham, Switzerland, 2019; pp. 255–351. [Google Scholar]
- Simoni, E.; Bartolini, M.; Abu, I.F.; Blockley, A.; Gotti, C.; Bottegoni, G.; Caporaso, R.; Bergamini, C.; Andrisano, V.; Cavalli, A.; et al. Multitarget drug design strategy in Alzheimer’s disease: Focus on cholinergic transmission and amyloid-β aggregation. Future Med. Chem. 2017, 9, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Huang, Z.; Meng, Q.; Liu, Z. Multi-target drug design of anti-alzheimer’s disease based on tacrine. Mini-Rev. Med. Chem. 2021, 21, 2039–2064. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef]
- Schneider, G.; Fechner, U. Computer-based de novo design of drug-like molecules. Nat. Rev. Drug Discov. 2005, 4, 649–663. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef]
- Zhong, H.; Mashinson, V.; Woolman, T.; Zha, M. Understanding the molecular properties and metabolism of top prescribed drugs. Curr. Top. Med. Chem. 2013, 13, 1290–1307. [Google Scholar] [CrossRef]
- Galano, A.; Pérez-González, A.; Castañeda-Arriaga, R.; Muñoz-Rugeles, L.; Mendoza-Sarmiento, G.; Romero-Silva, A.; Ibarra-Escutia, A.; Rebollar-Zepeda, A.M.; León-Carmona, J.R.; Hernández-Olivares, M.A.; et al. Empirically Fitted Parameters for Calculating pKaValues with Small Deviations from Experiments Using a Simple Computational Strategy. J. Chem. Inf. Model. 2016, 56, 1714–1724. [Google Scholar] [CrossRef]
- Pérez-González, A.; Castañeda-Arriaga, R.; Verastegui, B.; Carreón-González, M.; Alvarez-Idaboy, J.R.; Galano, A. Estimation of empirically fitted parameters for calculating pK a values of thiols in a fast and reliable way. Theor. Chem. Acc. 2018, 137, 5. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef]
- Miche, H.; Brumas, V.; Berthon, G. Copper(II) interactions with nonsteroidal antiinflammatory agents. II. Anthranilic acid as a potential OH-inactivating ligand. J. Inorg. Biochem. 1997, 68, 27–38. [Google Scholar] [CrossRef]
- Gaubert, S.; Bouchaut, M.; Brumas, V.; Berthon, G. Copper-ligand interactions and physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radic. Res. 2000, 32, 451–461. [Google Scholar] [CrossRef]
- Berthon, G. Is copper pro- or anti-inflammatory? A reconciling view and a novel approach for the use of copper in the control of inflammation. Agents Actions 1993, 39, 210–217. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ghosh, S.; Chatterji, A.; Chakraborty, K. Neuron-glia: Understanding cellular copper homeostasis, its cross-talk and their contribution towards neurodegenerative diseases. Metallomics 2020, 12, 1897–1911. [Google Scholar] [CrossRef]
- Giampietro, R.; Spinelli, F.; Contino, M.; Colabufo, N.A. The Pivotal Role of Copper in Neurodegeneration: A New Strategy for the Therapy of Neurodegenerative Disorders. Mol. Pharm. 2018, 15, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, H.; Luczkowski, M.; Remelli, M.; Valensin, D. Copper, zinc and iron in neurodegenerative diseases (Alzheimer’s, Parkinson’s and prion diseases). Coord. Chem. Rev. 2012, 256, 2129–2141. [Google Scholar] [CrossRef]
- Manto, M. Abnormal copper homeostasis: Mechanisms and roles in neurodegeneration. Toxics 2014, 2, 327–345. [Google Scholar] [CrossRef] [Green Version]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Viles, J.H. Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, zinc and iron in Alzheimer’s, Parkinson’s and prion diseases. Coord. Chem. Rev. 2012, 256, 2271–2284. [Google Scholar] [CrossRef]
- Castañeda-Arriaga, R.; Galano, A. Exploring Chemical Routes Relevant to the Toxicity of Paracetamol and Its meta-Analogue at a Molecular Level. Chem. Res. Toxicol. 2017, 30, 1286–1301. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. Cytoprotective antioxidant function of tyrosine and tryptophan residues in transmembrane proteins. Eur. J. Biochem. 2000, 267, 5687–5692. [Google Scholar] [CrossRef] [Green Version]
- Watts, Z.I.; Easton, C.J. Peculiar stability of amino acids and peptides from a radical perspective. J. Am. Chem. Soc. 2009, 131, 11323–11325. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Domazou, A.S.; Koppenol, W.H.; Gebicki, J.M. Efficient repair of protein radicals by ascorbate. Free Radic. Biol. Med. 2009, 46, 1049–1057. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Nauser, T.; Domazou, A.; Steinmann, D.; Bounds, P.L.; Koppenol, W.H. Reduction of protein radicals by GSH and ascorbate: Potential biological significance. Amino Acids 2010, 39, 1131–1137. [Google Scholar] [CrossRef]
- Oreilly, R.J.; Chan, B.; Taylor, M.S.; Ivanic, S.; Bacskay, G.B.; Easton, C.J.; Radom, L. Hydrogen abstraction by chlorine atom from amino acids: Remarkable influence of polar effects on regioselectivity. J. Am. Chem. Soc. 2011, 133, 16553–16559. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [Green Version]
- Reid, D.L.; Armstrong, D.A.; Rauk, A.; Von Sonntag, C. H-atom abstraction by thiyl radicals from peptides and cyclic dipeptides. A theoretical study of reaction rates. Phys. Chem. Chem. Phys. 2003, 5, 3994–3999. [Google Scholar] [CrossRef]
- Doan, H.Q.; Davis, A.C.; Francisco, J.S. Primary steps in the reaction of OH radicals with peptide systems: Perspective from a study of model amides. J. Phys. Chem. A 2010, 114, 5342–5357. [Google Scholar] [CrossRef]
- Chan, B.; O’Reilly, R.J.; Easton, C.J.; Radom, L. Reactivities of Amino Acid Derivatives Toward Hydrogen Abstraction by Cl• and OH•. J. Org. Chem. 2012, 77, 9807–9812. [Google Scholar] [CrossRef]
- Owen, M.C.; Szóri, M.; Csizmadia, I.G.; Viskolcz, B. Conformation-dependent OH/H2O2 hydrogen abstraction reaction cycles of Gly and Ala residues: A comparative theoretical study. J. Phys. Chem. B 2012, 116, 1143–1154. [Google Scholar] [CrossRef]
- Mujika, J.I.; Uranga, J.; Matxain, J.M. Computational study on the attack of ·oH radicals on aromatic amino acids. Chem. Eur. J. 2013, 19, 6862–6873. [Google Scholar] [CrossRef]
- Thomas, D.A.; Sohn, C.H.; Gao, J.; Beauchamp, J.L. Hydrogen bonding constrains free radical reaction dynamics at serine and threonine residues in peptides. J. Phys. Chem. A 2014, 118, 8380–8392. [Google Scholar] [CrossRef] [Green Version]
- Domazou, A.S.; Gebicka, L.; Didik, J.; Gebicki, J.L.; Van Der Meijden, B.; Koppenol, W.H. The kinetics of the reaction of nitrogen dioxide with iron(II)- and iron(III) cytochrome c. Free Radic. Biol. Med. 2014, 69, 172–180. [Google Scholar] [CrossRef]
- Medina, M.E.; Galano, A.; Alvarez-Idaboy, J.R. Site reactivity in the free radicals induced damage to leucine residues: A theoretical study. Phys. Chem. Chem. Phys. 2015, 17, 4970–4976. [Google Scholar] [CrossRef]
- Amos, R.I.J.; Chan, B.; Easton, C.J.; Radom, L. Hydrogen-atom abstraction from a model amino acid: Dependence on the attacking radical. J. Phys. Chem. B 2015, 119, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Rugeles, L.; Alvarez-Idaboy, J.R. A proton-electron sequential transfer mechanism: Theoretical evidence about its biological relevance. Phys. Chem. Chem. Phys. 2015, 17, 28525–28528. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Arriaga, R.; Mora-Diez, N.; Alvarez-Idaboy, J.R. Modelling the chemical repair of protein carbon-centered radicals formed via oxidative damage with dihydrolipoic acid. RSC Adv. 2015, 5, 96714–96719. [Google Scholar] [CrossRef]
- Seidel, C.A.M.; Schulz, A.; Sauer, M.H.M. Nucleobase-Specific Quenching of Fluorescent Dyes. 1. Nucleobase One-Electron Redox Potentials and Their Correlation with Static and Dynamic Quenching Efficiencies. J. Phys. Chem. 1996, 100, 5541–5553. [Google Scholar] [CrossRef]
- Sugiyama, H.; Saito, I. Theoretical Studies of GG-Specific Photocleavage of DNA via Electron Transfer: Significant Lowering of Ionization Potential and 5‘-Localization of HOMO of Stacked GG Bases in B-Form DNA. J. Am. Chem. Soc. 1996, 118, 7063–7068. [Google Scholar] [CrossRef]
- Melvin, T.; Botchway, S.W.; Parker, A.W.; O’Neill, P. Induction of Strand Breaks in Single-Stranded Polyribonucleotides and DNA by Photoionization: One Electron Oxidized Nucleobase Radicals as Precursors. J. Am. Chem. Soc. 1996, 118, 10031–10036. [Google Scholar] [CrossRef]
- Wetmore, S.D.; Boyd, R.J.; Eriksson, L.A. Electron affinities and ionization potentials of nucleotide bases. Chem. Phys. Lett. 2000, 322, 129–135. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively Generated Damage to the Guanine Moiety of DNA: Mechanistic Aspects and Formation in Cells. Acc. Chem. Res. 2008, 41, 1075–1083. [Google Scholar] [CrossRef]
- Steenken, S.; Jovanovic, S.V. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. On the evolution of one-electron-oxidized deoxyguanosine in damaged DNA under physiological conditions: A DFT and ONIOM study on proton transfer and equilibrium. Phys. Chem. Chem. Phys. 2012, 14, 12476–12484. [Google Scholar] [CrossRef]
- Tronche, C.; Goodman, B.K.; Greenberg, M.M. DNA damage induced via independent generation of the radical resulting from formal hydrogen atom abstraction from the C1’-position of a nucleotide. Chem. Biol. 1998, 5, 263–271. [Google Scholar] [CrossRef]
- Pogozelski, W.K.; Tullius, T.D. Oxidative Strand Scission of Nucleic Acids: Routes Initiated by Hydrogen Abstraction from the Sugar Moiety. Chem. Rev. 1998, 98, 1089–1108. [Google Scholar] [CrossRef]
- Dedon, P.C. The Chemical Toxicology of 2-Deoxyribose Oxidation in DNA. Chem. Res. Toxicol. 2007, 21, 206–219. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, Y.; Niedernhofer, L.J.; Wang, Y. Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage. Chem. Res. Toxicol. 2016, 29, 2008–2039. [Google Scholar] [CrossRef] [Green Version]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Roszkowski, K.; Jozwicki, W.; Blaszczyk, P.; Mucha-Malecka, A.; Siomek, A. Oxidative damage DNA: 8-oxogua and 8-oxodG as molecular markers of cancer. Med. Sci. Monit. 2011, 17, CR329–CR333. [Google Scholar] [CrossRef] [Green Version]
- Pérez-González, A.; Castañeda-Arriaga, R.; Álvarez-Idaboy, J.R.; Reiter, R.J.; Galano, A. Melatonin and its metabolites as chemical agents capable of directly repairing oxidized DNA. J. Pineal Res. 2019, 66, e12539. [Google Scholar] [CrossRef]
- Castañeda-Arriaga, R.; Pérez-González, A.; Reina, M.; Galano, A. Computer-designed melatonin derivatives: Potent peroxyl radical scavengers with no pro-oxidant behavior. Theor. Chem. Acc. 2020, 139, 133. [Google Scholar] [CrossRef]
- Castro-González, L.M.; Alvarez-Idaboy, J.R.; Galano, A. Computationally Designed Sesamol Derivatives Proposed as Potent Antioxidants. ACS Omega 2020, 5, 9566–9575. [Google Scholar] [CrossRef] [Green Version]
- Castro-González, L.M.; Galano, A.; Alvarez-Idaboy, J.R. Free radical scavenging activity of newly designed sesamol derivatives. New J. Chem. 2021, 45, 11960–11967. [Google Scholar] [CrossRef]
- Galano, A.; Guzmán-López, E.G.; Reiter, R.J. Potentiating the benefits of melatonin through chemical functionalization: Possible impact on multifactorial neurodegenerative disorders. Int. J. Mol. Sci. 2021, 22, 11584. [Google Scholar] [CrossRef] [PubMed]
- Millán-Pacheco, C.; Serratos, I.N.; del Rosario Sánchez González, S.; Galano, A. Newly designed melatonin analogues with potential neuroprotective effects. Theor. Chem. Acc. 2022, 141, 49. [Google Scholar] [CrossRef]
- Reina, M.; Guzmán-López, E.G.; Romeo, I.; Marino, T.; Russo, N.; Galano, A. Computationally designed: P -coumaric acid analogs: Searching for neuroprotective antioxidants. New J. Chem. 2021, 45, 14369–14380. [Google Scholar] [CrossRef]
- Reina, M.; Castañeda-Arriaga, R.; Pérez-González, A.; Guzman-Lopez, E.; Tan, D.-X.; Reiter, R.; Galano, A. A Computer-Assisted Systematic Search for Melatonin Derivatives with High Potential as Antioxidants. Melatonin Res. 2018, 1, 27–58. [Google Scholar] [CrossRef] [Green Version]
- Galano, A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys. Chem. Chem. Phys. 2011, 13, 7178–7188. [Google Scholar] [CrossRef]
- Alberto, M.E.; Russo, N.; Grand, A.; Galano, A. A physicochemical examination of the free radical scavenging activity of Trolox: Mechanism, kinetics and influence of the environment. Phys. Chem. Chem. Phys. 2013, 15, 4642–4650. [Google Scholar] [CrossRef]
- Ingold, K.U.; Bowry, V.W.; Stockers, R.; Walling, C. Autoxidation of lipids and antioxidation by a-tocopherol and ubiquinol in homogeneous solution and in aqueous dispersions of lipids: Unrecognized consequences of lipid particle size as exemplified by oxidation of human low density lipoprotein. Proc. Nati. Acad. Sci. USA 1993, 90, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O−2 radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal Res. 2015, 58, 107–116. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Diallo, M.S.; Goddard Iii, W.A. Computational Study of Copper(II) Complexation and Hydrolysis in Aqueous Solutions Using Mixed Cluster/Continuum Models. J. Phys. Chem. A 2009, 113, 9559–9567. [Google Scholar] [CrossRef] [Green Version]
- Learmonth, D.A.; Bonifácio, M.J.; Soares-da-Silva, P. Synthesis and Biological Evaluation of a Novel Series of “Ortho-Nitrated” Inhibitors of Catechol-O-methyltransferase. J. Med. Chem. 2005, 48, 8070–8078. [Google Scholar] [CrossRef]
- Shafferman, A.; Kronman, C.; Flashner, Y.; Leitner, M.; Grosfeld, H.; Ordentlich, A.; Gozes, Y.; Cohen, S.; Ariel, N.; Barak, D.; et al. Mutagenesis of human acetylcholinesterase. Identification of residues involved in catalytic activity and in polypeptide folding. J. Biol. Chem. 1992, 267, 17640–17648. [Google Scholar] [CrossRef]
- Fallarero, A.; Oinonen, P.; Gupta, S.; Blom, P.; Galkin, A.; Mohan, C.G.; Vuorela, P.M. Inhibition of acetylcholinesterase by coumarins: The case of coumarin 106. Pharmacol. Res. 2008, 58, 215–221. [Google Scholar] [CrossRef]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of Human Monoamine Oxidase B Complexes with Selective Noncovalent Inhibitors: Safinamide and Coumarin Analogs. J. Med. Chem. 2007, 50, 5848–5852. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Ahmed, J.; Pullattayil, S.A.K.; Robertson, N.J.; More, K. Melatonin for neuroprotection in neonatal encephalopathy: A systematic review & meta-analysis of clinical trials. Eur. J. Paediatr. Neurol. 2021, 31, 38–45. [Google Scholar]
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef] [Green Version]
- Balmik, A.A.; Chinnathambi, S. Multi-Faceted Role of Melatonin in Neuroprotection and Amelioration of Tau Aggregates in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1481–1493. [Google Scholar] [CrossRef]
- Melhuish Beaupre, L.M.; Brown, G.M.; Gonçalves, V.F.; Kennedy, J.L. Melatonin’s neuroprotective role in mitochondria and its potential as a biomarker in aging, cognition and psychiatric disorders. Transl. Psychiatry 2021, 11, 339. [Google Scholar] [CrossRef]
- Calculation of Molecular Properties and Bioactivity Score. 2021. Available online: https://www.molinspiration.com/cgi-bin/properties (accessed on 3 March 2022).
- Drug Likeness Tool (DruLiTo 1). Available online: http://www.niper.gov.in/pi_dev_tools/DruLiToWeb/DruLiTo_index.html (accessed on 8 February 2020).
- Zhu, H.; Tropsha, A.; Fourches, D.; Varnek, A.; Papa, E.; Gramatica, P.; Öberg, T.; Dao, P.; Cherkasov, A.; Tetko, I.V. Combinatorial QSAR Modeling of Chemical Toxicants Tested against Tetrahymena pyriformis. J. Chem. Inf. Model. 2008, 48, 766–784. [Google Scholar] [CrossRef] [Green Version]
- Boda, K.; Seidel, T.; Gasteiger, J. Structure and reaction based evaluation of synthetic accessibility. J. Comput. Aided Mol. Des. 2007, 21, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, P. Is chemical synthetic accessibility computationally predictable for drug and lead-like molecules? A comparative assessment between medicinal and computational chemists. Eur. J. Med. Chem. 2012, 54, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ellermann, M.; Lerner, C.; Burgy, G.; Ehler, A.; Bissantz, C.; Jakob-Roetne, R.; Paulini, R.; Allemann, O.; Tissot, H.; Grünstein, D.; et al. Catechol-O-methyltransferase in complex with substituted 3’-deoxyribose bisubstrate inhibitors. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 253–260. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- BIOVIA. Available online: https://www.3ds.com/products-services/biovia/ (accessed on 10 September 2022).



| Lipinski’s Rule | Ghose’s Rule | Veber’s Criteria | |
|---|---|---|---|
| H bond donors (HBD) | No more than 5 | ||
| H bond acceptors (HBA) | No more than 10 | ||
| Molecular weight (MW) | Under 500 | From 160 to 480 | |
| Octanol/water partition coefficient (logP) | Lower than 5 | From −0.4 to 5.6 | |
| Molar refractivity (MR) | From 40 to 130 | ||
| Number of non-hydrogen atoms (XAt) | From 20 to 70 | ||
| Polar surface area (PSA) | No larger than 140 Å2 | ||
| HBDA = HBD + HBA | No higher than 12 |
| Functional Group | m | C0 | Ref. |
|---|---|---|---|
| Phenol | 0.316 | −81.497 | [102] |
| Carboxylic acid | 0.356 | −94.380 | [102] |
| Amine | 0.464 | −121.000 | [102] |
| Thiol | 0.357 | −94.639 | [103] |
| Reductant | Cu(II) | ΔG (kcal/mol) | ΔG≠ (kcal/mol) | k (M−1 s−1) |
|---|---|---|---|---|
| Ascorbate | ‘free’ | −1.53 | 6.89 | 5.44 × 107 |
| in CDCM-cSO | 17.46 | 19.07 | 6.46 × 10−2 | |
| O2•− | ‘free’ | −20.86 | 3.89 | 2.07 × 109 |
| in CDCM-cSO | −1.88 | 11.46 | 2.46 × 104 |
| Lipid (a) | Site Numbering | ||||
|---|---|---|---|---|---|
| site 1 | −0.37 | 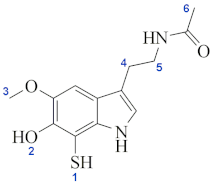 | |||
| site 2 | 5.69 | ||||
| site 3 | 26.25 | ||||
| site 4 | 15.43 | ||||
| site 5 | 23.02 | ||||
| site 6 | 24.75 | ||||
| Residues (b) | Leu | Cys | Tyr | His | Met |
| site 2 | −23.56 | −15.03 | −18.83 | −19.10 | −22.99 |
| site 3 | 6.18 | 14.70 | 10.90 | 10.64 | 6.74 |
| site 4 | −3.02 | 5.51 | 1.71 | 1.44 | −2.45 |
| site 5 | 2.86 | 11.39 | 7.59 | 7.32 | 3.43 |
| site 6 | 12.92 | 21.45 | 17.65 | 17.38 | 13.49 |
| DNA (b) | C4● | 8-OH-dG | DNA (b) | C4● | 8-OH-dG |
| site 2 | −24.41 | −6.32 | site 5 | 2.01 | 20.10 |
| site 3 | 5.32 | 23.42 | site 6 | 12.07 | 30.16 |
| site 4 | −3.87 | 14.22 | |||
| (ΔG≠) (kcal/mol) | if (cm−1) | κ | k (M−1 s−1) | |
|---|---|---|---|---|
| LM | 21.88 | 1564.19 | 17.60 | 1.00 × 10−2 |
| Leu | 15.93 | 2306.68 | 10.43 | 1.35 × 102 |
| Cys | 5.72 | 1166.33 | 1.00 | 3.96 × 108 |
| Tyr | 7.57 | 1575.39 | 1.00 | 1.74 × 107 |
| His | 16.15 | 3005.12 | 24.37 | 2.20 × 102 |
| Met | 12.44 | 1969.26 | 1.00 | 4.69 × 103 |
| 2dG, C4● | 15.92 | 2623.21 | 34.16 | 4.50 × 102 |
| 8-OH-dG | 9.25 | 1219.05 | 1.00 | 1.03 × 106 |
| DBM | dM38 | ΔG (kcal/mol) | ΔG≠ (kcal/mol) | k (M−1 s−1) | k Mf (M−1 s−1) | ktot, SET (M−1 s−1) |
|---|---|---|---|---|---|---|
| Tyr | Neutral | −16.43 | 0.52 | 7.98 × 109 | 2.44 × 108 | |
| Anion | −38.53 | 23.25 | 5.63 × 10−5 | 5.45 × 10−5 | 2.44 × 108 | |
| Trp | Neutral | −3.41 | 1.10 | 7.93 × 109 | 2.43 × 108 | |
| Anion | −25.52 | 10.13 | 2.35 × 105 | 2.28 × 105 | 2.43 × 108 | |
| 2dG+● | Neutral | −11.00 | 0.06 | 7.99 × 109 | 2.45 × 108 | |
| Anion | −33.10 | 12.21 | 6.90 × 103 | 6.69 × 103 | 2.45 × 108 |
| Compound | COMT | MAOB | AChE | |||
|---|---|---|---|---|---|---|
| ΔGb (Kcal/mol) | Ki (μmol/L) | ΔGb (Kcal/mol) | Ki (μmol/L) | ΔGb (Kcal/mol) | Ki (μmol/L) | |
| dM38 | −7.42 | 3.59 | −7.30 | 4.40 | −8.04 | 1.26 |
| melatonin | −6.28 | 24.64 | −5.29 | 131.24 | −6,90 | 8.64 |
| substrate | −6.16 | 30.18 | −5.10 | 129.05 | −4.72 | 343.85 |
| inhibitor | −8.60 | 0.49 | −9.50 | 0.11 | −12.16 | 1.2 × 10−3 |
| Software | Details | Used for | Ref. |
|---|---|---|---|
| Smile-It | As implemented. | Generation of the derivatives structures and theirs smiles. | a |
| Molinspiration Property Calculation Service and DruLiTo software. | As implemented. | Number of donors in H-bond interactions (HBD), number of acceptors in H-bond interactions (HBA), molecular weight (MW), octanol/water partition coefficient (log P), Molar refractivity (MR), Number of non-hydrogen atoms (AtX), Number of rotatable bonds (RB) and Polar surface area (PSA). | [169,170] |
| Toxicity Estimation Software Tool (T.E.S.T.), version 4.1 | Consensus method. | Ames mutagenicity (M) and the oral rat 50 percent lethal dose (LD50) descriptors. | [171] |
| SYLVIA-XT 1.4 program (Molecular Networks, Erlangen, Germany) | Values from 1 to 10. High values imply more difficult synthesis. | Synthetic accessibility (SA). | [172,173] |
| Gaussian 09 | M05-2X/6-311+G(d,p) and the SMD [174] continuum solvation model (with water and/or pentyl ethanoate as solvent). | Ionization energies (IE), electron affinities (EA), bond dissociation energies (BDE), energies of the free radical scavenging reactions, energies of reactions involved in repairing biological molecules. | [175] |
| M05/6-311+G(d,p) and the SMD [174] continuum solvation model (with water as solvent). | Energies of reactions involving copper (i.e., chelation reactions, and reactions of the CDCM-cSO complex with ascorbate and superoxide radical anion). | ||
| Chimera 1.16 | Crystalline structures of COMT (ID: 4YL), [176] MAOB (ID: 2V5Z) [163] and AChE (ID:4EY7) [164] were obtained from the protein data bank. | Protein preparation. | [177] |
| AutoDock Vina | Lamarckian genetic algorithm, 150 individual steps in population with 2.5 × 104 evaluations. | Docking simulations: free binding energy (ΔGU) and inhibition constants (Ki) | [178] |
| Discovery Studio 2021. | As implemented. | Processing and analysis of the best conformations. | [179] |
| MarcusKin | At 298.15 K and 1M standard state, considering diffusion. | Calculating rate constants for electron transfer reactions. | b |
| EasyRate 1.0 | At 298.15 K and 1M standard state; considering diffusion, reaction path degeneracy, and tunneling corrections. | Calculating rate constants for H transfer and radical adduct formation reactions. | c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzman-Lopez, E.G.; Reina, M.; Perez-Gonzalez, A.; Francisco-Marquez, M.; Hernandez-Ayala, L.F.; Castañeda-Arriaga, R.; Galano, A. CADMA-Chem: A Computational Protocol Based on Chemical Properties Aimed to Design Multifunctional Antioxidants. Int. J. Mol. Sci. 2022, 23, 13246. https://doi.org/10.3390/ijms232113246
Guzman-Lopez EG, Reina M, Perez-Gonzalez A, Francisco-Marquez M, Hernandez-Ayala LF, Castañeda-Arriaga R, Galano A. CADMA-Chem: A Computational Protocol Based on Chemical Properties Aimed to Design Multifunctional Antioxidants. International Journal of Molecular Sciences. 2022; 23(21):13246. https://doi.org/10.3390/ijms232113246
Chicago/Turabian StyleGuzman-Lopez, Eduardo Gabriel, Miguel Reina, Adriana Perez-Gonzalez, Misaela Francisco-Marquez, Luis Felipe Hernandez-Ayala, Romina Castañeda-Arriaga, and Annia Galano. 2022. "CADMA-Chem: A Computational Protocol Based on Chemical Properties Aimed to Design Multifunctional Antioxidants" International Journal of Molecular Sciences 23, no. 21: 13246. https://doi.org/10.3390/ijms232113246
APA StyleGuzman-Lopez, E. G., Reina, M., Perez-Gonzalez, A., Francisco-Marquez, M., Hernandez-Ayala, L. F., Castañeda-Arriaga, R., & Galano, A. (2022). CADMA-Chem: A Computational Protocol Based on Chemical Properties Aimed to Design Multifunctional Antioxidants. International Journal of Molecular Sciences, 23(21), 13246. https://doi.org/10.3390/ijms232113246







