Siponimod Modulates the Reaction of Microglial Cells to Pro-Inflammatory Stimulation
Abstract
:1. Introduction
2. Results
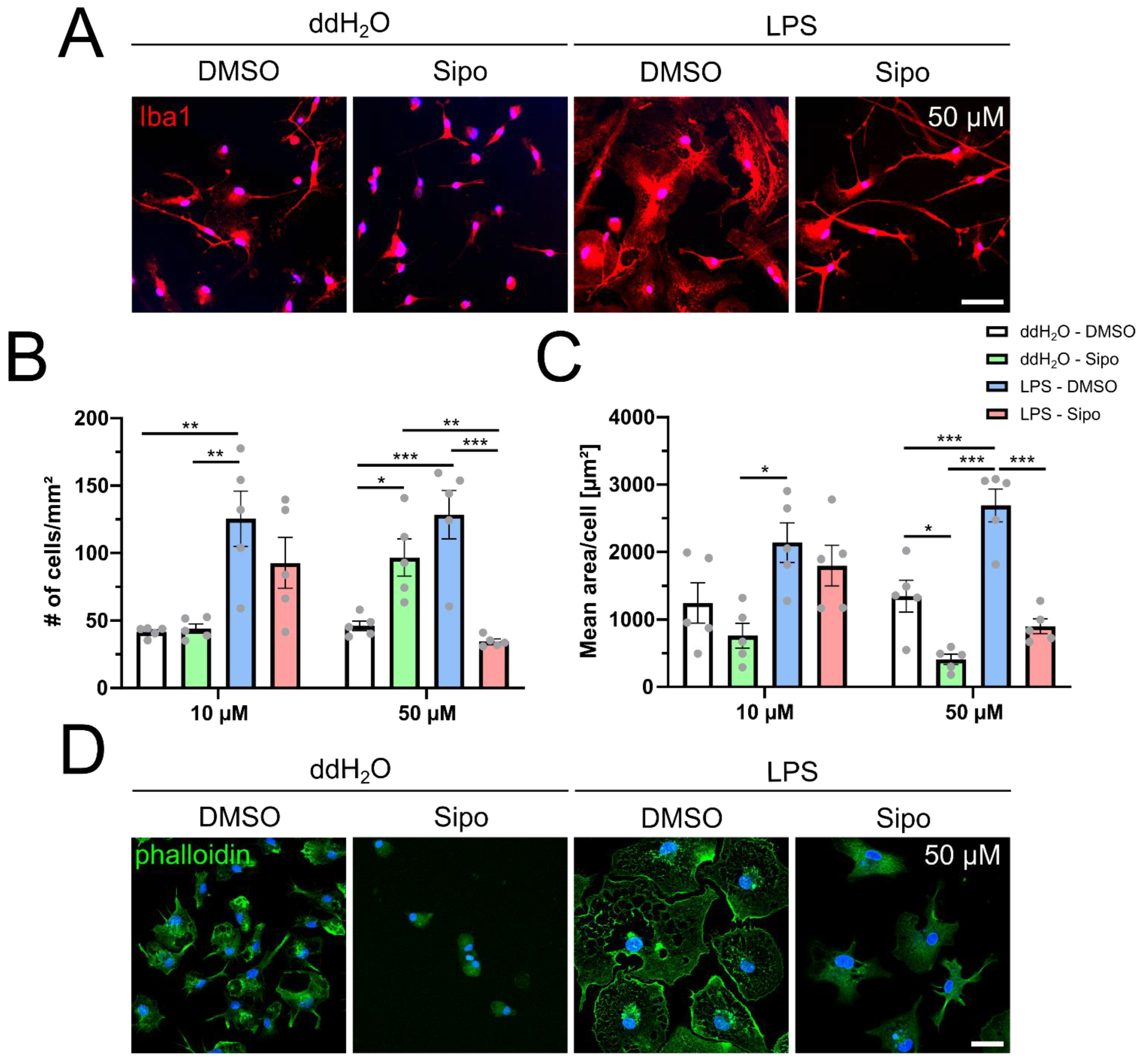
2.1. Siponimod Modulates Microglial Morphology and Actin Filament Organization
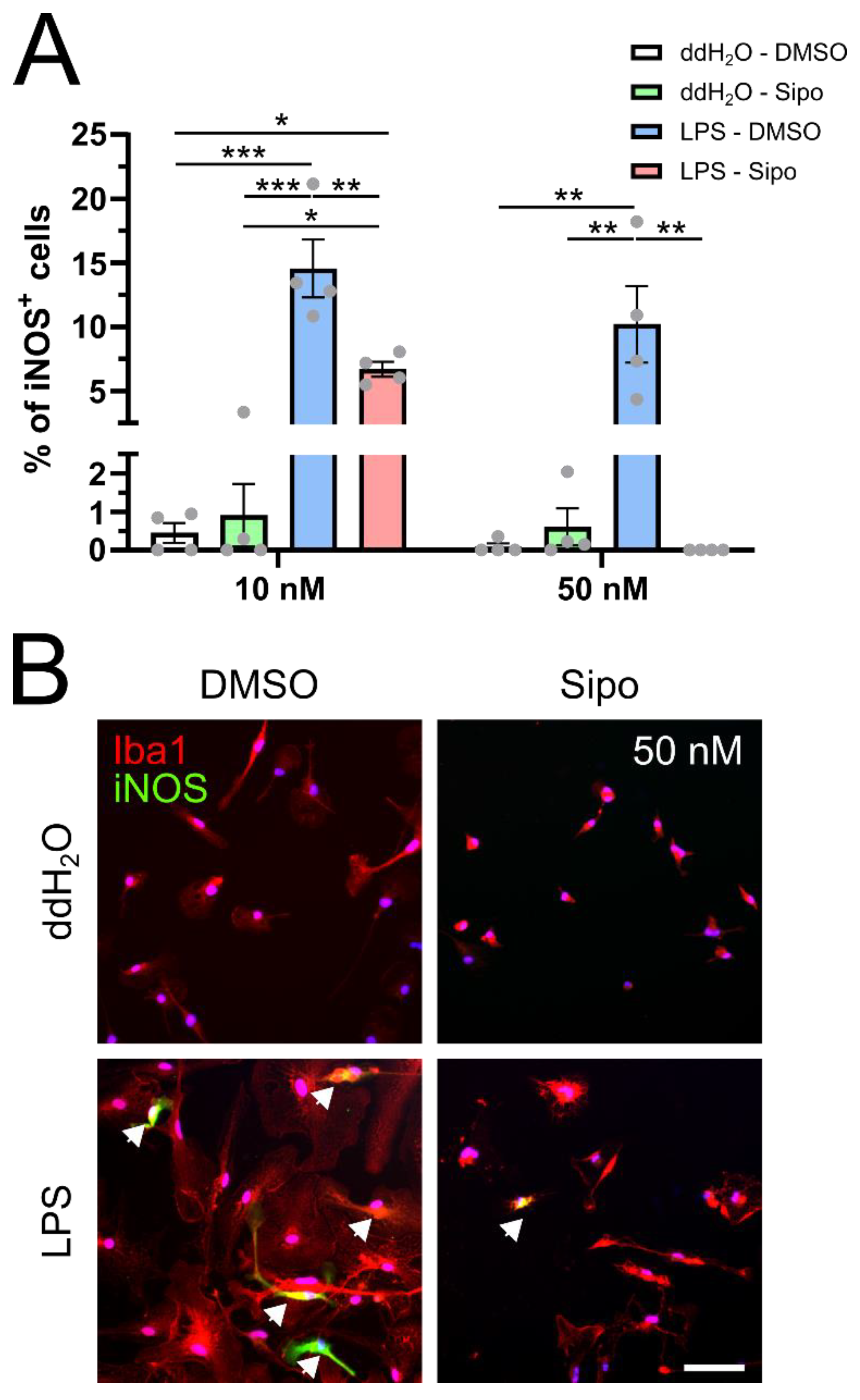
2.2. Siponimod Modulates iNOS Protein Expression
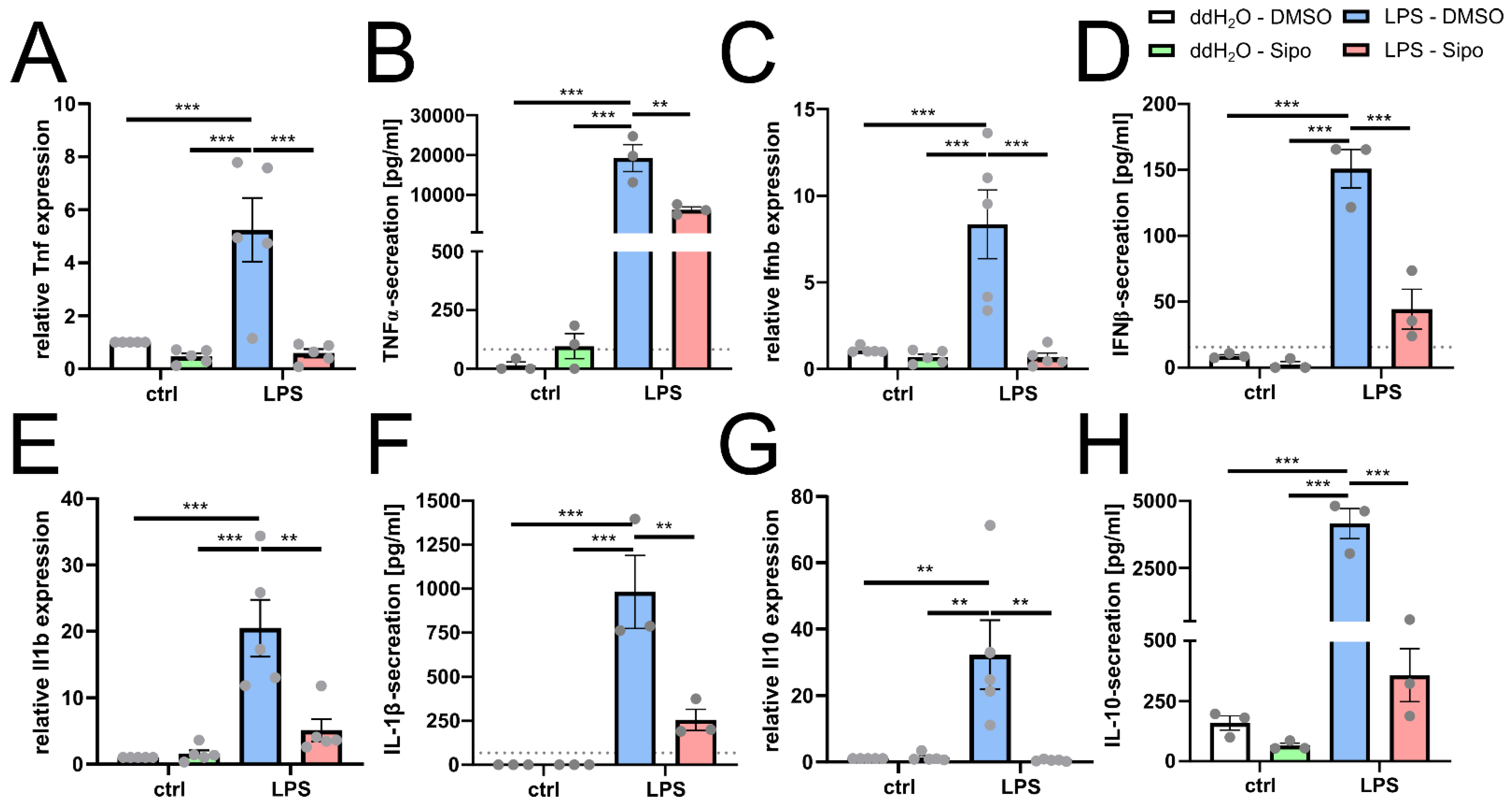
2.3. Siponimod Modulates Microglial Cytokine Gene Expression
2.4. Siponimod Modulates Immunological Signature in Pro-Inflammatory Triggered Microglial Cells
3. Discussion
4. Materials and Methods
4.1. Primary Rat Microglial Cell Culture
4.2. Immunocytochemistry
4.3. RNA Preparation, cDNA Synthesis and Quantitative Reverse Transcription (RT)-Polymerase Chain Reaction (PCR)
4.4. Bulk RNA Sequencing
4.5. ELISA
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trapp, B.D.; Peterson, J.; Ransohoff, R.M.; Rudick, R.; Mork, S.; Bo, L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998, 338, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Li, D.K.; Stuve, O.; Hartung, H.P.; Freedman, M.S.; Hemmer, B.; Rieckmann, P.; Montalban, X.; Ziemssen, T.; Hunter, B.; et al. Safety and Efficacy of Siponimod (BAF312) in Patients With Relapsing-Remitting Multiple Sclerosis: Dose-Blinded, Randomized Extension of the Phase 2 BOLD Study. JAMA Neurol. 2016, 73, 1089–1098. [Google Scholar] [CrossRef] [Green Version]
- Kappos, L.; Bar-Or, A.; Cree, B.A.C.; Fox, R.J.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnold, D.L.; Arnould, S.; Scherz, T.; et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018, 391, 1263–1273. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Schubart, A.; Mir, A.K.; Dev, K.K. The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J. Neuroinflamm. 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, A.; Musella, A.; Bullitta, S.; Fresegna, D.; De Vito, F.; Fantozzi, R.; Piras, E.; Gargano, F.; Borsellino, G.; Battistini, L.; et al. Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. J. Neuroinflamm. 2016, 13, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipp, M. Does Siponimod Exert Direct Effects in the Central Nervous System? Cells 2020, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Prinz, M.; van Loo, G. Microglia in Central Nervous System Inflammation and Multiple Sclerosis Pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Karamita, M.; Barnum, C.; Mobius, W.; Tansey, M.G.; Szymkowski, D.E.; Lassmann, H.; Probert, L. Therapeutic inhibition of soluble brain TNF promotes remyelination by increasing myelin phagocytosis by microglia. JCI Insight 2017, 2, e87455. [Google Scholar] [CrossRef] [Green Version]
- Tham, C.S.; Lin, F.F.; Rao, T.S.; Yu, N.; Webb, M. Microglial activation state and lysophospholipid acid receptor expression. Int. J. Dev. Neurosci. 2003, 21, 431–443. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Jack, C.S.; Arbour, N.; Manusow, J.; Montgrain, V.; Blain, M.; McCrea, E.; Shapiro, A.; Antel, J.P. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 2005, 175, 4320–4330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardin, A.; Dodman, A.; Kalluri, S.; Neelakantham, S.; Tan, X.; Legangneux, E.; Shakeri-Nejad, K. Pharmacokinetics, safety, and tolerability of siponimod (BAF312) in subjects with severe renal impairment: A single-dose, open-label, parallel-group study. Int. J. Clin. Pharmacol. Ther. 2017, 55, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Bigaud, M.; Rudolph, B.; Briard, E.; Beerli, C.; Hofmann, A.; Hermes, E.; Muellershausen, F.; Schubart, A.; Gardin, A. Siponimod (BAF312) penetrates, distributes, and acts in the central nervous system: Preclinical insights. Mult. Scler. J.-Exp. Transl. Clin. 2021, 7, 20552173211049168. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Basset, E.; Fedoroff, S. Effect of bacterial wall lipopolysaccharide (LPS) on morphology, motility, and cytoskeletal organization of microglia in cultures. J. Neurosci. Res. 1995, 41, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Lassmann, H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002, 1, 232–241. [Google Scholar] [CrossRef]
- Tang, X.; Lan, M.; Zhang, M.; Yao, Z. Effect of nitric oxide to axonal degeneration in multiple sclerosis via downregulating monocarboxylate transporter 1 in oligodendrocytes. Nitric Oxide 2017, 67, 75–80. [Google Scholar] [CrossRef]
- Gobel, K.; Ruck, T.; Meuth, S.G. Cytokine signaling in multiple sclerosis: Lost in translation. Mult. Scler. 2018, 24, 432–439. [Google Scholar] [CrossRef]
- Tolosa, L.; Caraballo-Miralles, V.; Olmos, G.; Llado, J. TNF-alpha potentiates glutamate-induced spinal cord motoneuron death via NF-kappaB. Mol. Cell. Neurosci. 2011, 46, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Sharief, M.K.; Hentges, R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N. Engl. J. Med. 1991, 325, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Motta, C.; Studer, V.; Macchiarulo, G.; Volpe, E.; Barbieri, F.; Ruocco, G.; Buttari, F.; Finardi, A.; Mancino, R.; et al. Interleukin-1beta causes excitotoxic neurodegeneration and multiple sclerosis disease progression by activating the apoptotic protein p53. Mol. Neurodegener. 2014, 9, 56. [Google Scholar] [CrossRef]
- Imitola, J.; Chitnis, T.; Khoury, S.J. Cytokines in multiple sclerosis: From bench to bedside. Pharmacol. Ther. 2005, 106, 163–177. [Google Scholar] [CrossRef]
- Kwilasz, A.J.; Grace, P.M.; Serbedzija, P.; Maier, S.F.; Watkins, L.R. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 2015, 96, 55–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cua, D.J.; Hutchins, B.; LaFace, D.M.; Stohlman, S.A.; Coffman, R.L. Central nervous system expression of IL-10 inhibits autoimmune encephalomyelitis. J. Immunol. 2001, 166, 602–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolivar, S.; Anfossi, R.; Humeres, C.; Vivar, R.; Boza, P.; Munoz, C.; Pardo-Jimenez, V.; Olivares-Silva, F.; Diaz-Araya, G. IFN-beta Plays Both Pro- and Anti-inflammatory Roles in the Rat Cardiac Fibroblast Through Differential STAT Protein Activation. Front. Pharmacol. 2018, 9, 1368. [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 2013, 256, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Giovannoni, G.; Baker, D. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflamm. 2011, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Sucksdorff, M.; Rissanen, E.; Tuisku, J.; Nuutinen, S.; Paavilainen, T.; Rokka, J.; Rinne, J.; Airas, L. Evaluation of the Effect of Fingolimod Treatment on Microglial Activation Using Serial PET Imaging in Multiple Sclerosis. J. Nucl. Med. 2017, 58, 1646–1651. [Google Scholar] [CrossRef] [Green Version]
- Raffaele, S.; Lombardi, M.; Verderio, C.; Fumagalli, M. TNF Production and Release from Microglia via Extracellular Vesicles: Impact on Brain Functions. Cells 2020, 9, 2145. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.Y.; Crews, F.T. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: Neuroprotection by NF kappa B inhibition. Brain Res. 2005, 1034, 11–24. [Google Scholar] [CrossRef]
- Kollias, G.; Douni, E.; Kassiotis, G.; Kontoyiannis, D. The function of tumour necrosis factor and receptors in models of multi-organ inflammation, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Ann. Rheum. Dis. 1999, 58 (Suppl. S1), I32–I39. [Google Scholar] [CrossRef]
- Bonora, M.; De Marchi, E.; Patergnani, S.; Suski, J.M.; Celsi, F.; Bononi, A.; Giorgi, C.; Marchi, S.; Rimessi, A.; Duszynski, J.; et al. Tumor necrosis factor-alpha impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ. 2014, 21, 1198–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurewicz, A.; Matysiak, M.; Tybor, K.; Selmaj, K. TNF-induced death of adult human oligodendrocytes is mediated by c-jun NH2-terminal kinase-3. Brain 2003, 126, 1358–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. TNF neutralization in MS: Results of a randomized, placebo-controlled multicenter study. Neurology 1999, 53, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Ronchi, F.; Basso, C.; Preite, S.; Reboldi, A.; Baumjohann, D.; Perlini, L.; Lanzavecchia, A.; Sallusto, F. Experimental priming of encephalitogenic Th1/Th17 cells requires pertussis toxin-driven IL-1beta production by myeloid cells. Nat. Commun. 2016, 7, 11541. [Google Scholar] [CrossRef] [Green Version]
- Argaw, A.T.; Zhang, Y.; Snyder, B.J.; Zhao, M.L.; Kopp, N.; Lee, S.C.; Raine, C.S.; Brosnan, C.F.; John, G.R. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 2006, 177, 5574–5584. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.L.; Doolittle, T.H.; Lincoln, R.; Brown, R.H.; Dinarello, C.A. Cytokine accumulations in CSF of multiple sclerosis patients: Frequent detection of interleukin-1 and tumor necrosis factor but not interleukin-6. Neurology 1990, 40, 1735–1739. [Google Scholar] [CrossRef]
- Dujmovic, I.; Pekmezovic, T.; Obrenovic, R.; Nikolic, A.; Spasic, M.; Mostarica Stojkovic, M.; Drulovic, J. Cerebrospinal fluid and serum uric acid levels in patients with multiple sclerosis. Clin. Chem. Lab. Med. 2009, 47, 848–853. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Edelson, B.T. New Insights into the Role of IL-1beta in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef] [Green Version]
- Jakimovski, D.; Kolb, C.; Ramanathan, M.; Zivadinov, R.; Weinstock-Guttman, B. Interferon beta for Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a032003. [Google Scholar] [CrossRef]
- Guo, B.; Chang, E.Y.; Cheng, G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Investig. 2008, 118, 1680–1690. [Google Scholar] [CrossRef]
- Prinz, M.; Schmidt, H.; Mildner, A.; Knobeloch, K.P.; Hanisch, U.K.; Raasch, J.; Merkler, D.; Detje, C.; Gutcher, I.; Mages, J.; et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 2008, 28, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Ledeboer, A.; Breve, J.J.; Wierinckx, A.; van der Jagt, S.; Bristow, A.F.; Leysen, J.E.; Tilders, F.J.; Van Dam, A.M. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur. J. Neurosci. 2002, 16, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.K.; Ireland, G.; Sullivan, G.; Becher, J.C.; Smith, C.; Boardman, J.P.; Gressens, P.; Miron, V.E. Microglial inflammasome activation drives developmental white matter injury. Glia 2021, 69, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Rice, R.A.; Pham, J.; Lee, R.J.; Najafi, A.R.; West, B.L.; Green, K.N. Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia 2017, 65, 931–944. [Google Scholar] [CrossRef]
- Clayton, K.; Delpech, J.C.; Herron, S.; Iwahara, N.; Ericsson, M.; Saito, T.; Saido, T.C.; Ikezu, S.; Ikezu, T. Plaque associated microglia hyper-secrete extracellular vesicles and accelerate tau propagation in a humanized APP mouse model. Mol. Neurodegener. 2021, 16, 18. [Google Scholar] [CrossRef]
- Kovacs, M.; Trias, E.; Varela, V.; Ibarburu, S.; Beckman, J.S.; Moura, I.C.; Hermine, O.; King, P.H.; Si, Y.; Kwon, Y.; et al. CD34 Identifies a Subset of Proliferating Microglial Cells Associated with Degenerating Motor Neurons in ALS. Int. J. Mol. Sci. 2019, 20, 3880. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.B.; He, L.N.; Jiang, B.C.; Shi, H.; Bai, X.Q.; Zhang, W.W.; Gao, Y.J. Spinal CXCL9 and CXCL11 are not involved in neuropathic pain despite an upregulation in the spinal cord following spinal nerve injury. Mol. Pain 2018, 14, 1744806918777401. [Google Scholar] [CrossRef]
- Mayo, L.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Moutin, M.J.; Lund, F.E.; Stein, R. Dual role of CD38 in microglial activation and activation-induced cell death. J. Immunol. 2008, 181, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Cheng, L.; Tian, D.; Li, Z.; Yao, F.; Luo, Y.; Liu, Y.; Zhu, Z.; Zheng, M.; Jing, J. Fascin-1 is Highly Expressed Specifically in Microglia After Spinal Cord Injury and Regulates Microglial Migration. Front. Pharmacol. 2021, 12, 729524. [Google Scholar] [CrossRef]
- Park, W.J.; Park, J.W. The role of sphingolipids in endoplasmic reticulum stress. FEBS Lett. 2020, 594, 3632–3651. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Lee, J.H.; Li, Q.; Diaz, K.; Chang, Y.T.; Chung, S.K. Divergent syntheses of all stereoisomers of phytosphingosine and their use in the construction of a ceramide library. Bioorganic Chem. 2008, 36, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Zabala, A.; Sierra-Torre, V.; Sierra, A. Assessing Autophagy in Microglia: A Two-Step Model to Determine Autophagosome Formation, Degradation, and Net Turnover. Front. Immunol. 2020, 11, 620602. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef] [Green Version]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, T.L.; Sagar; Dautzenberg, J.; Grun, D.; Prinz, M. Unique microglia recovery population revealed by single-cell RNAseq following neurodegeneration. Acta Neuropathol. Commun. 2018, 6, 87. [Google Scholar] [CrossRef]
- Pulido-Salgado, M.; Vidal-Taboada, J.M.; Barriga, G.G.; Sola, C.; Saura, J. RNA-Seq transcriptomic profiling of primary murine microglia treated with LPS or LPS + IFNgamma. Sci. Rep. 2018, 8, 16096. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Jalabi, W.; Shpargel, K.B.; Farabaugh, K.T.; Dutta, R.; Yin, X.; Kidd, G.J.; Bergmann, C.C.; Stohlman, S.A.; Trapp, B.D. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J. Neurosci. 2012, 32, 11706–11715. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Zhu, M.; Che, X.; Wang, H.; Liang, X.J.; Wu, C.; Xue, X.; Yang, J. Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation. J. Neuroinflamm. 2020, 17, 18. [Google Scholar] [CrossRef] [Green Version]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-inflammatory Stimuli (LPS, IFNgamma+TNFalpha) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Kremer, D.; Gruchot, J.; Weyers, V.; Oldemeier, L.; Göttle, P.; Healy, L.; Ho Jang, J.; Kang, T.X.Y.; Volsko, C.; Dutta, R.; et al. pHERV-W envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 15216–15225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, D.; Schichel, T.; Forster, M.; Tzekova, N.; Bernard, C.; van der Valk, P.; van Horssen, J.; Hartung, H.P.; Perron, H.; Küry, P. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann. Neurol. 2013, 74, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Forster, M.; Schichel, T.; Göttle, P.; Hartung, H.P.; Perron, H.; Küry, P. The neutralizing antibody GNbAC1 abrogates HERV-W envelope protein-mediated oligodendroglial maturation blockade. Mult. Scler. 2015, 21, 1200–1203. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | log2 Fold Change | p Adjusted |
|---|---|---|
| Lta | −4.42 | 2.07 × 10−6 |
| Cd38 | −4.04 | 4.58 × 10−24 |
| C3ar1 | −2.69 | 8.27 × 10−10 |
| Pycard | −2.69 | 8.97 × 10−8 |
| Card11 | −2.59 | 1.60 × 10−12 |
| Tifa | −2.53 | 3.81 × 10−13 |
| Cd180 | −2.47 | 1.37 × 10−6 |
| Nlrp3 | −2.10 | 2.02 × 10−13 |
| Tfrc | −1.91 | 1.31 × 10−6 |
| Lat2 | −1.82 | 3.68 × 10−11 |
| Slamf1 | −1.76 | 5.45 × 10−6 |
| RT1-N3 | −1.76 | 8.05 × 10−8 |
| Cmklr1 | −1.74 | 2.19 × 10−5 |
| Ctsh | −1.72 | 4.42 × 10−6 |
| Cd81 | −1.71 | 1.79 × 10−5 |
| Dhx58 | −1.65 | 4.29 × 10−6 |
| Ada | −1.60 | 3.02 × 10−6 |
| Xrcc5 | −1.59 | 1.21 × 10−6 |
| Lacc1 | −1.57 | 3.02 × 10−6 |
| Trex1 | −1.57 | 1.51 × 10−6 |
| Nectin2 | −1.52 | 2.44 × 10−5 |
| Cyrib | −1.36 | 5.69 × 10−6 |
| Tlr6 | −1.32 | 3.37 × 10−6 |
| Nod1 | −1.29 | 8.86 × 10−6 |
| Ifi35 | −1.25 | 7.32 × 10−6 |
| Gene Symbol | log2 Fold Change | p Adjusted |
|---|---|---|
| Il21r | −3.66 | 1.46 × 10−7 |
| P2ry12 | −3.44 | 4.64 × 10−7 |
| Cxcl13 | −3.35 | 7.65 × 10−7 |
| Fgl2 | −3.20 | 4.82 × 10−11 |
| Cxcl10 | −3.14 | 1.62 × 10−10 |
| Cd244 | −2.94 | 1.36 × 10−15 |
| Slamf9 | −2.88 | 2.15 × 10−7 |
| Tnfrsf11a | −2.81 | 1.94 × 10−7 |
| Klf2 | −2.81 | 2.27 × 10−7 |
| Pycard | −2.69 | 8.97 × 10−8 |
| Anxa3 | −2.60 | 2.16 × 10−7 |
| Card11 | −2.59 | 1.60 × 10−12 |
| Clec4a3 | −2.54 | 7.55 × 10−7 |
| Cd180 | −2.47 | 1.37 × 10−6 |
| Trpm2 | −2.43 | 6.88 × 10−13 |
| Sh3pxd2a | −2.37 | 1.25 × 10−7 |
| Actb | −2.33 | 5.90 × 10−8 |
| Il6r | −2.30 | 5.13 × 10−10 |
| Lrrk1 | −2.09 | 1.13 × 10−6 |
| Tfrc | −1.91 | 1.31 × 10−6 |
| Axl | −1.86 | 5.20 × 10−8 |
| Lat2 | −1.82 | 3.68 × 10−11 |
| Ubash3b | −1.61 | 4.55 × 10−8 |
| Xrcc5 | −1.59 | 1.21 × 10−6 |
| Prdx2 | −1.35 | 1.84 × 10−7 |
| Gene Symbol | log2 Fold Change | p Adjusted |
|---|---|---|
| Cxcl11 | −4.20 | 9.69 × 10−34 |
| Il16 | −4.05 | 5.43 × 10−7 |
| P2ry12 | −3.44 | 4.64 × 10−7 |
| Cxcl13 | −3.35 | 7.65 × 10−7 |
| Siglec8 | −3.28 | 1.76 × 10−9 |
| Fgl2 | −3.20 | 4.82 × 10−11 |
| Cxcl10 | −3.14 | 1.62 × 10−10 |
| Cd244 | −2.94 | 1.36 × 10−15 |
| Tnfrsf11a | −2.81 | 1.94 × 10−7 |
| Klf2 | −2.81 | 2.27 × 10−7 |
| Samhd1 | −2.72 | 2.29 × 10−7 |
| C3ar1 | −2.69 | 8.27 × 10−10 |
| Pycard | −2.69 | 8.97 × 10−8 |
| Card11 | −2.59 | 1.60 × 10−12 |
| Clec4a3 | −2.54 | 7.55 × 10−7 |
| Dagla | −2.32 | 2.04 × 10−7 |
| Il6r | −2.30 | 5.13 × 10−10 |
| Cxcl17 | −2.28 | 8.05 × 10−8 |
| Nlrp3 | −2.10 | 2.02 × 10−13 |
| Ezr | −1.91 | 1.30 × 10−7 |
| Ccr5 | −1.90 | 1.45 × 10−7 |
| Axl | −1.86 | 5.20 × 10−8 |
| Ctnnbip1 | −1.77 | 1.13 × 10−6 |
| Nt5e | −1.48 | 2.00 × 10−7 |
| Prdx2 | −1.35 | 1.84 × 10−7 |
| Gene Symbol | log2 Fold Change | p Adjusted |
|---|---|---|
| Cx3cr1 | −3.68 | 2.18 × 10−6 |
| Wfdc21 | −3.22 | 6.08 × 10−10 |
| Evl | −3.17 | 1.72 × 10−11 |
| Cxcl10 | −3.14 | 1.62 × 10−10 |
| Clec4a1 | −2.78 | 6.22 × 10−7 |
| Pycard | −2.69 | 8.97 × 10−8 |
| Ccl12 | −2.59 | 1.23 × 10−6 |
| Clec4a3 | −2.54 | 7.55 × 10−7 |
| Tifa | −2.53 | 3.81 × 10−13 |
| Mrc1 | −2.43 | 4.99 × 10−10 |
| Gbp4 | −2.34 | 4.46 × 10−9 |
| Fes | −2.33 | 1.35 × 10−6 |
| Ly86 | −2.10 | 2.17 × 10−5 |
| Nlrp3 | −2.10 | 2.02 × 10−13 |
| Lrrk1 | −2.09 | 1.13 × 10−6 |
| Aif1 | −1.90 | 5.24 × 10−6 |
| Coro1a | −1.73 | 4.18 × 10−5 |
| Dhx58 | −1.65 | 4.29 × 10−6 |
| Sla | −1.59 | 5.03 × 10−6 |
| Trim25 | −1.58 | 1.22 × 10−6 |
| Trex1 | −1.57 | 1.51 × 10−6 |
| Tmem106a | −1.43 | 1.22 × 10−5 |
| Tlr6 | −1.32 | 3.37 × 10−6 |
| Nod1 | −1.29 | 8.86 × 10−6 |
| Ifi35 | −1.25 | 7.32 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruchot, J.; Lein, F.; Lewen, I.; Reiche, L.; Weyers, V.; Petzsch, P.; Göttle, P.; Köhrer, K.; Hartung, H.-P.; Küry, P.; et al. Siponimod Modulates the Reaction of Microglial Cells to Pro-Inflammatory Stimulation. Int. J. Mol. Sci. 2022, 23, 13278. https://doi.org/10.3390/ijms232113278
Gruchot J, Lein F, Lewen I, Reiche L, Weyers V, Petzsch P, Göttle P, Köhrer K, Hartung H-P, Küry P, et al. Siponimod Modulates the Reaction of Microglial Cells to Pro-Inflammatory Stimulation. International Journal of Molecular Sciences. 2022; 23(21):13278. https://doi.org/10.3390/ijms232113278
Chicago/Turabian StyleGruchot, Joel, Ferdinand Lein, Isabel Lewen, Laura Reiche, Vivien Weyers, Patrick Petzsch, Peter Göttle, Karl Köhrer, Hans-Peter Hartung, Patrick Küry, and et al. 2022. "Siponimod Modulates the Reaction of Microglial Cells to Pro-Inflammatory Stimulation" International Journal of Molecular Sciences 23, no. 21: 13278. https://doi.org/10.3390/ijms232113278
APA StyleGruchot, J., Lein, F., Lewen, I., Reiche, L., Weyers, V., Petzsch, P., Göttle, P., Köhrer, K., Hartung, H. -P., Küry, P., & Kremer, D. (2022). Siponimod Modulates the Reaction of Microglial Cells to Pro-Inflammatory Stimulation. International Journal of Molecular Sciences, 23(21), 13278. https://doi.org/10.3390/ijms232113278







