A Case Series Exploration of Multi-Regional Expression Heterogeneity in Triple-Negative Breast Cancer Patients
Abstract
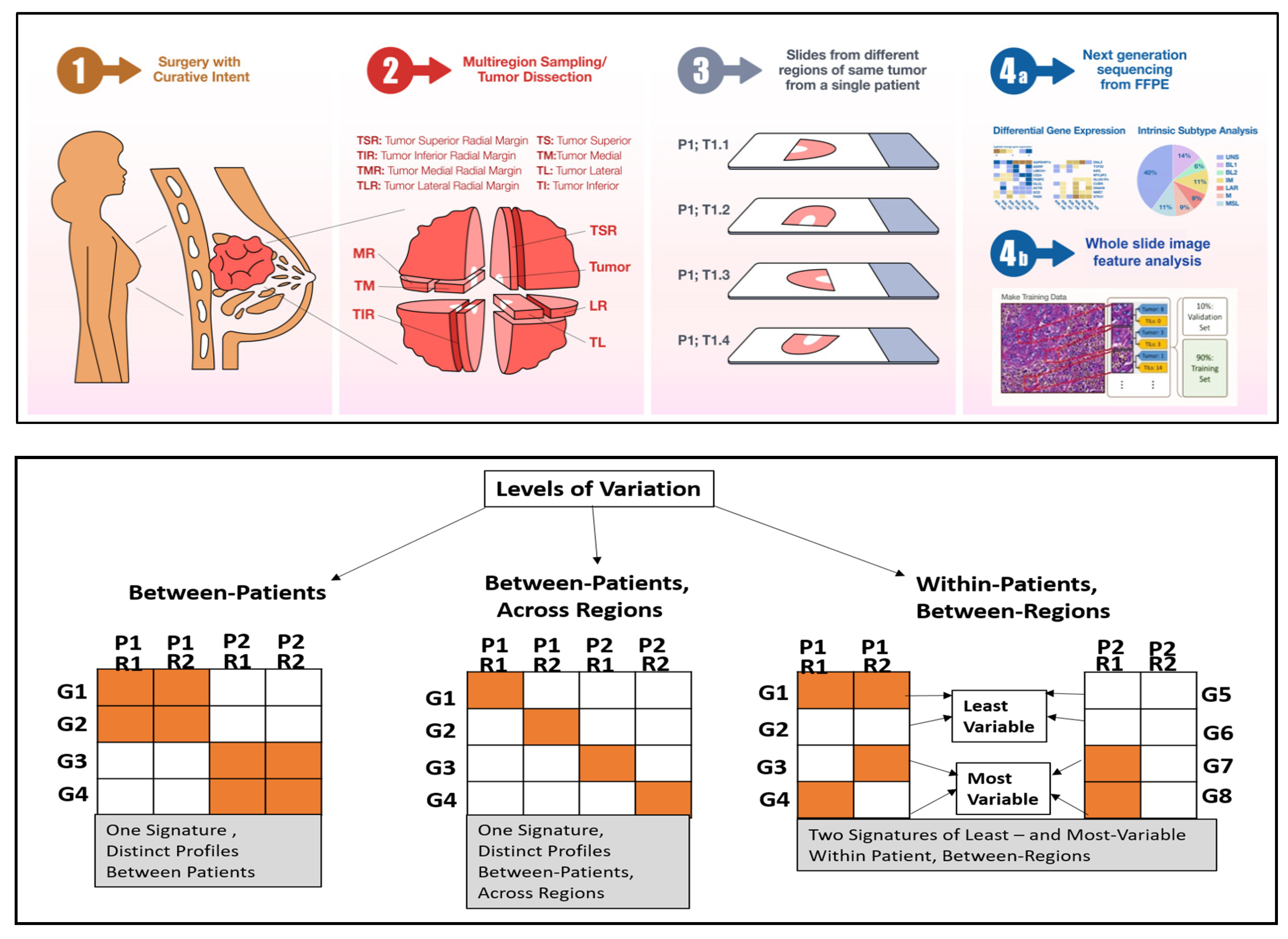
:1. Introduction
2. Results
2.1. Tumor and Patient Characteristics
2.2. Between-Patients (Intertumoral) Gene Expression Heterogeneity
2.3. Gene Expression Heterogeneity within Patient, between Regions
3. Discussion
4. Materials and Methods
4.1. TNBC Samples
4.2. RNA-seq Data Processing
4.3. Variability Analysis of Gene Expression
4.4. Enrichment Analysis
4.5. Association Analysis
4.6. Subtyping
4.7. Prediction of Immune, Stroma, and Tumor Purity Scores
4.8. Slide Annotation
4.9. Divergent Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval and Consent to Participate
References
- Qiu, J.; Xue, X.; Hu, C.; Xu, H.; Kou, D.; Li, R.; Li, M. Comparison of Clinicopathological Features and Prognosis in Triple-Negative and Non-Triple Negative Breast Cancer. J. Cancer 2016, 7, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietze, E.C.; Sistrunk, C.; Miranda-Carboni, G.; O’Regan, R.; Seewaldt, V.L. Triple-negative breast cancer in African-American women: Disparities versus biology. Nat. Rev. Cancer 2015, 15, 248–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Di, G. Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance. Chin. J. Cancer Res. 2017, 29, 237. [Google Scholar] [CrossRef]
- Fan, Y.; He, S. The Characteristics of Tumor Microenvironment in Triple Negative Breast Cancer. Cancer Manag. Res. 2022, 14, 1–17. [Google Scholar] [CrossRef]
- Dieci, M.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef] [Green Version]
- Kvokačková, B.; Remšík, J.; Jolly, M.; Souček, K. Phenotypic Heterogeneity of Triple-Negative Breast Cancer Mediated by Epithelial–Mesenchymal Plasticity. Cancers 2021, 13, 2188. [Google Scholar] [CrossRef] [PubMed]
- Tchou, J.; Kossenkov, A.V.; Chang, L.; Satija, C.; Herlyn, M.; Showe, L.C.; Puré, E. Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles. BMC Med. Genom. 2012, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- LLiedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Jerusalem, G.; Collignon, J.; Schroeder, H.; Lousberg, L. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer: Targets Ther. 2016, 8, 93–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, P.L.; Hansen, A.R.; Ratain, M.J.; Siu, L.L. Tumour heterogeneity in the clinic. Nature 2013, 501, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navin, N.; Krasnitz, A.; Rodgers, L.; Cook, K.; Meth, J.; Kendall, J.; Riggs, M.; Eberling, Y.; Troge, J.; Grubor, V.; et al. Inferring tumor progression from genomic heterogeneity. Genome Res. 2009, 20, 68–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Ellis, M.J.; Li, S.; Larson, D.E.; Chen, K.; Wallis, J.W.; Harris, C.C.; McLellan, M.D.; Fulton, R.S.; Fulton, L.L.; et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 2010, 464, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Navin, N.; Kendall, J.; Troge, J.; Andrews, P.; Rodgers, L.; McIndoo, J.; Cook, K.; Stepansky, A.; Levy, D.; Esposito, D.; et al. Tumour evolution inferred by single-cell sequencing. Nature 2011, 472, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Banerji, S.; Cibulskis, K.; Rangel-Escareno, C.; Brown, K.K.; Carter, S.L.; Frederick, A.M.; Lawrence, M.S.; Sivachenko, A.Y.; Sougnez, C.; Zou, L.; et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 2012, 486, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Tarpey, P.S.; Davies, H.; Van Loo, P.; Greenman, C.; Wedge, D.C.; Nik-Zainal, S.; Martin, S.; Varela, I.; Bignell, G.R.; et al. The landscape of cancer genes and mutational processes in breast cancer. Nature 2012, 486, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Waters, J.; Leung, M.L.; Unruh, A.; Roh, W.; Shi, X.; Chen, K.; Scheet, P.; Vattathil, S.; Liang, H.; et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nat. 2014, 512, 155–160. [Google Scholar] [CrossRef]
- araayvaz, M.; Cristea, S.; Gillespie, S.M.; Patel, A.P.; Mylvaganam, R.; Luo, C.C.; Specht, M.C.; Bernstein, B.E.; Michor, F.; Ellisen, L.W. Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat. Commun. 2018, 9, 3588. [Google Scholar] [CrossRef] [Green Version]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalski, J.; Tu, X.M. Modern Applied U-Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Hubalek, M.; Czech, T.; Müller, H. Biological Subtypes of Triple-Negative Breast Cancer. Breast Care 2017, 12, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santonja, A.; Sánchez-Muñoz, A.; Lluch, A.; Chica-Parrado, M.R.; Albanell, J.; Chacón, J.I.; Antolín, S.; Jerez, J.M.; de la Haba, J.; de Luque, V.; et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget 2018, 9, 26406–26416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Giuli, M.V.; Giuliani, E.; Screpanti, I.; Bellavia, D.; Checquolo, S. Notch Signaling Activation as a Hallmark for Triple-Negative Breast Cancer Subtype. J. Oncol. 2019, 2019, 8707053. [Google Scholar] [CrossRef]
- Qiu, M.; Peng, Q.; Jiang, I.; Carroll, C.; Han, G.; Rymer, I.; Lippincott, J.; Zachwieja, J.; Gajiwala, K.; Kraynov, E.; et al. Specific inhibition of Notch1 signaling enhances the antitumor efficacy of chemotherapy in triple negative breast cancer through reduction of cancer stem cells. Cancer Lett. 2013, 328, 261–270. [Google Scholar] [CrossRef]
- Lim, J.S.; Ibaseta, A.; Fischer, M.M.; Cancilla, B.; O’Young, G.; Cristea, S.; Luca, V.C.; Yang, D.; Jahchan, N.S.; Hamard, C.; et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 2017, 545, 360–364. [Google Scholar] [CrossRef] [Green Version]
- Zou, B.; Zhou, X.; Lai, S.; Liu, J. Notch signaling and non-small cell lung cancer (Review). Oncol. Lett. 2018, 15, 3415–3421. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.F.; Zhang, X.; Su, Y.G.; Zhao, R.; Wang, Y.Y. The role of the deubiquitinating enzyme DUB3/USP17 in cancer: A narrative review. Cancer Cell Int. 2021, 21, 455. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Yuan, J.; Chen, H. Ubiquitin-specific peptidase 17 promotes cisplatin resistance via PI3K/AKT activation in non-small cell lung cancer. Oncol. Lett. 2020, 20, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, C.; Liao, K.; Zhou, S.; Cao, L.; Chen, J.; Xu, C.; Lin, Y. USP17 Suppresses Tumorigenesis and Tumor Growth through Deubiquitinating AEP. Int. J. Biol. Sci. 2019, 15, 738–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, R.; Carvalho, M.J.; Goncalves, J.; Moreira, J.N. Immunotherapy in triple-negative breast cancer: Insights into tumor immune landscape and therapeutic opportunities. Front. Mol. Biosci. 2022, 9, 903065. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef]
- Kim, I.S.; Zhang, X.H.-F. One microenvironment does not fit all: Heterogeneity beyond cancer cells. Cancer Metastasis Rev. 2016, 35, 601–629. [Google Scholar] [CrossRef] [Green Version]
- Finak, G.; Bertos, N.; Pepin, F.; Sadekova, S.; Souleimanova, M.; Zhao, H.; Chen, H.; Omeroglu, G.; Meterissian, S.; Omeroglu, A.; et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 2008, 14, 518–527. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Perou, C.M. Molecular stratification of triple-negative breast cancers. Oncologist 2011, 16 (Suppl. 1), 61–70. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef] [PubMed]
- RRakha, E.A.; Elsheikh, S.E.; Aleskandarany, M.A.; Habashi, H.O.; Green, A.R.; Powe, D.G.; El-Sayed, M.E.; Benhasouna, A.; Brunet, J.-S.; Akslen, L.A.; et al. Triple-Negative Breast Cancer: Distinguishing between Basal and Nonbasal Subtypes. Clin. Cancer Res. 2009, 15, 2302–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd El-Rehim, D.M.; Ball, G.; Pinder, S.E.; Rakha, E.; Paish, C.; Robertson, J.F.; Macmillan, D.; Blamey, R.W.; Ellis, I.O. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int. J. Cancer 2005, 116, 340–350. [Google Scholar] [CrossRef]
- Ellis, I.O.; Al-Sam, S.; Anderson, N.; Carder, P.; Deb, R.; Girling, A.; Hales, S.; Hanby, A.; Ibrahim, M.; Lee, A.; et al. Pathology reporting of breast disease in surgical excision specimens incorporating the dataset for histological reporting of breast cancer, June 2016. R. Coll. Pathol. 2019. Available online: https://www.rcpath.org/asset/693DB661-0592-4D7E-9644357FBFA00A76/ (accessed on 20 September 2022).
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Res 2015, 4, 1521. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Parker, H.S.; Fertig, E.J.; Jaffe, A.E.; Storey, J.D.; Zhang, Y.; Torres, L.C. Surrogate Variable Analysis, R package version 3.24.4; University of Washington: Washington, DC, USA, 2017. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [Green Version]
- Erdman, C.; Emerson, J.W. bcp: An R package for performing a Bayesian analysis of change point problems. J. Stat. Softw. 2008, 23, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, A.; Bader, G.D.; Reimand, J. HyperModules: Identifying clinically and phenotypically significant network modules with disease mutations for biomarker discovery. Bioinformatics 2014, 30, 2230–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picornell, A.C.; Echavarria, I.; Alvarez, E.; López-Tarruella, S.; Jerez, Y.; Hoadley, K.; Parker, J.S.; del Monte-Millán, M.; Ramos-Medina, R.; Gayarre, J.; et al. Breast cancer PAM50 signature: Correlation and concordance between RNA-Seq and digital multiplexed gene expression technologies in a triple negative breast cancer series. BMC Genom. 2019, 20, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gendoo, D.; Ratanasirigulchai, N.; Schröder, M.; Pare, L.; Parker, J.S.; Prat, A.; Haibe-Kains, B. Genefu: An R/Bioconductor package for computation of gene expression-based signatures in breast cancer. Bioinformatics 2015, 32, 1097–1099. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, J.; Gray, W.H.; Lehmann, B.D.; Bauer, J.A.; Shyr, Y.; Pietenpol, J.A. TNBCtype: A Subtyping Tool for Triple-Negative Breast Cancer. Cancer Inform. 2012, 11, 147–156. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J.; Freidank, M.; Cai, J.; Protivinsky, Y. Package ‘Corrplot’: Visualization of a Correlation Matrix, Version 0.92. 2021. Available online: https://github.com/taiyun/corrplot (accessed on 20 September 2022).






| Menopausal Status, n (%) | |
|---|---|
| Pre | 6 (60%) |
| Post | 4 (40%) |
| Age at diagnosis, n (%) | |
| <50 | 4 (40%) |
| ≥50 | 6 (60%) |
| Tumor size, n (%) | |
| <2.0 | 2 (20%) |
| ≥2.0 | 8 (80%) |
| Grade, n (%) | |
| 1 | 1 (10%) |
| 2 | 1 (10%) |
| 3 | 8 (80%) |
| LN stage, n (%) | |
| 1 | 2 (20%) |
| 2 | 5 (50%) |
| 3 | 3 (30%) |
| Chemotherapy, n (%) | |
| CMF | 7 (70%) |
| No therapy | 1 (10%) |
| Missing Data | 2 (20%) |
| Recurrence, n (%) | |
| Yes | 4 (40%) |
| No | 6 (60%) |
| Distant metastasis, n (%) | |
| Yes | 4 (40%) |
| No | 6 (60%) |
| Alive or dead, n (%) | |
| Alive | 6 (60%) |
| Died from Breast Cancer | 4 (40%) |
| Patient | RNA-Seq-Derived Gene Expression | Image-Derived Cell Counts | ||||||
|---|---|---|---|---|---|---|---|---|
| PAM50 | TNBC | Immune Cell | Immune | Stroma | Tumor | Tumor | TILs | |
| Subtype | Subtype | Type | Score | Score | Purity | Cell Counts | Cell Counts | |
| P1 | D | D | D | D | D | C | D | D |
| P2 | D | D | C | D | D | D | ||
| P3 | D | D | D | D | C | D | C | D |
| P4 | D | C | D | D | C | D | C | D |
| P5 | C | D | C | D | C | D | D | C |
| P6 | D | C | C | C | C | C | D | D |
| P7 | D | C | C | C | C | D | D | D |
| P8 | D | D | D | C | C | C | ||
| P9 | D | C | D | C | C | C | C | C |
| P10 | C | D | C | C | C | C | ||
| Divergent | CMF | Lymph Node Stage | CMF | Lymph Node Stage | Grade | |||
| Association | Chemotherapy | Chemotherapy | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Kaur, J.; Wylie, D.; Mittal, K.; Li, H.; Kolachina, R.; Aleskandarany, M.; Toss, M.S.; Green, A.R.; Yang, J.; et al. A Case Series Exploration of Multi-Regional Expression Heterogeneity in Triple-Negative Breast Cancer Patients. Int. J. Mol. Sci. 2022, 23, 13322. https://doi.org/10.3390/ijms232113322
Xu Q, Kaur J, Wylie D, Mittal K, Li H, Kolachina R, Aleskandarany M, Toss MS, Green AR, Yang J, et al. A Case Series Exploration of Multi-Regional Expression Heterogeneity in Triple-Negative Breast Cancer Patients. International Journal of Molecular Sciences. 2022; 23(21):13322. https://doi.org/10.3390/ijms232113322
Chicago/Turabian StyleXu, Qi, Jaspreet Kaur, Dennis Wylie, Karuna Mittal, Hongxiao Li, Rishab Kolachina, Mohammed Aleskandarany, Michael S. Toss, Andrew R. Green, Jianchen Yang, and et al. 2022. "A Case Series Exploration of Multi-Regional Expression Heterogeneity in Triple-Negative Breast Cancer Patients" International Journal of Molecular Sciences 23, no. 21: 13322. https://doi.org/10.3390/ijms232113322
APA StyleXu, Q., Kaur, J., Wylie, D., Mittal, K., Li, H., Kolachina, R., Aleskandarany, M., Toss, M. S., Green, A. R., Yang, J., Yankeelov, T. E., Bhattarai, S., Janssen, E. A. M., Kong, J., Rakha, E. A., Kowalski, J., & Aneja, R. (2022). A Case Series Exploration of Multi-Regional Expression Heterogeneity in Triple-Negative Breast Cancer Patients. International Journal of Molecular Sciences, 23(21), 13322. https://doi.org/10.3390/ijms232113322









