Enhanced Water Solubility and Anti-Tumor Activity of Oleanolic Acid through Chemical Structure Modification
Abstract
1. Introduction
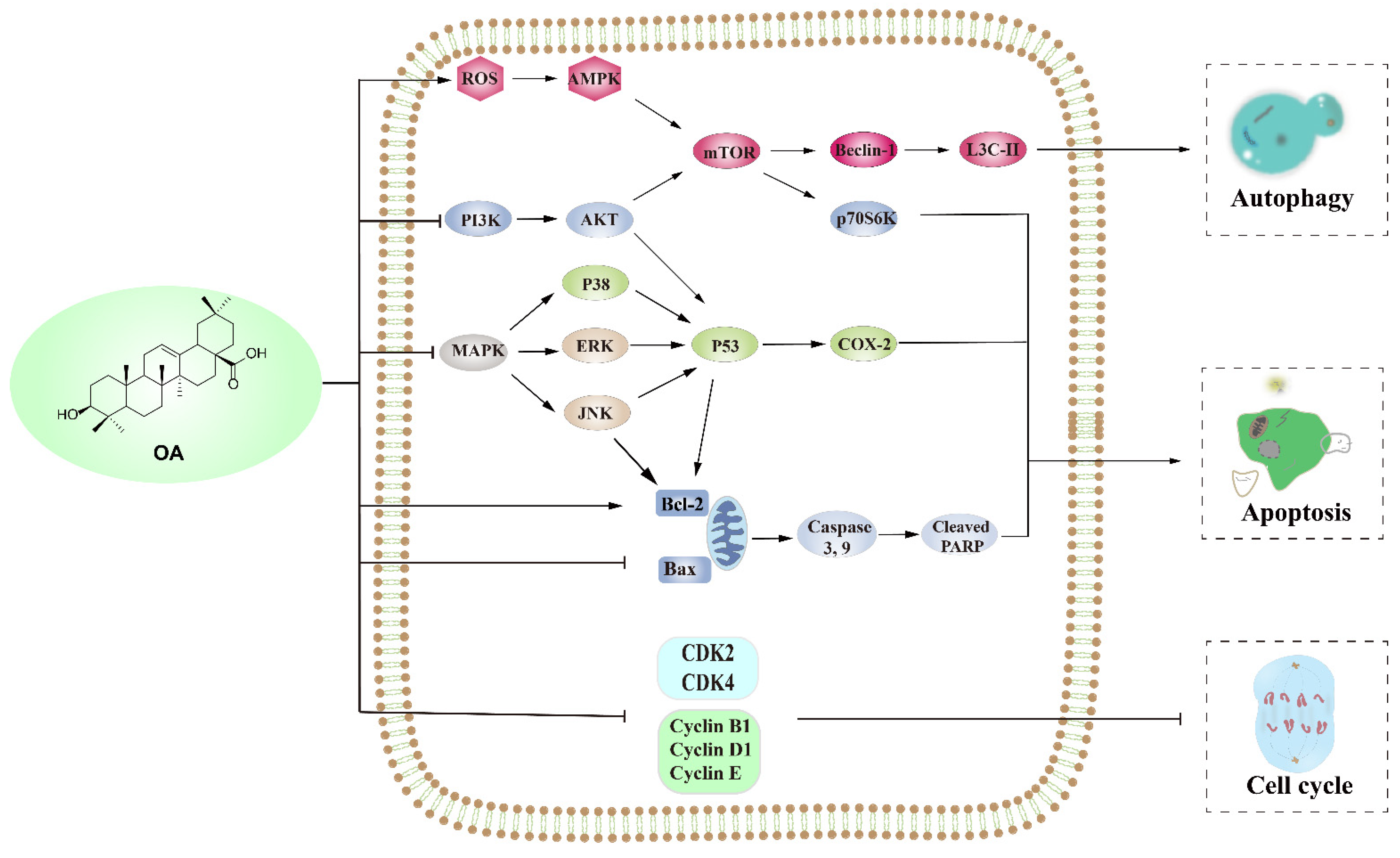
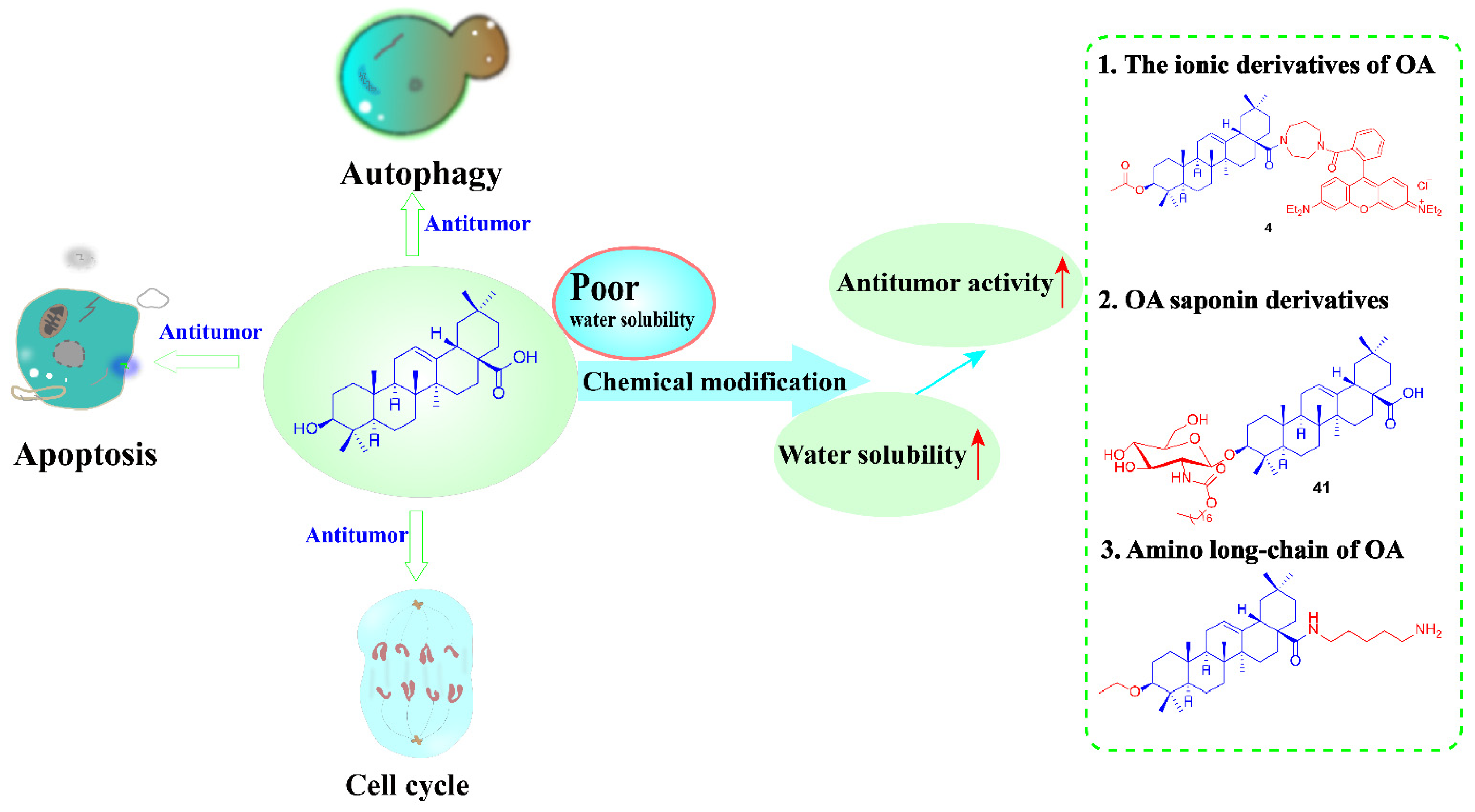
2. Anti-Tumor Effect of OA
2.1. OA Induces Apoptosis
2.1.1. Upregulation Pathway of ERK/p53
2.1.2. Inducing Mitochondria-Mediated Apoptosis by Increasing the Bax/Bcl-2 Ratio
2.1.3. PI3K/AKT and MAPK Pathways
2.2. OA Induced Autophagy Death of Tumor Cells
2.2.1. PI3K/AKT/mTOR Pathways
2.2.2. AMPK/mTOR Pathways
2.3. Oleanolic Acid Regulates the Cell Cycle
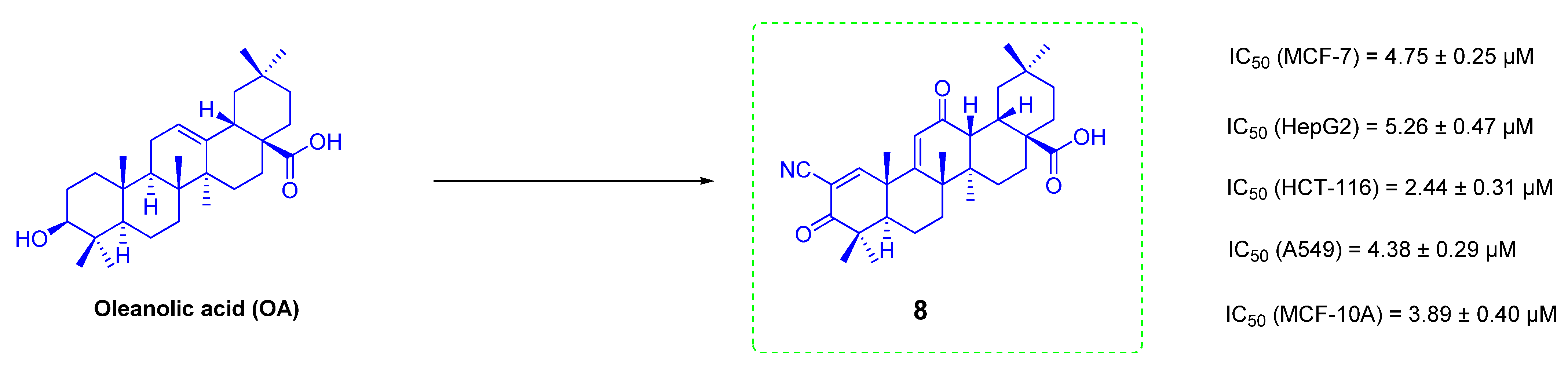
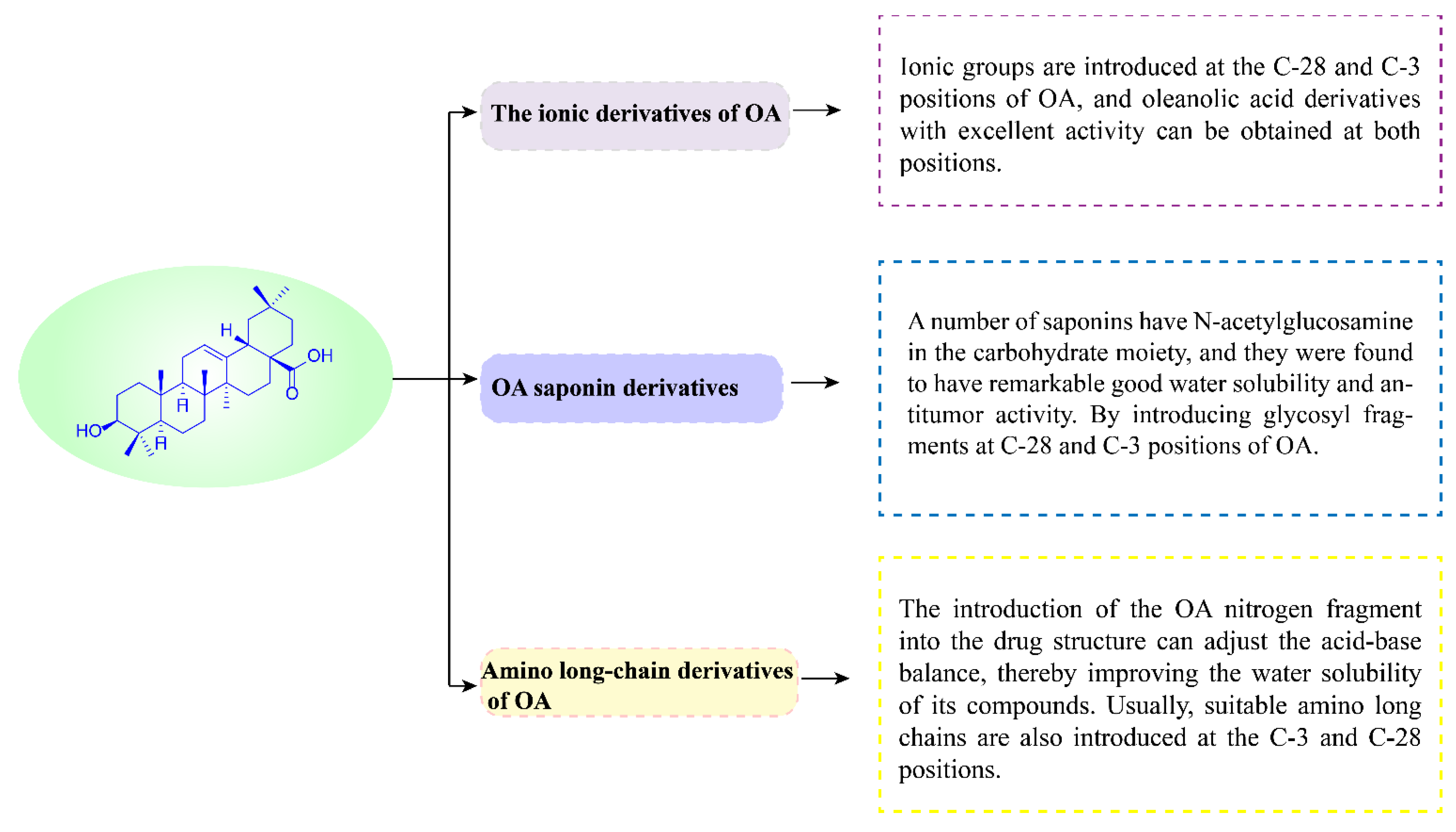
3. Anti-Tumor Study of OA Derivatives
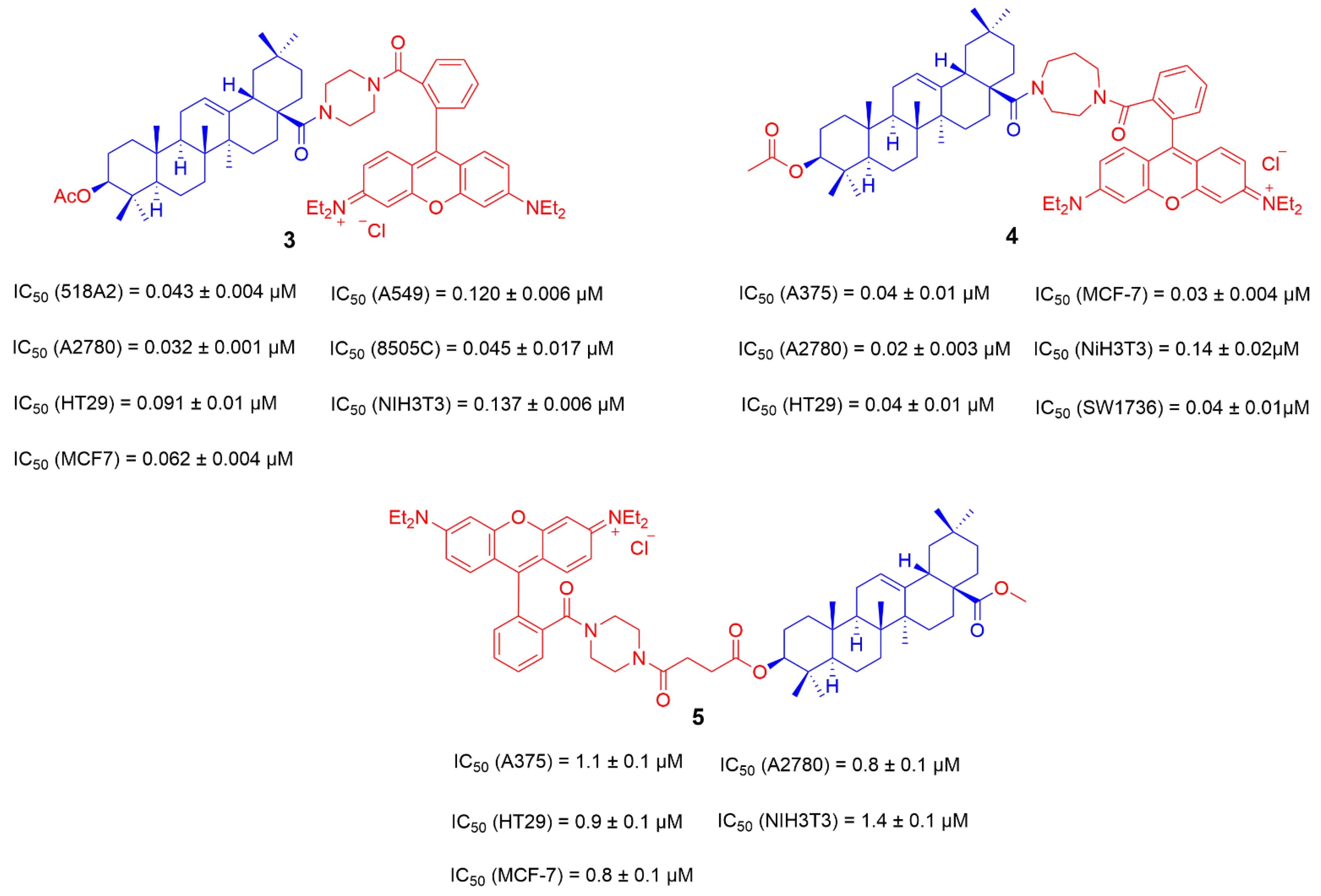
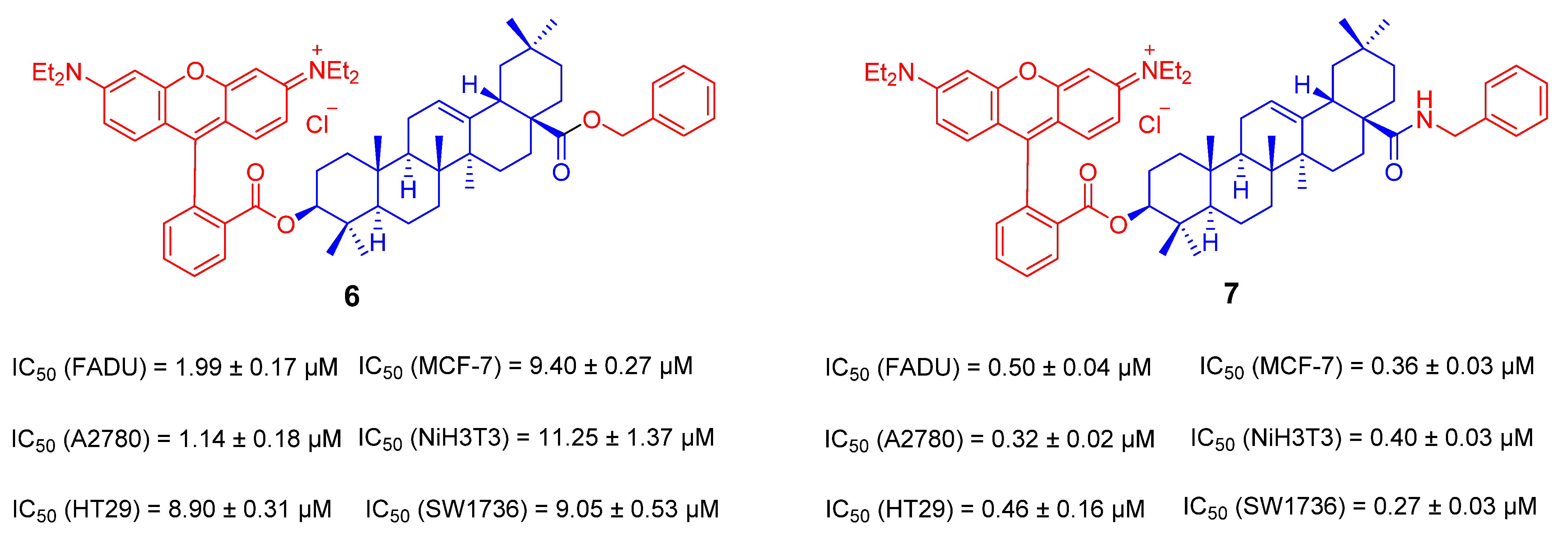
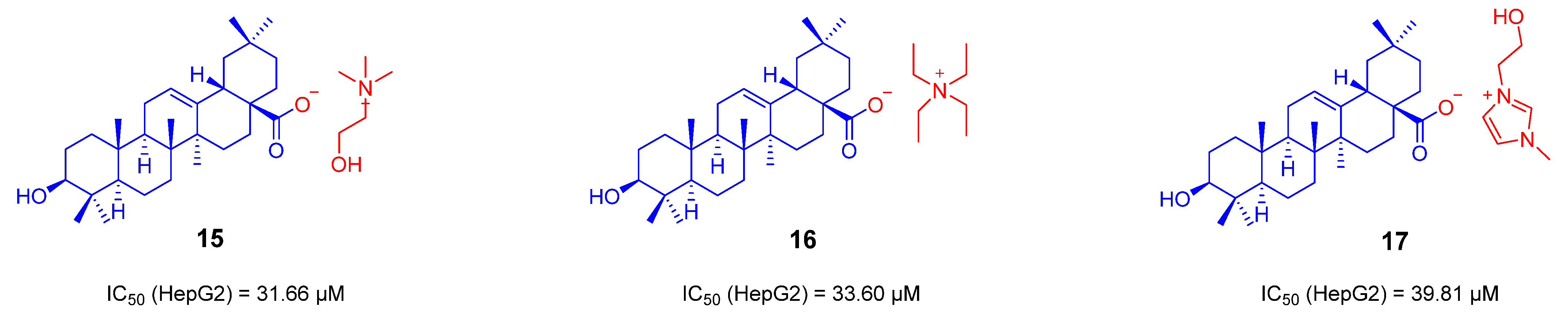
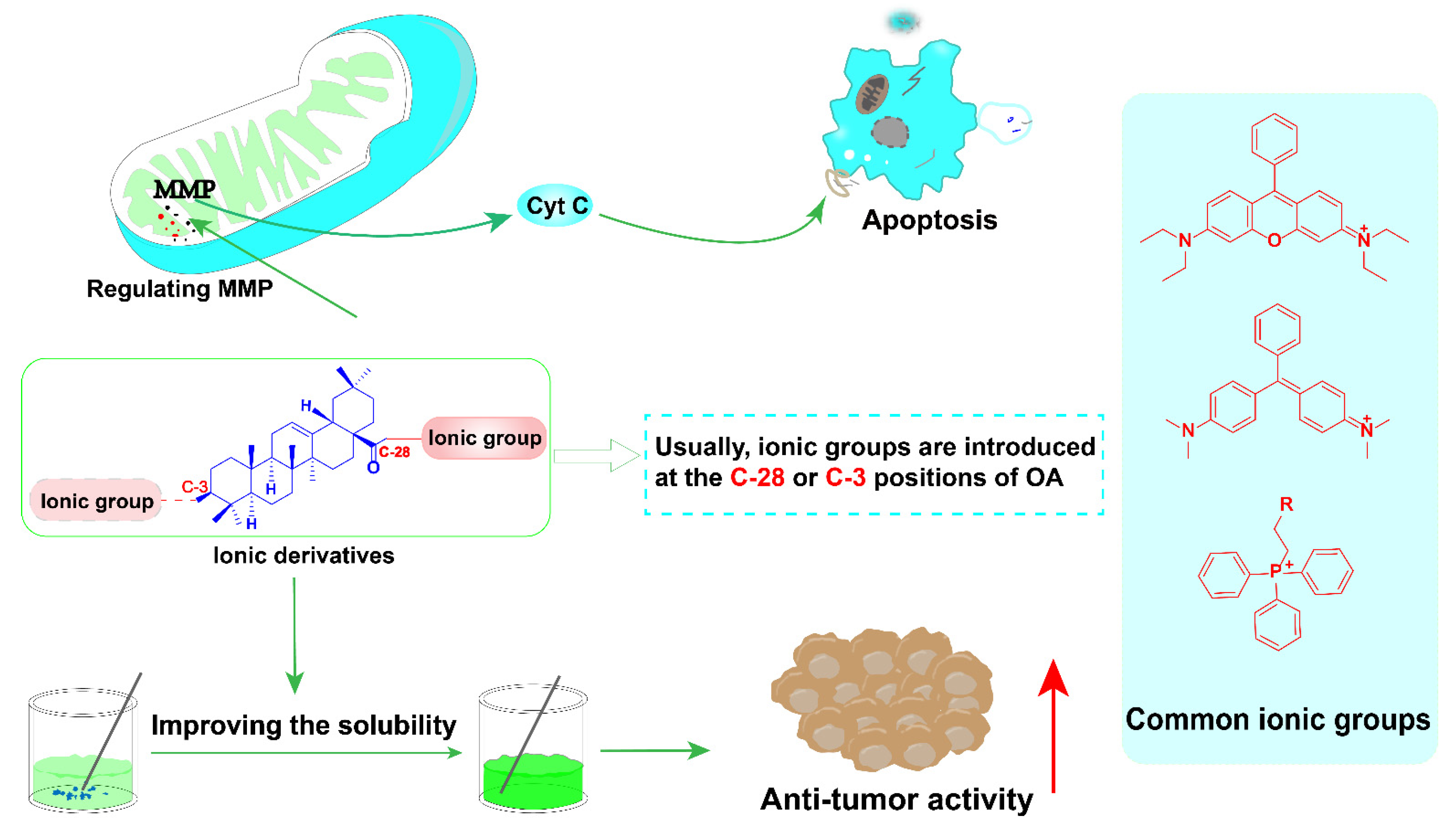
3.1. The Ionic Derivatives of OA
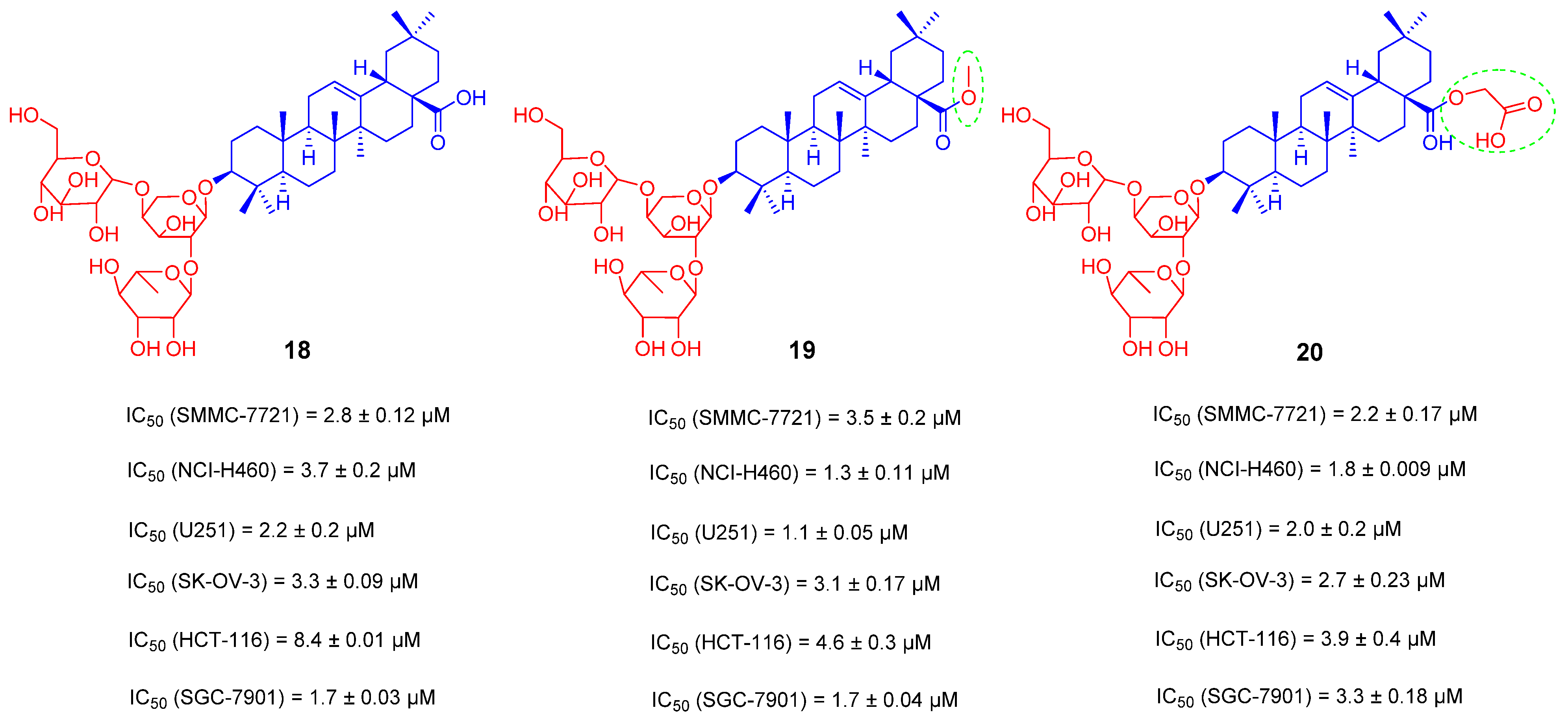
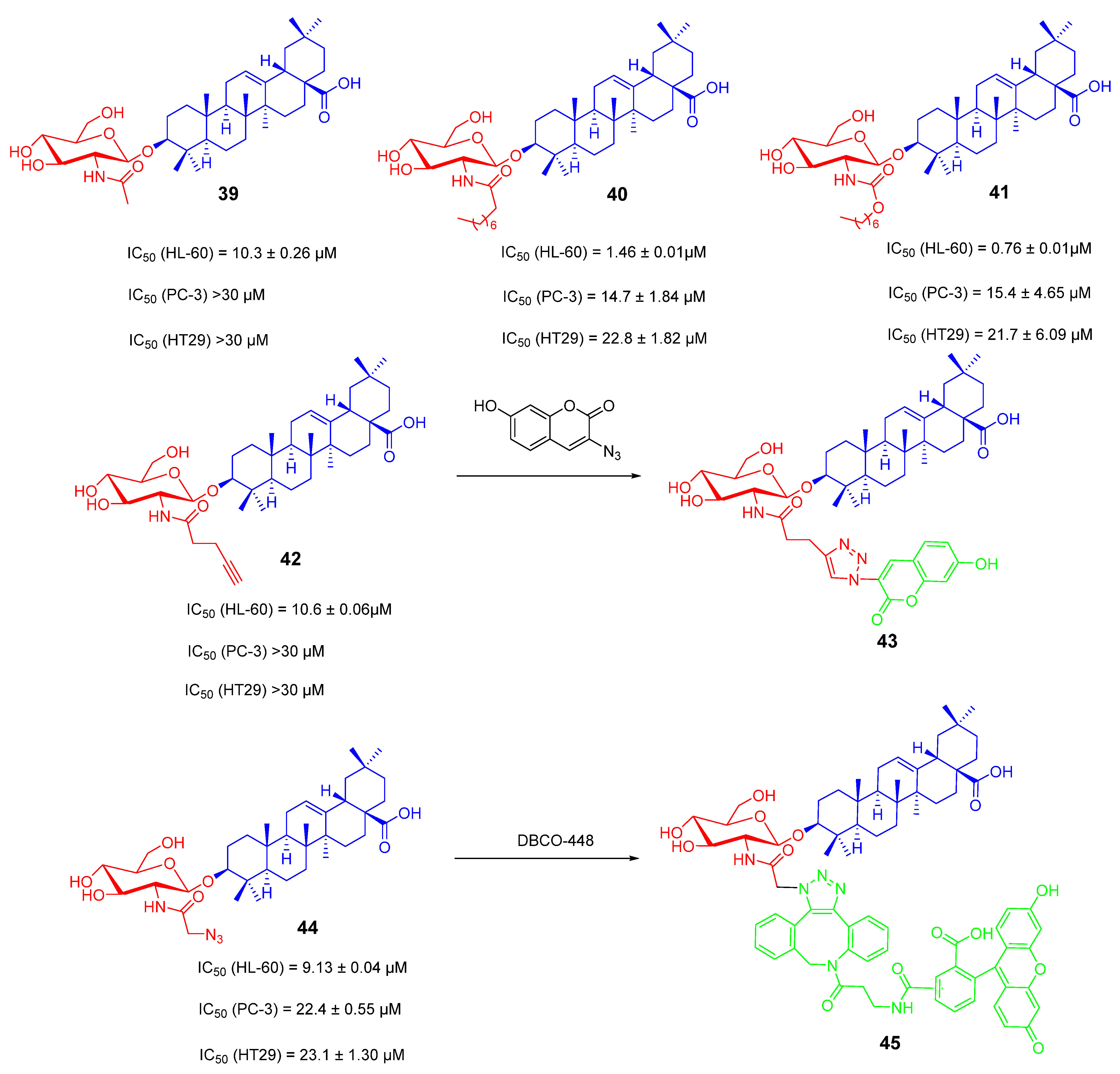
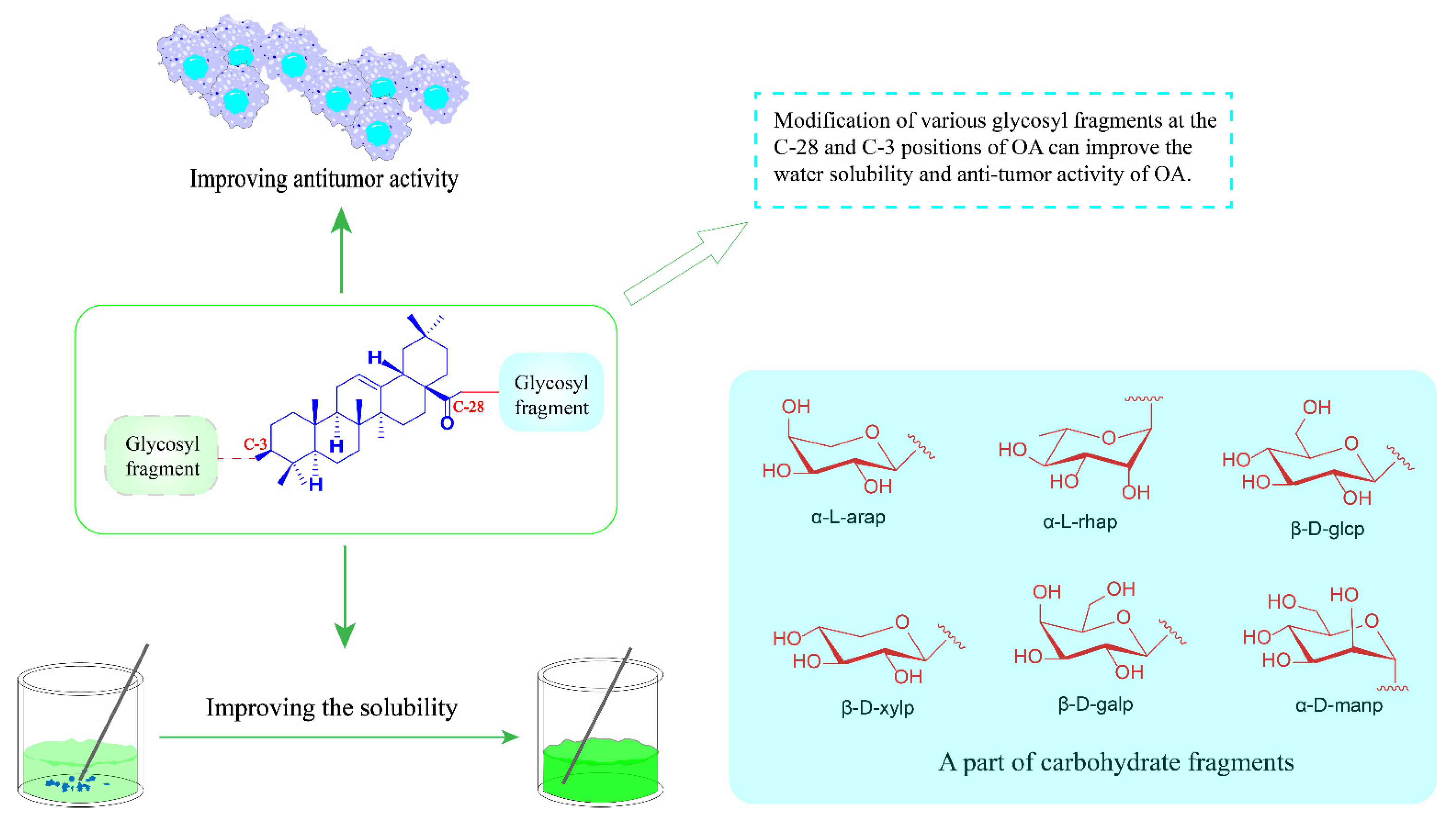
3.2. OA Saponin Derivatives
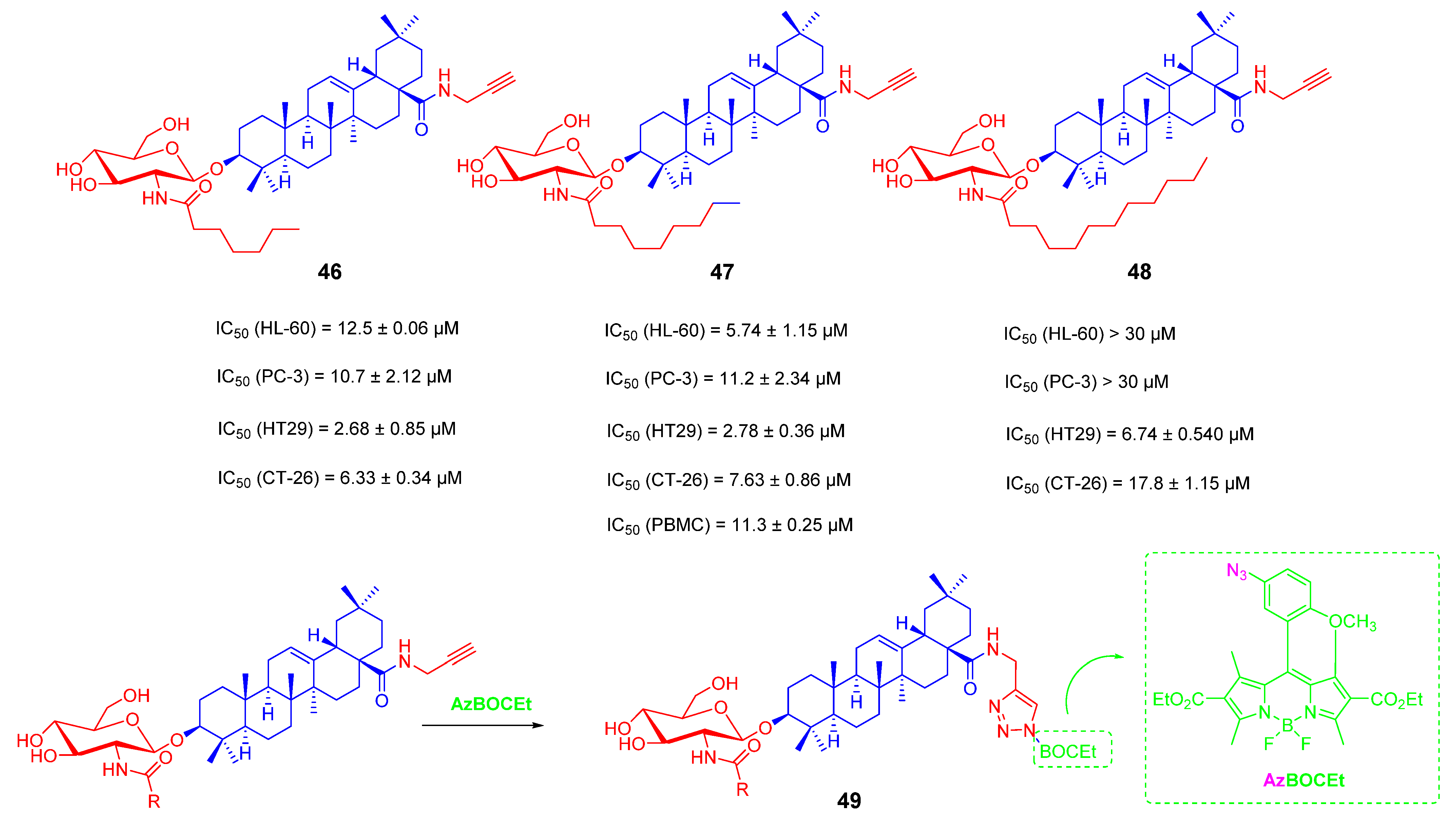
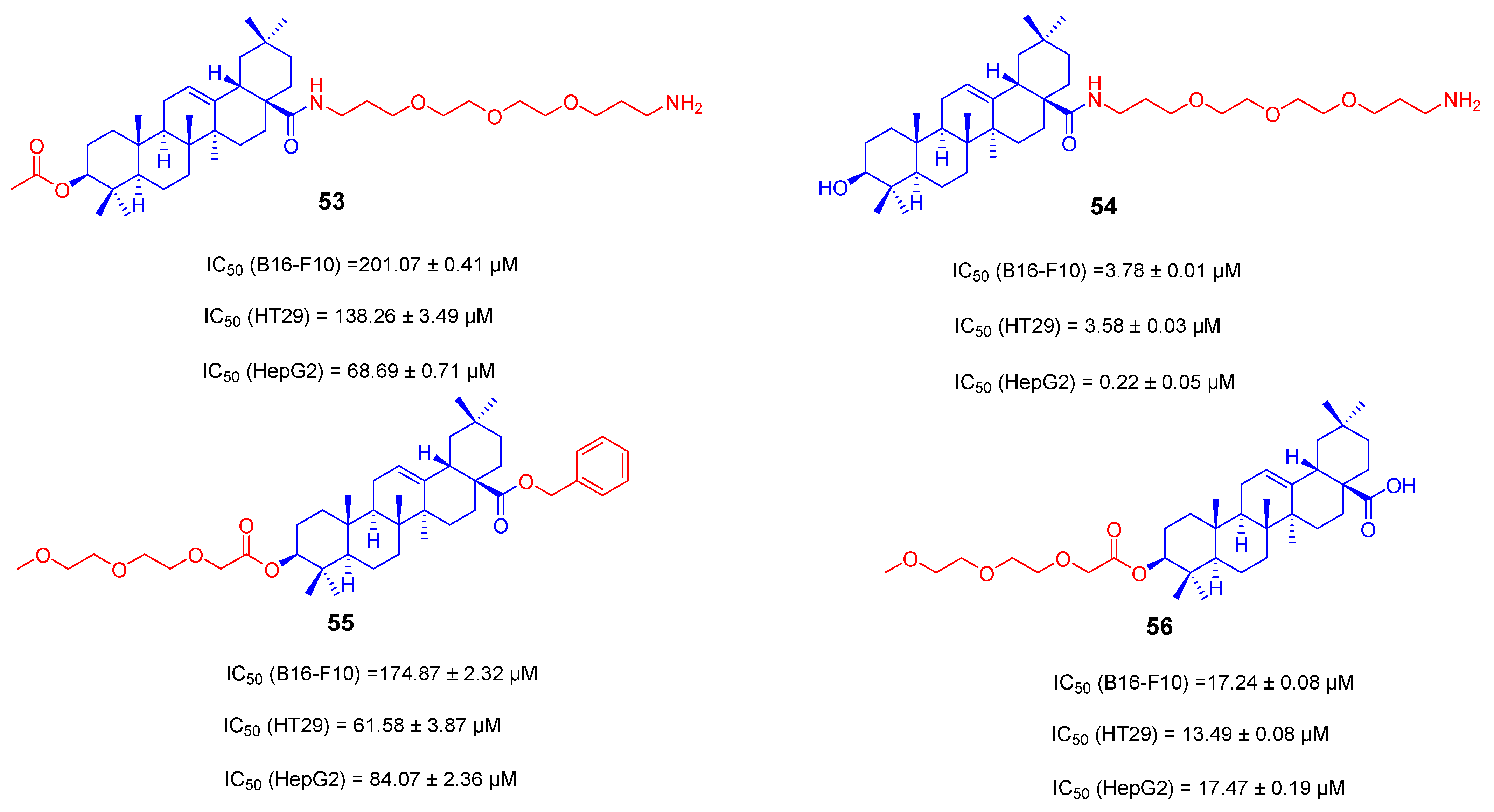
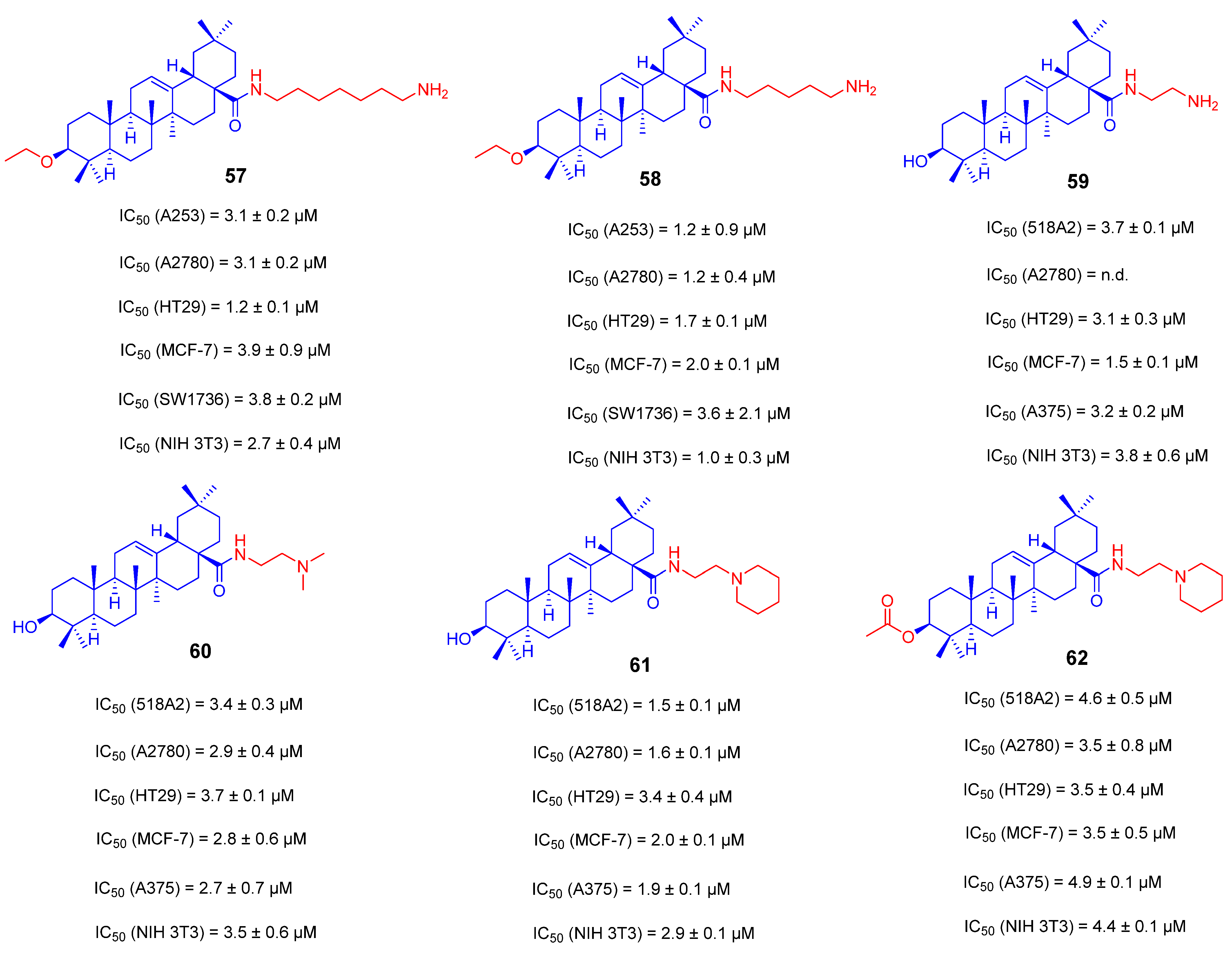
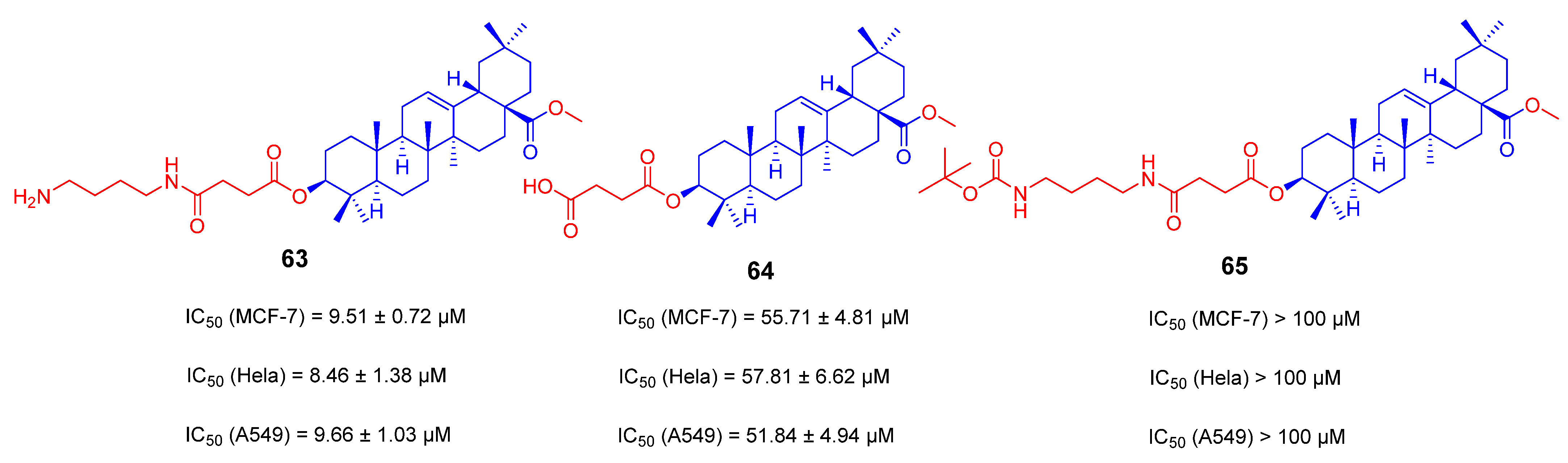
3.3. Amino Long-Chain Derivatives of OA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassannia, B.; Vandenabeele, P.; Berghe, T.V. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, S.; Wang, K.; Lu, J.; Bao, X.; Wang, R.; Qiu, Y.; Wang, T.; Yu, H. Cellular senescence and cancer: Focusing on traditional Chinese medicine and natural products. Cell Prolif. 2020, 53, e12894. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Lei, K.F.; Han, F. Tumor microenvironment: Recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3855–3864. [Google Scholar]
- Behranvand, N.; Nasri, F.; Emameh, R.Z.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Sarkar, B.K.; Lee, S.-S. The novel strategies for next-generation cancer treatment: miRNA combined with chemotherapeutic agents for the treatment of cancer. Oncotarget 2018, 9, 10164–10174. [Google Scholar] [CrossRef]
- Fu, R.G.; Sun, Y.; Sheng, W.B.; Liao, D.F. Designing multi-targeted agents: An emerging anticancer drug discovery paradigm. Eur. J. Med. Chem. 2017, 136, 195–211. [Google Scholar] [CrossRef]
- Luo, B.; Song, X. A comprehensive overview of β-carbolines and its derivatives as anticancer agents. Eur. J. Med. Chem. 2021, 224, 113688. [Google Scholar] [CrossRef]
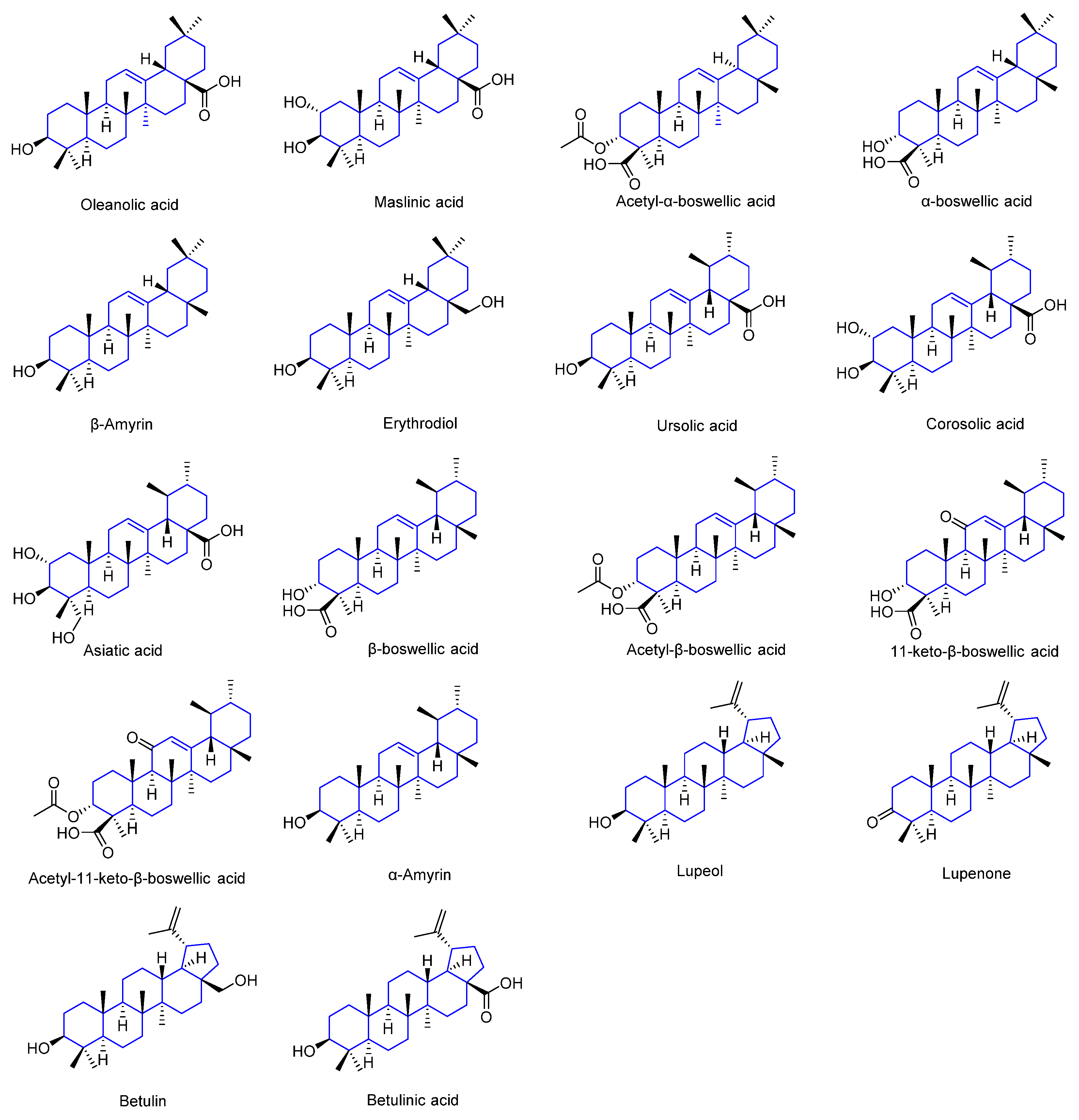
- Fabio, G.D.; Romanucci, V.; De Marco, A.; Zarrelli, A. Triterpenoids from Gymnema sylvestre and their pharmacological activities. Molecules 2014, 19, 10956–10981. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Sheng, H.; Sun, H. Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011, 28, 543–593. [Google Scholar] [CrossRef] [PubMed]
- Żwawiak, J.; Pawełczyk, A.; Olender, D.; Zaprutko, L. Structure and Activity of Pentacyclic Triterpenes Codrugs. A Review. Mini. Rev. Med. Chem. 2021, 21, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Jaswal, V.; Sharma, A.; Kashyap, D.; Tuli, H.S.; Garg, V.K.; Das, S.K.; Srinivas, R. Celastrol as a pentacyclic triterpenoid with chemopre-ventive properties. Pharm. Pat. Anal. 2018, 7, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic triterpenes: New tools to fight metabolic syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Valdés, K.; Morales, J.; Rodríguez, L.; Günther, G. Potential use of nanocarriers with pentacyclic triterpenes in cancer treatments. Nanomedicine 2016, 11, 3139–3156. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, Z.; Wang, Q. Ursane triterpenoids inhibit atherosclerosis and xanthoma in LDL receptor knockout mice. Cardiovasc. Drugs Ther. 2006, 20, 349–357. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Suzuki, H.; Mukainaka, T.; Ichiishi, E.; Yasukawa, K.; Kasahara, Y.; Nishino, H. Constituents of Compositae plants III. Anti-tumor promoting effects and cytotoxic activity against human cancer cell lines of triterpene diols and triols from edible chrysanthemum flowers. Cancer Lett. 2002, 177, 7–12. [Google Scholar] [CrossRef]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigbe, B.A. Pentacyclic Triterpenoids with Nitrogen-Containing Heterocyclic Moiety, Privileged Hybrids in Anticancer Drug Discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Pádua, T.A.; de Abreu, B.S.; Costa, T.E.; Nakamura, M.J.; Valente, L.M.; Henriques, M.D.G.; Siani, A.C.; Rosas, E.C. Anti-inflammatory effects of methyl ursolate obtained from a chemically derived crude extract of apple peels: Potential use in rheumatoid arthritis. Arch. Pharm. Res. 2014, 37, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Zhu, Y.; Guan, Y.-J.; Chen, Q.-Z.; Yuan, L.-H.; Xu, Q.-Q.; Zhou, M.-L.; Liu, H.; Lin, W.; Zhang, Z.-D.; Zhou, Z.-L.; et al. Pentacyclic Triterpenes from the resin of Liquidambar formosana have anti-angiogenic properties. Phytochemistry 2021, 184, 112676. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; STuli, H.; Punia, S.; KSharma, A. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.-F.; Wu, Q.; Xu, S.-F.; Shi, F.-G.; Klaassen, C.D. Oleanolic acid reprograms the liver to protect against hepatotoxicants, but is hepatotoxic at high doses. Liver Int. 2019, 39, 427–439. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Narożna, M.; Krajka-Kuźniak, V. Anti-cancer potential of synthetic oleanolic acid derivatives and their conjugates with NSAIDs. Molecules 2021, 26, 4957. [Google Scholar] [CrossRef]
- Oboh, M.; Govender, L.; Siwela, M.; Mkhwanazi, B.N. Anti-diabetic potential of plant-based pentacyclic triterpene derivatives: Progress made to improve efficacy and bioavailability. Molecules 2021, 26, 7243. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Liu, Q.; Wang, P.; Zhang, L.; Zhang, W.; Li, Y. Facile synthesis of three bidesmosidic oleanolic acid saponins with strong inhibitory activity on pancreatic lipase. Carbohydr. Res. 2009, 344, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.; Yang, S.; Sun, Y.; Zhang, L.; Bo, F.; Li, L. Development and Evaluation of Oleanolic Acid Dosage Forms and Its Derivatives. BioMed Res. Int. 2020, 2020, 1308749. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Jia, J.; Yin, Z.; Gao, Y.; Sha, L.; Lai, Y.; Ping, Q.; Zhang, Y. Ethylene glycol-linked amino acid diester prodrugs of oleanolic acid for PepT1-mediated transport: Synthesis, intestinal permeability and pharmacokinetics. Mol. Pharm. 2012, 9, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, M.; Gou, S.; Liu, X.; Zhang, H.; Cao, F. Combination of amino acid/dipeptide with nitric oxide donating oleanolic acid derivatives as PepT1 targeting anti-tumor prodrugs. J. Med. Chem. 2014, 57, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Han, S.I.; Kim, Y.S.; Kim, T.H. Role of apoptotic and necrotic cell death under physiologic conditions. BMB Rep. 2008, 41, 1–10. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef]
- Liby, K.T.; Sporn, M.B. Synthetic oleanane triterpenoids: Multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients. 2022, 14, 623. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Li, Y.; Tang, Y.-T.; Ma, X.-D.; Tang, Z.-Y. Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Ucker, D.S.; Levine, J.S. Exploitation of Apoptotic Regulation in Cancer. Front. Immunol. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef]
- Al-Alem, L.F.; Baker, A.T.; Pandya, U.M.; Eisenhauer, E.L.; Rueda, B.R. Understanding and Targeting Apoptotic Pathways in Ovarian Cancer. Cancers 2019, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Maddika, S.; Ande, S.R.; Panigrahi, S.; Paranjothy, T.; Weglarczyk, K.; Zuse, A.; Eshraghi, M.; Manda, K.D.; Wiechec, E.; Los, M. Cell survival, cell death and cell cycle pathways are interconnected: Implications for cancer therapy. Drug Resist. Updates 2007, 10, 13–29. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Duan, L.; Yang, Z.; Jiang, X.; Zhang, J.; Guo, X. Oleanolic acid inhibits cell proliferation migration and invasion and induces SW579 thyroid cancer cell line apoptosis by targeting forkhead transcription factor A. Anticancer Drugs 2019, 30, 812–820. [Google Scholar] [CrossRef]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020, 10, 54. [Google Scholar] [CrossRef]
- Dent, P. Crosstalk between ERK, AKT, and cell survival. Cancer Biol. Ther. 2014, 15, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y.; Zhang, P.; Zhu, H.; Chen, L.; Xiao, Y.; Xing, Y. Oleanolic acid inhibits cell survival and proliferation of prostate cancer cells in vitro and in vivo through the PI3K/Akt pathway. Tumor Biol. 2016, 37, 7599–7613. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, L.; Shen, A.; Chu, J.; Lin, J.; Peng, J. Oleanolic acid modulates multiple intracellular targets to inhibit colorectal cancer growth. Int. J. Oncol. 2015, 47, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Potočnjak, I.; Šimić, L.; Vukelić, I.; Batičić, L.; Domitrović, R. Oleanolic acid induces HCT116 colon cancer cell death through the p38/FOXO3a/Sirt6 pathway. Chem.-Biol. Interact. 2022, 363, 110010. [Google Scholar] [CrossRef] [PubMed]
- Msibi, Z.N.P.; Mabandla, M.V. Oleanolic Acid Mitigates 6-Hydroxydopamine Neurotoxicity by Attenuating Intracellular ROS in PC12 Cells and Striatal Microglial Activation in Rat Brains. Front. Physiol. 2019, 10, 1059. [Google Scholar] [CrossRef]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef]
- Levy, J.M.; Thorburn, A. Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacol. Ther. 2011, 131, 130–141. [Google Scholar] [CrossRef]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer. Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef]
- Wu, X.L.; Wang, L.K.; Yang, D.D.; Qu, M.; Yang, Y.J.; Guo, F.; Han, L.; Xue, J. Effects of Glut1 gene silencing on proliferation, differentiation, and apoptosis of colorectal cancer cells by targeting the TGF-β/PI3K-AKT-mTOR signaling pathway. J. Cell. Biochem. 2018, 119, 2356–2367. [Google Scholar] [CrossRef]
- Shi, Y.; Song, Q.; Hu, D.; Zhuang, X.; Yu, S.; Teng, D. Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway. Korean J. Physiol. Pharmacol. 2016, 20, 237–243. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yoo, E.-S.; Han, S.-H.; Jung, G.-H.; Han, E.-J.; Jung, S.-H.; Kim, B.S.; Cho, S.-D.; Nam, J.-S.; Choi, C.; et al. Oleanolic acid induces apoptosis and autophagy via the PI3K/AKT/mTOR pathway in AGS human gastric cancer cells. J. Funct. Foods 2021, 87, 104854. [Google Scholar] [CrossRef]
- Howell, J.J.; Ricoult, S.J.; Ben-Sahra, I.; Manning, B.D. A growing role for mTOR in promoting anabolic metabolism. Biochem. Soc. Trans. 2013, 41, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Liu, D.; Zhang, G.; Chen, L.; Song, Z. AMPK/mTOR/ULK1 Axis-Mediated Pathway Participates in Apoptosis and Autophagy Induction by Oridonin in Colon Cancer DLD-1 Cells. OncoTargets Ther. 2020, 13, 8533–8545. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cao, Y.; Li, P.; Tang, X.; Yang, M.; Gu, S.; Xiong, K.; Li, T.; Xiao, T. Oleanolic Acid Induces Autophagy and Apoptosis via the AMPK-mTOR Signaling Pathway in Colon Cancer. J. Oncol. 2021, 2021, 8281718. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Elazar, Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007, 17, 422–427. [Google Scholar] [CrossRef]
- Wang, S.S.; Zhang, Q.L.; Chu, P.; Kong, L.Q.; Li, G.Z.; Li, Y.Q.; Yang, L.; Zhao, X.J.; Guo, X.H.; Tang, Z.Y. Synthesis and antitumor activity of α, β-unsaturated carbonyl moiety- containing oleanolic acid derivatives targeting PI3K/AKT/mTOR signaling pathway. Bioorg. Chem. 2020, 101, 104036. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Guan, F.; Zhao, X.; Wang, M.; Chen, Y.; Wang, Q.; Feng, X. Macranthoside B Induces Apoptosis and Autophagy Via Reactive Oxygen Species Accumulation in Human Ovarian Cancer A2780 Cells. Nutr. Cancer 2016, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; He, Z.; Kitazato, K.; Wang, Y. Selective Autophagy Regulates Cell Cycle in Cancer Therapy. Theranostics 2019, 9, 104–125. [Google Scholar] [CrossRef]
- Kim, G.-J.; Jo, H.-J.; Lee, K.-J.; Choi, J.W.; An, J.H. Oleanolic acid induces p53-dependent apoptosis via the ERK/JNK/AKT pathway in cancer cell lines in prostatic cancer xenografts in mice. Oncotarget 2018, 9, 26370–26386. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, W.; Zhao, J.; Zhang, H.; Zhang, Q.; Liang, Y.; Chen, S.; Liu, H.; Zong, S.; Tian, Y.; et al. Oleanolic Acid Inhibits Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma by Promoting iNOS Dimerization. Mol. Cancer. Ther. 2019, 18, 62–74. [Google Scholar] [CrossRef]
- Modica-Napolitano, J.S.; Aprille, J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug. Deliv. Rev 2001, 49, 63–70. [Google Scholar] [CrossRef]
- Modica-Napolitano, J.S.; Aprille, J.R. Basis for the selective cytotoxicity of rhodamine 123. Cancer Res. 1987, 47, 4361–4365. [Google Scholar]
- Cai, H.; Zhang, Y.; Han, T.K.; Everett, R.S.; Thakker, D.R. Cation-selective transporters are critical to the AMPK-mediated antiproliferative effects of metformin in human breast cancer cells. Int. J. Cancer 2016, 138, 2281–2292. [Google Scholar] [CrossRef]
- Brown, K.K.; Spinelli, J.B.; Asara, J.M.; Toker, A. Adaptive Reprogramming of De Novo Pyrimidine Synthesis Is a Metabolic Vulnerability in Triple-Negative Breast Cancer. Cancer Discov. 2017, 7, 391–399. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Q.; Dong, C. Metabolic reprogramming in triple-negative breast cancer. Cancer Biol. Med. 2020, 17, 44–59. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, T.; Lou, H.; Song, H.; Li, C.; Fan, P. Anticancer Effects of Honokiol via Mitochondrial Dysfunction Are Strongly Enhanced by the Mitochondria-Targeting Carrier Berberine. J. Med. Chem. 2020, 63, 11786–11800. [Google Scholar] [CrossRef]
- Friedrich, S.; Serbian, I.; Hoenke, S.; Wolfram, R.K.; Csuk, R. Synthesis and cytotoxic evaluation of malachite green derived oleanolic and ursolic acid piperazineamides. Med. Chem. Res. 2020, 29, 926–933. [Google Scholar] [CrossRef]
- Xie, C.; Chang, J.; Hao, X.-D.; Yu, J.-M.; Liu, H.-R.; Sun, X. Mitochondrial-targeted prodrug cancer therapy using a rhodamine B labeled fluorinated docetaxel. Eur. J. Pharm. Biopharm. 2013, 85 Pt A, 541–549. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kerzig, C.; Kramell, A.E.; Csuk, R. Rhodamine B conjugates of triterpenoic acids are cytotoxic mitocans even at nanomolar concentrations. Eur. J. Med. Chem. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Wolfram, R.K.; Fischer, L.; Kluge, R.; Ströhl, D.; Al-Harrasi, A.; Csuk, R. Homopiperazine-rhodamine B adducts of triterpenoic acids are strong mitocans. Eur. J. Med. Chem. 2018, 155, 869–879. [Google Scholar] [CrossRef]
- Heise, N.; Hoenke, S.; Simon, V.; Deigner, H.-P.; Al-Harrasi, A.; Csuk, R. Type and position of linkage govern the cytotoxicity of oleanolic acid rhodamine B hybrids. Steroids 2021, 172, 108876. [Google Scholar] [CrossRef] [PubMed]
- Masullo, M.; Pizza, C.; Piacente, S. Oleanane derivatives for pharmaceutical use: A patent review (2000–2016). Expert Opin. Ther. Pat. 2017, 27, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Snowden, R.T.; Dyer, M.; Cohen, G.M. CDDO induces apoptosis via the intrinsic pathway in lymphoid cells. Leukemia 2004, 18, 948–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ju, W.; Li, N.; Wang, J.; Yu, N.; Lei, Z.; Zhang, L.; Sun, J.; Chen, L. Design and synthesis of novel mitochondria-targeted CDDO derivatives as potential anti-cancer agents. Bioorg. Chem. 2021, 115, 105249. [Google Scholar] [CrossRef]
- Spivak, A.; Khalitova, R.; Nedopekina, D.; Dzhemileva, L.; Yunusbaeva, M.; Odinokov, V.; D’yakonov, V.; Dzhemilev, U. Synthesis and Evaluation of Anticancer Activities of Novel C-28 Guanidine-Functionalized Triterpene Acid Derivatives. Molecules 2018, 23, 3000. [Google Scholar] [CrossRef]
- Fan, J.P.; Lai, X.H.; Zhang, X.H.; Yang, L.; Yuan, T.T.; Chen, H.P.; Liang, X. Synthesis and evaluation of the cancer cell growth inhibitory activity of the ionic derivatives of oleanolic acid and ursolic acid with improved solubility. J. Mol. Liq. 2021, 332, 115837. [Google Scholar] [CrossRef]
- Biswas, T.; Dwivedi, U.N. Plant triterpenoid saponins: Biosynthesis, in vitro production, and pharmacological relevance. Protoplasma 2019, 256, 1463–1486. [Google Scholar] [CrossRef]
- Xiao, G.; Shao, X.; Zhu, D.; Yu, B. Chemical Synthesis of Marine Saponins. Nat. Prod. Rep. 2019, 36, 769–787. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Lin, X.P.; Zhou, X.F.; Liu, Y. Sesterterpenoids. Nat. Prod. Rep. 2013, 30, 455–473. [Google Scholar] [CrossRef]
- Krief, S.; Thoison, O.; Sévenet, T.; Wrangham, R.W.; Lavaud, C. Triterpenoid saponin anthranilates from Albizia grandibracteata leaves ingested by primates in Uganda. J. Nat. Prod. 2005, 68, 897–903. [Google Scholar] [CrossRef]
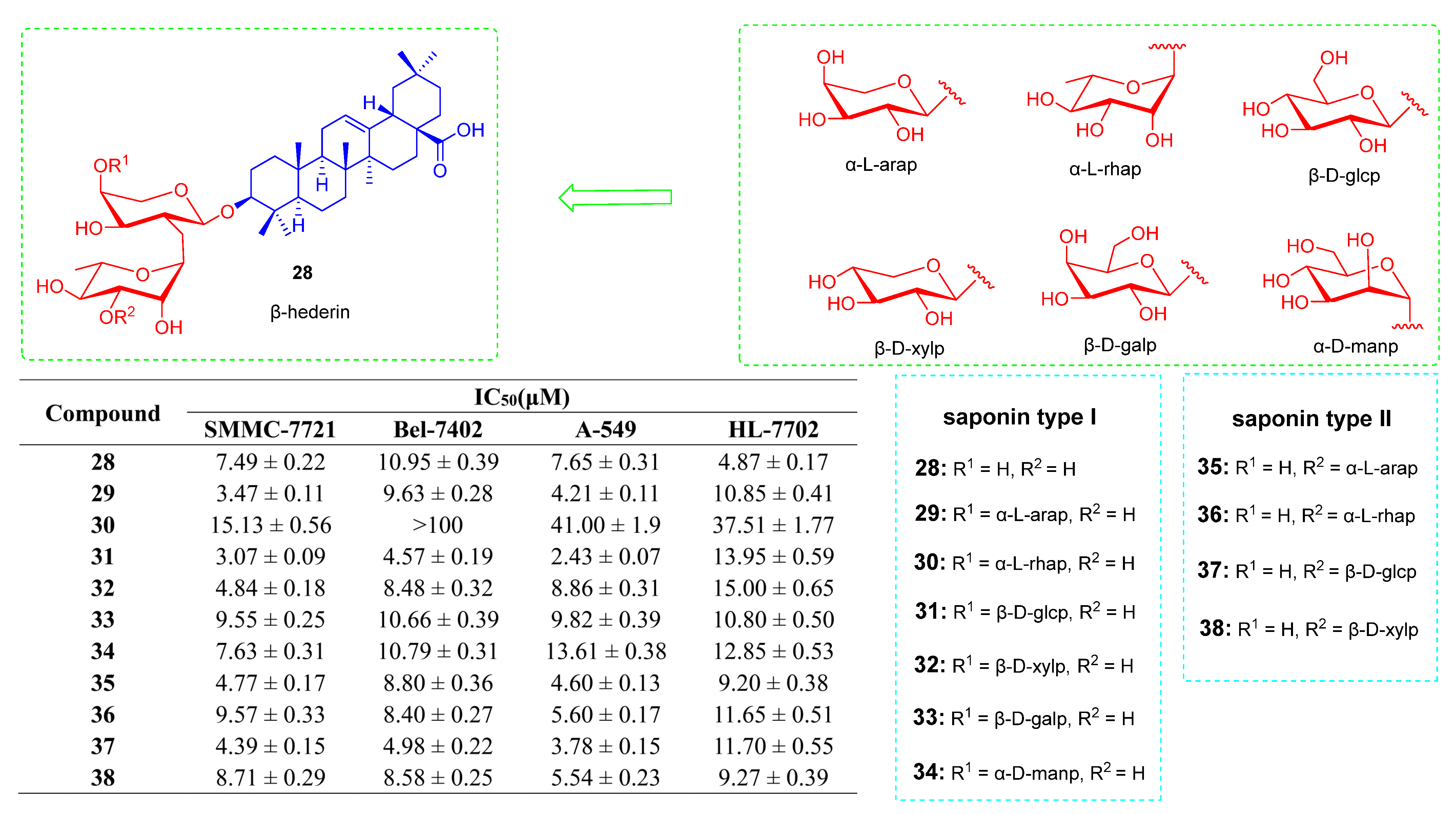
- Liu, Q.; Liu, H.; Zhang, L.; Guo, T.; Wang, P.; Geng, M.; Li, Y. Synthesis and anti-tumor activities of naturally occurring oleanolic acid triterpenoid saponins and their derivatives. Eur. J. Med. Chem. 2013, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Juang, Y.P.; Liang, P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yang, Z.; Ouyang, H.; Wang, R.; Li, J.; Huang, H.; Jin, Y.; Feng, Y.; Yang, S. Synthesis and biological evaluation of Hederacolchiside A1 derivatives as anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Duan, H.; Wang, M.; Han, L.; Liu, Y.; Zhu, Y.; Yang, S. Synthesis, cytotoxicity and haemolytic activity of Pulsatilla saponin A, D derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.; Beshr, E.A.; Ali, T. New nitric oxide donating 1,2,4-triazole/oxime hybrids: Synthesis, investigation of anti-inflammatory, ulceroginic liability and antiproliferative activities. Bioorg. Med. Chem. 2013, 21, 3839–3849. [Google Scholar] [CrossRef]
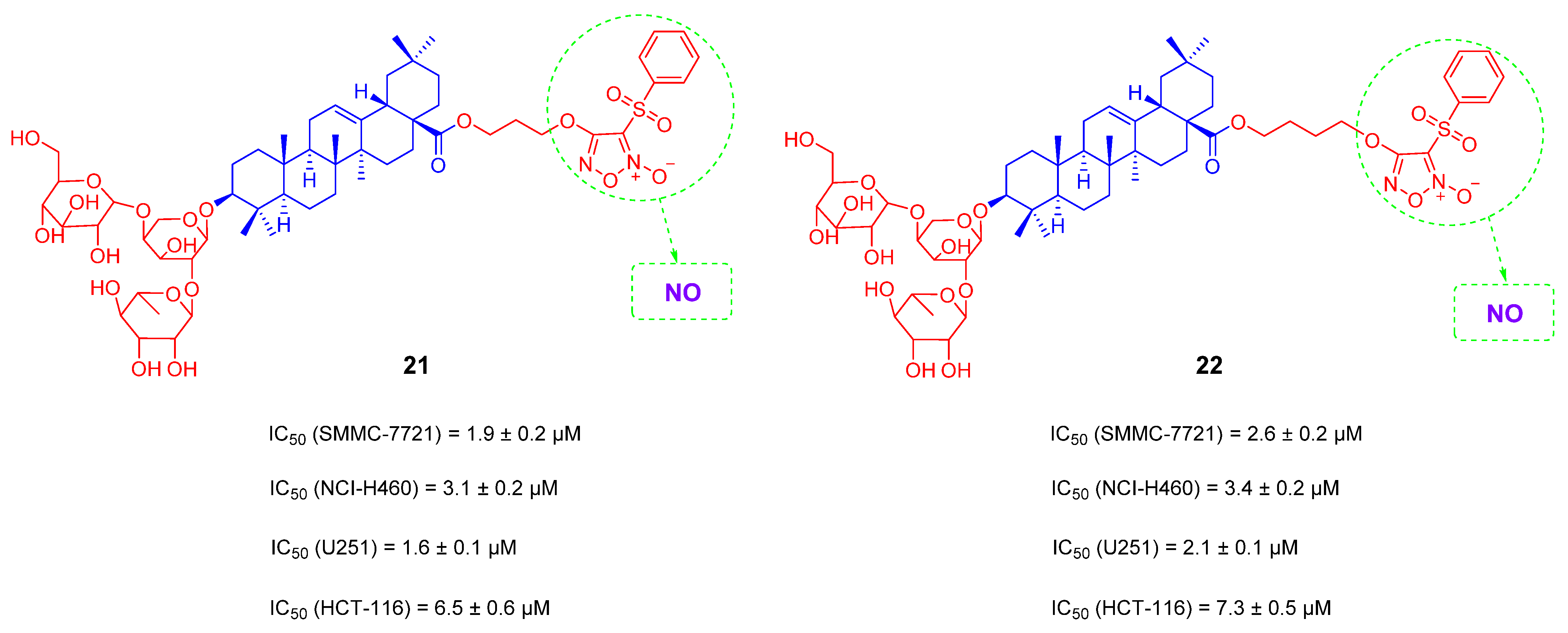
- Zou, Y.; Yan, C.; Liu, J.-C.; Huang, Z.-J.; Xu, J.-Y.; Zhou, J.-P.; Zhang, H.-B.; Zhang, Y.-H. Synthesis and anti-hepatocellular carcinoma activity of novel O2-vinyl diazeniumdiolate-based nitric oxide-releasing derivatives of oleanolic acid. Chin. J. Nat. Med. 2017, 15, 928–937. [Google Scholar]
- Fang, Y.; Wang, R.; He, M.; Huang, H.; Wang, Q.; Yang, Z.; Li, Y.; Yang, S.; Jin, Y. Nitric oxide-donating derivatives of hederacolchiside A1: Synthesis and biological evaluation in vitro and in vivo as potential anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 98–101. [Google Scholar] [CrossRef]
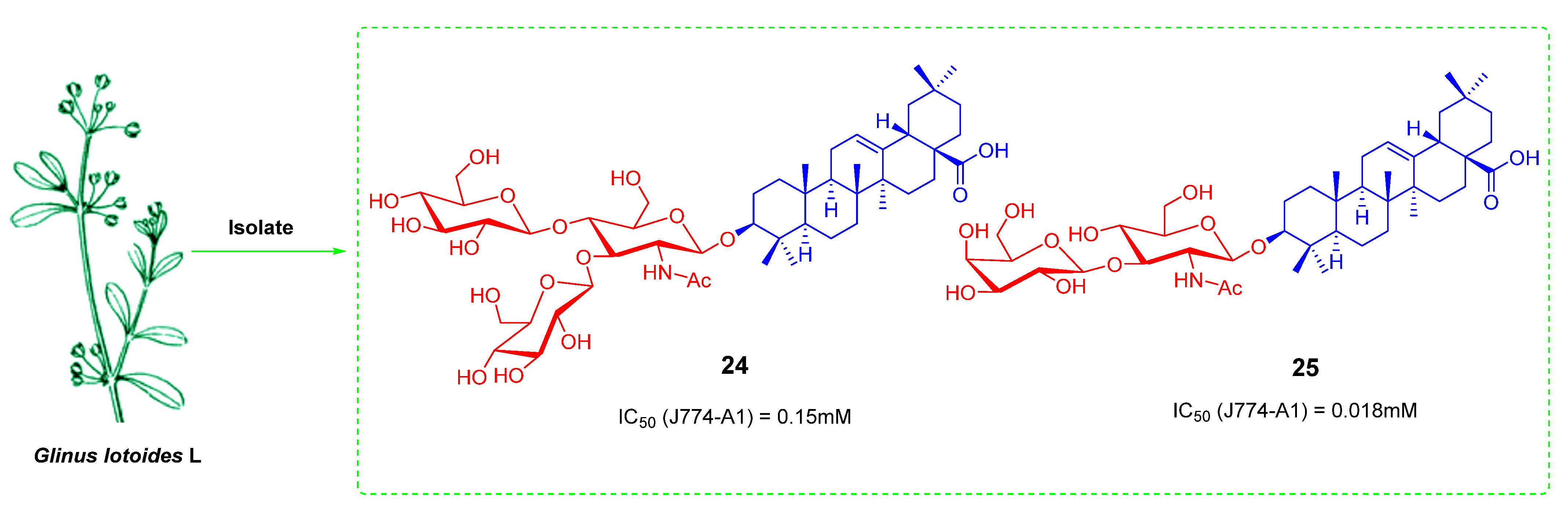
- Hamed, A.I.; Piacente, S.; Autore, G.; Marzocco, S.; Pizza, C.; Oleszek, W. Antiproliferative hopane and oleanane glycosides from the roots of Glinus lotoides. Planta Med. 2005, 71, 554–560. [Google Scholar] [CrossRef]
- Wei, G.; Cui, S.; Luan, W.; Wang, S.; Hou, Z.; Liu, Y.; Liu, Y.; Cheng, M. Natural product-based design, synthesis and biological evaluation of Albiziabioside A derivatives that selectively induce HCT116 cell death. Eur. J. Med. Chem. 2016, 113, 92–101. [Google Scholar] [CrossRef]
- Zhang, H.; Samadi, A.K.; Rao, K.V.; Cohen, M.S.; Timmermann, B.N. Cytotoxic oleanane-type saponins from Albizia inundata. J. Nat. Prod. 2011, 74, 477–482. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Su, S.; Xing, Y.; Li, Y.; Li, M.; Liu, J.; Yang, S. Synthesis and cytotoxicity of oleanolic acid trisaccharide saponins. Carbohydr. Res. 2017, 442, 9–16. [Google Scholar] [CrossRef]
- Seo, Y.; Hoch, J.; Abdel-Kader, M.; Malone, S.; Derveld, I.; Adams, H.; Werkhoven, M.C.M.; Wisse, J.H.; Mamber, S.W.; Dalton, J.M.; et al. Bioactive saponins from Acacia tenuifolia from the suriname rainforest. J. Nat. Prod. 2002, 65, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.C.; Liu, Y.; Chen, H.; Ke, Y.; Xu, Q.C.; Cheng, M.S. Synthesis and anti-tumor activity of two natural N-acetylglucosamine-bearing triterpenoid saponins: Lotoidoside D and E. Bioorg. Med. Chem. Lett. 2006, 16, 4200–4204. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Chan, S.-H.; Juang, Y.-P.; Hsiao, H.-M.; Guh, J.-H.; Liang, P.-H. Design, synthesis and cytotoxic activity of N-Modified oleanolic saponins bearing A glucosamine. Eur. J. Med. Chem. 2018, 143, 1942–1958. [Google Scholar] [CrossRef] [PubMed]
- Juang, Y.-P.; Lin, Y.-Y.; Chan, S.-H.; Chang, C.-K.; Shie, J.-J.; Hsieh, Y.S.; Guh, J.-H.; Liang, P.-H. Synthesis, distribution analysis and mechanism studies of N-acyl glucosamine-bearing oleanolic saponins. Bioorg. Chem. 2020, 99, 103835. [Google Scholar] [CrossRef]
- Jung, H.-J.; Lee, C.O.; Lee, K.-T.; Choi, J.; Park, H.-J. Structure-activity relationship of oleanane disaccharides isolated from Akebia quinata versus cytotoxicity against cancer cells and NO inhibition. Biol. Pharm. Bull. 2004, 27, 744–747. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, J.; Sun, W.; Zhang, T.; Zuo, D.; Wang, H.; Wang, G.; Xu, J.; Yin, F.; Mao, M.; et al. Anti-tumor activity of Raddeanin A is mediated by Jun amino-terminal kinase activation and signal transducer and activator of transcription 3 inhibition in human osteosarcoma. Cancer Sci. 2019, 110, 1746–1759. [Google Scholar] [CrossRef]
- Li, H.N.; Wang, H.; Wang, Z.P.; Yan, H.N.; Zhang, M.; Liu, Y.; Cheng, M.S. Synthesis, anti-tumor activity evaluation and mechanistic study of novel hederacolchiside A1 derivatives bearing an aryl triazole moiety. Bioorg. Med. Chem. 2018, 26, 4025–4033. [Google Scholar] [CrossRef]
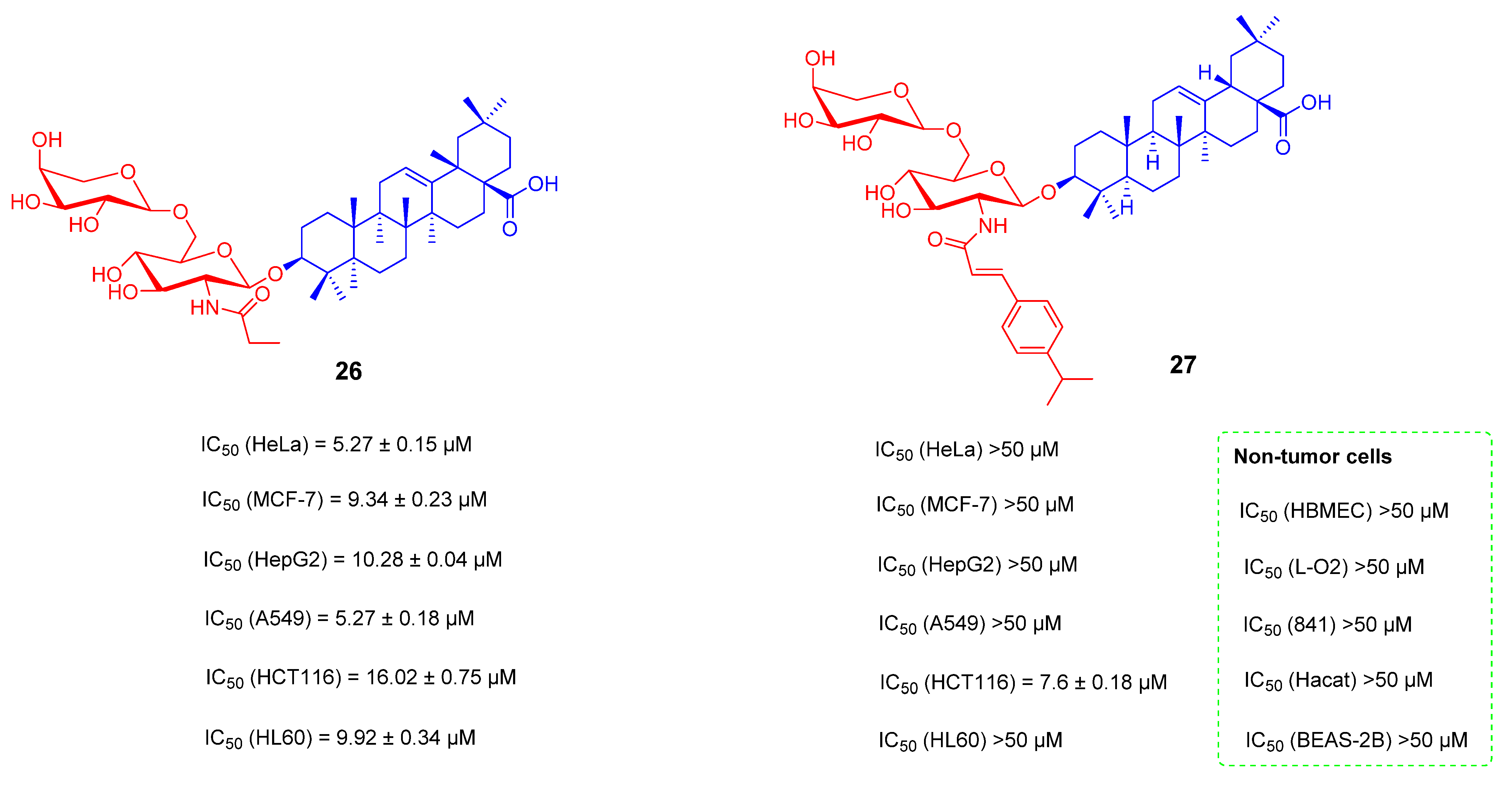
- Zhong, Y.; Li, H.N.; Zhou, L.; Su, H.S.; Cheng, M.S.; Liu, Y. Synthesis and anti-tumor activity evaluation of oleanolic acid saponins bearing an acetylated l-arabinose moiety. Carbohydr. Res. 2021, 503, 108311. [Google Scholar] [CrossRef]
- Soica, C.; Trandafirescu, C.; Danciu, C.; Muntean, D.; Dehelean, C.; Simu, G. New improved drug delivery technologies for pentacyclic triterpenes: A review. Protein Pept. Lett. 2014, 21, 1137–1145. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Abrego, G.; Garduño-Ramirez, M.L.; Clares, B.; Calpena, A.C.; García, M.L. Design and optimization of oleanolic/ursolic acid-loaded nanoplatforms for ocular anti-inflammatory applications. Nanomedicine 2015, 11, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Man, D.K.W.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F.; Sze, S.C.W.; Leung, G.P.H.; Lam, J.; Kwok, P.C.L. Oleanolic Acid Loaded PEGylated PLA and PLGA Nanoparticles with Enhanced Cytotoxic Activity against Cancer Cells. Mol. Pharm. 2015, 12, 2112–2125. [Google Scholar] [CrossRef]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Martinez, A.; Martin-Fonseca, S.; Garcia-Granados, A.; Ferrer-Martín, R.M.; Lupianez, J.A.; Parra, A. Semi-synthesis and antiproliferative evaluation of PEGylated pentacyclic triterpenes. Eur. J. Med. Chem. 2016, 118, 64–78. [Google Scholar] [CrossRef]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977, 252, 3582–3586. [Google Scholar] [CrossRef]
- Heller, L.; Knorrscheidt, A.; Flemming, F.; Wiemann, J.; Sommerwerk, S.; Pavel, I.Z.; Al-Harrasi, A.; Csuk, R. Synthesis and proapoptotic activity of oleanolic acid derived amides. Bioorg. Chem. 2016, 68, 137–151. [Google Scholar] [CrossRef]
- Jannus, F.; Medina-O’Donnell, M.; Rivas, F.; Díaz-Ruiz, L.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Parra, A.; Reyes-Zurita, F.J. A Diamine-PEGylated Oleanolic Acid Derivative Induced Efficient Apoptosis through a Death Receptor and Mitochondrial Apoptotic Pathway in HepG2 Human Hepatoma Cells. Biomolecules 2020, 10, 1375. [Google Scholar] [CrossRef]
- Kahnt, M.; Loesche, A.; Serbian, I.; Hoenke, S.; Fischer, L.; Al-Harrasi, A.; Csuk, R. The cytotoxicity of oleanane derived aminocarboxamides depends on their aminoalkyl substituents. Steroids 2019, 149, 108422. [Google Scholar] [CrossRef]
- Feng, B.; Zhao, C.; Li, J.; Yu, J.; Zhang, Y.; Zhang, X.; Tian, T.; Zhao, L. The Novel Synthetic Triterpene Methyl 3β-O-[4-(2-Aminoethylamino)-4-oxo-butyryl] olean-12-ene-28-oate Inhibits Breast Tumor Cell Growth in Vitro and in Vivo. Chem. Pharm. Bull. 2020, 68, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Elhassan, R.M.; Hou, X.; Fang, H. Recent Advances in Small Molecule PROTACs for the Treatment of Cancer. Curr. Med. Chem. 2021, 28, 4893–4909. [Google Scholar] [CrossRef]
- Wu, T.; Yoon, H.; Xiong, Y.; Dixon-Clarke, S.E.; Nowak, R.P.; Fischer, E.S. Targeted protein degradation as a powerful research tool in basic biology and drug target discovery. Nat. Struct. Mol. Biol. 2020, 27, 605–614. [Google Scholar] [CrossRef]
- Spradlin, J.N.; Hu, X.; Ward, C.C.; Brittain, S.M.; Jones, M.D.; Ou, L.; To, M.; Proudfoot, A.; Ornelas, E.; Woldegiorgis, M.; et al. Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat. Chem. Biol. 2019, 15, 747–755. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Lin, S.; Yan, W.; Chen, D.; Zeng, Z.; Chen, L.; Li, Y.; He, B. Enhanced Water Solubility and Anti-Tumor Activity of Oleanolic Acid through Chemical Structure Modification. Int. J. Mol. Sci. 2022, 23, 13291. https://doi.org/10.3390/ijms232113291
Gu Z, Lin S, Yan W, Chen D, Zeng Z, Chen L, Li Y, He B. Enhanced Water Solubility and Anti-Tumor Activity of Oleanolic Acid through Chemical Structure Modification. International Journal of Molecular Sciences. 2022; 23(21):13291. https://doi.org/10.3390/ijms232113291
Chicago/Turabian StyleGu, Zhicheng, Shuxian Lin, Wanli Yan, Di Chen, Ziwei Zeng, Lei Chen, Yan Li, and Bin He. 2022. "Enhanced Water Solubility and Anti-Tumor Activity of Oleanolic Acid through Chemical Structure Modification" International Journal of Molecular Sciences 23, no. 21: 13291. https://doi.org/10.3390/ijms232113291
APA StyleGu, Z., Lin, S., Yan, W., Chen, D., Zeng, Z., Chen, L., Li, Y., & He, B. (2022). Enhanced Water Solubility and Anti-Tumor Activity of Oleanolic Acid through Chemical Structure Modification. International Journal of Molecular Sciences, 23(21), 13291. https://doi.org/10.3390/ijms232113291




