Preinitiation Complex Loading onto mRNAs with Long versus Short 5′ TLs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Translation Initiation Can Occur on Very Short 5′ TLs
2.2. TISU Reduces Leaky Scanning on a Short 5′ TL
2.3. Translation from a Short 5′ TL Is Cap Dependent
- RRLs are a poor in vitro system to monitor 5′ cap dependence of translation, an observation already noted by a number of investigators [34].
- The expression profiles in both systems reveals no evident difference in the behavior of the short and long 5′ TLs with regards to cap-dependence. Translation from the short 5′ TL is consequently cap-dependent.
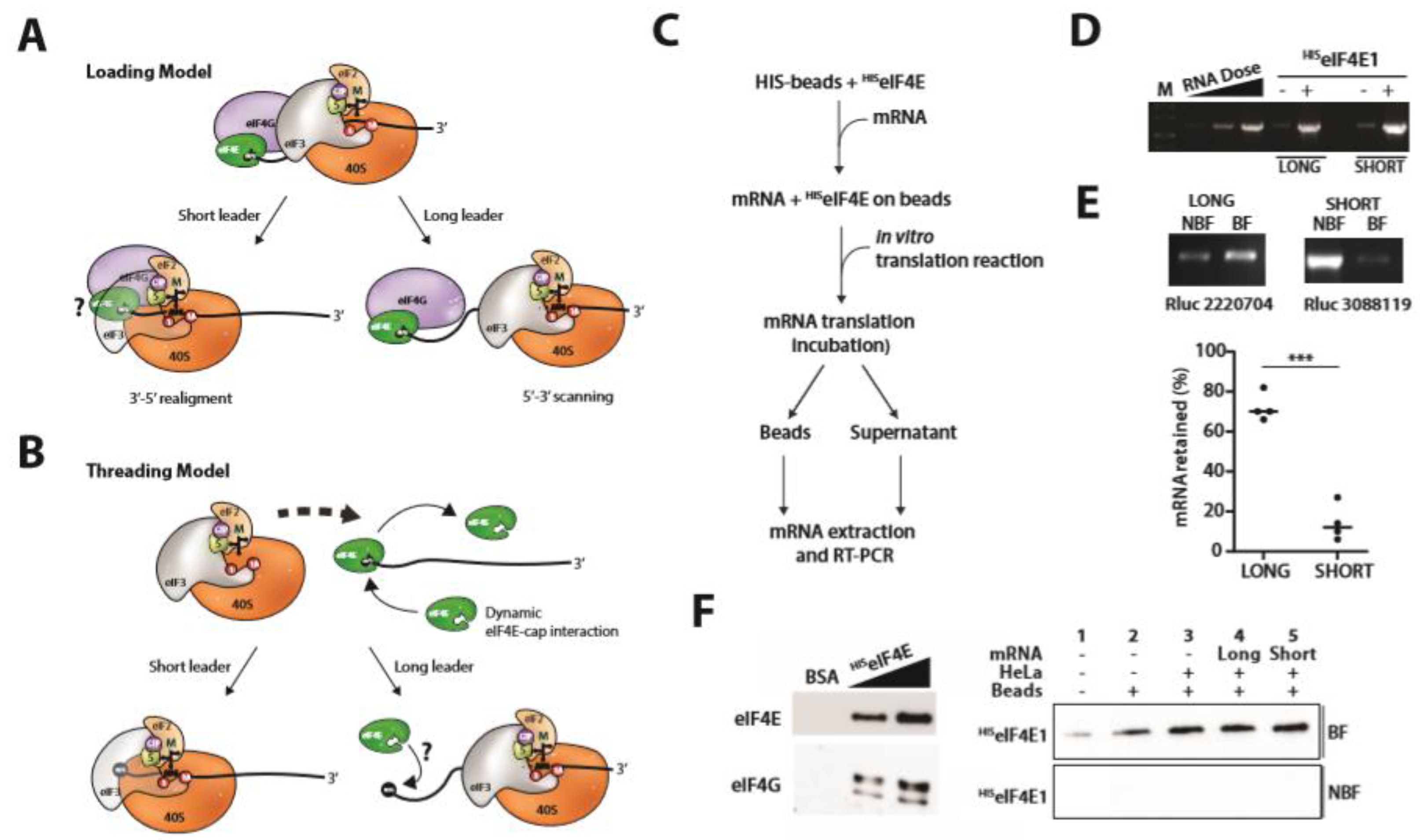
2.4. PIC Loading
2.5. Future Directions
3. Materials and Methods
- G: 5′TAATACGACTCACTATAGATGGACCCATCTGTG
- GGG: 5′TAATACGACTCACTATAGGGATGACTTCGAAAGTTTATGA
- Rdm:5′CGCTAGCTAATACGACTCACTATAGGGAGAAAATGACTTCGAAAGTTTAT
- TISU: 5′-TAATACGACTCACTATAGGGACAAGATGGCGGCATCTGTGACGCTGTGG
- Reverse: 5′-TCAGCGAGCTCTAGCATTTAGGTG
- (-VE): GAACACCACGGTAGGCTGCGAAATG
- (+VE) GATCAAAGCAATAGTTCACGCTGAAAGTGTAG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buttgereit, F.; Brand, M.D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 1995, 312, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Szamecz, B.; Rutkai, E.; Cuchalová, L.; Munzarová, V.; Herrmannová, A.; Nielsen, K.H.; Burela, L.; Hinnebusch, A.G.; Valášek, L. eIF3a cooperates with sequences 5′ of uORF1 to promote resumption of scanning by post-termination ribosomes for reinitiation on GCN4 mRN.A. Genes Dev. 2008, 22, 2414–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinnebusch, A.G. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol. Mol. Biol. Rev. MMBR 2011, 75, 434–467. [Google Scholar] [CrossRef] [Green Version]
- Merrick, W.C. eIF4F: A Retrospective. J. Biol. Chem. 2015, 290, 24091–24099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozak, M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987, 15, 8125–8148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.P.; Firth, A.E.; Michel, A.M.; Atkins, J.F.; Baranov, P.V. Identification of evolutionarily conserved non-AUG-initiated N-terminal extensions in human coding sequences. Nucleic Acids Res. 2011, 39, 4220–4234. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Marintchev, A.; Kolupaeva, V.G.; Unbehaun, A.; Veryasova, T.; Lai, S.-C.; Hong, P.; Wagner, G.; Hellen, C.U.T.; Pestova, T.V. Position of eukaryotic translation initiation factor eIF1A on the 40S ribosomal subunit mapped by directed hydroxyl radical probing. Nucleic Acids Res. 2009, 37, 5167–5182. [Google Scholar] [CrossRef]
- Weisser, M.; Voigts-Hoffmann, F.; Rabl, J.; Leibundgut, M.; Ban, N. The crystal structure of the eukaryotic 40S ribosomal subunit in complex with eIF1 and eIF1A. Nat. Struct. Mol. Biol. 2013, 20, 1015–1017. [Google Scholar] [CrossRef]
- Aitken, C.E.; Lorsch, J.R. A mechanistic overview of translation initiation in eukaryotes. Nat. Struct. Mol. Biol. 2012, 19, 568–576. [Google Scholar] [CrossRef]
- Passmore, L.A.; Schmeing, T.M.; Maag, D.; Applefield, D.J.; Acker, M.G.; Algire, M.A.; Lorsch, J.R.; Ramakrishnan, V. The Eukaryotic Translation Initiation Factors eIF1 and eIF1A Induce an Open Conformation of the 40S Ribosome. Mol. Cell 2007, 26, 41–50. [Google Scholar] [CrossRef]
- Algire, M.A.; Maag, D.; Lorsch, J.R. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 2005, 20, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. A short leader sequence impairs the fidelity of initiation by eukaryotic ribosomes. Gene Expr. 1991, 1, 111–115. [Google Scholar]
- Ingolia, N.T. Chapter 6—Genome-Wide Translational Profiling by Ribosome Footprinting. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2010; pp. 119–142. [Google Scholar]
- Pisarev, A.V.; Kolupaeva, V.G.; Yusupov, M.M.; Hellen, C.U.; Pestova, T.V. Ribosomal position and contacts of mRNA in eukaryotic translation initiation complexes. EMBO J. 2008, 27, 1609–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curran, J.A.; Weiss, B. What Is the Impact of mRNA 5′ TL Heterogeneity on Translational Start Site Selection and the Mammalian Cellular Phenotype? Front. Genet. 2016, 7, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaji, H.; Kasukawa, T.; Fukuda, S.; Katayama, S.; Kai, C.; Kawai, J.; Carninci, P.; Hayashizaki, Y. CAGE Basic/Analysis Databases: The CAGE resource for comprehensive promoter analysis. Nucleic Acids Res. 2006, 34, D632–D636. [Google Scholar] [CrossRef] [PubMed]
- Carninci, P.; Sandelin, A.; Lenhard, B.; Katayama, S.; Shimokawa, K.; Ponjavic, J.; Semple, C.; Taylor, M.; Engström, P.; Frith, M.; et al. Genome-wide analysis of mammalian promoter architecture and evolution. Nat. Genet. 2006, 38, 626–635. [Google Scholar] [CrossRef]
- Elfakess, R.; Sinvani, H.; Haimov, O.; Svitkin, Y.; Sonenberg, N.; Dikstein, R. Unique translation initiation of mRNAs-containing TISU element. Nucleic Acids Res. 2011, 39, 7598–7609. [Google Scholar] [CrossRef]
- Elfakess, R.; Dikstein, R. A translation initiation element specific to mRNAs with very short 5′UTR that also regulates transcription. PLoS ONE 2008, 3, e3094. [Google Scholar] [CrossRef] [Green Version]
- Pestova, T.V.; Kolupaeva, V.G. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 2002, 16, 2906–2922. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Hellen, C.U.; Pestova, T.V. Toward the mechanism of eIF4F-mediated ribosomal attachment to mammalian capped mRNAs. Genes Dev. 2016, 30, 1573–1588. [Google Scholar] [CrossRef] [Green Version]
- Dikstein, R. Transcription and translation in a package deal: The TISU paradigm. Gene 2012, 491, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Legrand, N.; Araud, T.; Conne, B.; Kuijpers, O.; Jaquier-Gubler, P.; Curran, J. An AUG codon conserved for protein function rather than translational initiation: The story of the protein sElk1. PLoS ONE 2014, 9, e102890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahim, G.; Araud, T.; Jaquier-Gubler, P.; Curran, J. Alternative splicing within the elk-1 5′ untranslated region serves to modulate initiation events downstream of the highly conserved upstream open reading frame 2. Mol. Cell Biol. 2012, 32, 1745–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weston, B.F.; Kuzmine, I.; Martin, C.T. Positioning of the start site in the initiation of transcription by bacteriophage T7 RNA polymerase. J. Mol. Biol. 1997, 272, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Fuerst, T.R.; Earl, P.L.; Moss, B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol. Cell Biol. 1987, 7, 2538–2544. [Google Scholar]
- Dai, A.; Cao, S.; Dhungel, P.; Luan, Y.; Liu, Y.; Xie, Z.; Yang, Z. Ribosome Profiling Reveals Translational Upregulation of Cellular Oxidative Phosphorylation mRNAs during Vaccinia Virus-Induced Host Shutoff. J. Virol. 2017, 91, e01858-16. [Google Scholar] [CrossRef] [Green Version]
- Szczerba, M.; Subramanian, S.; Trainor, K.; McCaughan, M.; Kibler, K.V.; Jacobs, B.L. Small Hero with Great Powers: Vaccinia Virus E3 Protein and Evasion of the Type I IFN Response. Biomedicines 2022, 10, 235. [Google Scholar] [CrossRef]
- Elroy-Stein, O.; Moss, B. Cytoplasmic expression system based on constitutive synthesis of bacteriophage T7 RNA polymerase in mammalian cells. Proc. Natl. Acad. Sci. USA 1990, 87, 6743–6747. [Google Scholar] [CrossRef] [Green Version]
- Shenvi, C.L.; Dong, K.C.; Friedman, E.M.; Hanson, J.A.; Cate, J.H. Accessibility of 18S rRNA in human 40S subunits and 80S ribosomes at physiological magnesium ion concentrations--implications for the study of ribosome dynamics. RNA 2005, 11, 1898–1908. [Google Scholar] [CrossRef] [Green Version]
- ten Asbroek, A.L.; van Groenigen, M.; Nooij, M.; Baas, F. The involvement of human ribonucleases H1 and H2 in the variation of response of cells to antisense phosphorothioate oligonucleotides. Eur. J. Biochem. 2002, 269, 583–592. [Google Scholar] [CrossRef]
- Genolet, R.; Rahim, G.; Gubler-Jaquier, P.; Curran, J. The translational response of the human mdm2 gene in HEK293T cells exposed to rapamycin: A role for the 5′-UTRs. Nucleic Acids Res. 2011, 39, 989–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreev, D.E.; Dmitriev, S.E.; Terenin, I.M.; Prassolov, V.S.; Merrick, W.C.; Shatsky, I.N. Differential contribution of the m7G-cap to the 5′ end-dependent translation initiation of mammalian mRNAs. Nucleic Acids Res. 2009, 37, 6135–6147. [Google Scholar] [CrossRef] [PubMed]
- Svitkin, Y.V.; Ovchinnikov, L.P.; Dreyfuss, G.; Sonenberg, N. General RNA binding proteins render translation cap dependent. EMBO J. 1996, 15, 7147–7155. [Google Scholar] [CrossRef]
- Berthelot, K.; Muldoon, M.; Rajkowitsch, L.; Hughes, J.; McCarthy, J.E. Dynamics and processivity of 40S ribosome scanning on mRNA in yeast. Mol. Microbiol. 2004, 51, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Abaeva, I.S.; Pestova, T.V.; Hellen, C.U. Attachment of ribosomal complexes and retrograde scanning during initiation on the Halastavi árva virus IRE.S. Nucleic Acids Res. 2016, 44, 2362–2377. [Google Scholar] [CrossRef]
- Haimov, O.; Sinvani, H.; Martin, F.; Ulitsky, I.; Emmanuel, R.; Tamarkin-Ben-Harush, A.; Vardy, A.; Dikstein, R. Efficient and Accurate Translation Initiation Directed by TISU Involves RPS3 and RPS10e Binding and Differential Eukaryotic Initiation Factor 1A Regulation. Mol. Cell Biol. 2017, 37, e00150-17. [Google Scholar] [CrossRef] [Green Version]
- Sinvani, H.; Haimov, O.; Svitkin, Y.; Sonenberg, N.; Tamarkin-Ben-Harush, A.; Viollet, B.; Dikstein, R. Translational tolerance of mitochondrial genes to metabolic energy stress involves TISU and eIF1-eIF4GI cooperation in start codon selection. Cell Metab. 2015, 21, 479–492. [Google Scholar] [CrossRef] [Green Version]
- DiTursi, M.K.; Cha, J.; Newman, M.R.; Dordick, J.S. Simultaneous in vitro protein synthesis using solid-phase DNA template. Biotechnol. Prog. 2004, 20, 1705–1709. [Google Scholar] [CrossRef]
- Ivanov, I.P.; Shin, B.-S.; Loughran, G.; Tzani, I.; Young-Baird, S.K.; Cao, C.; Atkins, J.F.; Dever, T.E. Polyamine Control of Translation Elongation Regulates Start Site Selection on Antizyme Inhibitor mRNA via Ribosome Queuing. Mol. Cell 2018, 70, 254–264.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, J.; Lewdorowicz, M.; Zuberek, J.; Grudzien-Nogalska, E.; Bojarska, E.; Stepinski, J.; Rhoads, R.E.; Darzynkiewicz, E.; Davis, R.E.; Jemielity, J. Synthesis and characterization of mRNA cap analogs containing phosphorothioate substitutions that bind tightly to eIF4E and are resistant to the decapping pyrophosphatase DcpS. RNA 2008, 14, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Bednarek, S.; Madan, V.; Sikorski, P.J.; Bartenschlager, R.; Kowalska, J.; Jemielity, J. mRNAs biotinylated within the 5′ cap and protected against decapping: New tools to capture RNA–protein complexes. Philos. Trans. B 2018, 373, 20180167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Breyne, S.; Monney, R.S.; Curran, J. Proteolytic Processing and Translation Initiation: Two Independent Mechanisms for The Expression of The Sendai Virus Y Proteins. J. Biol. Chem. 2004, 279, 16571–16580. [Google Scholar] [CrossRef] [PubMed]
- Terenin, I.M.; Andreev, D.E.; Dmitriev, S.E.; Shatsky, I.N. A novel mechanism of eukaryotic translation initiation that is neither m7G-cap-, nor IRES-dependent. Nucleic Acids Res. 2013, 41, 1807–1816. [Google Scholar] [CrossRef] [PubMed]


| LEAKINESS | ||||
|---|---|---|---|---|
| VacT7/HeLa | mRNA/HEK293T | RRL | HeLa Extract | |
| G | 95 | 95 | 36 | 68 |
| GGG | 30 | 30 | 22 | 43 |
| Rdm | 85 | 85 | 22 | 32 |
| TISU | 34 | 34 | 0 | 19 |
| Kozak | 5 | 5 | 0 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, B.; Jaquier-Gubler, P.; Curran, J.A. Preinitiation Complex Loading onto mRNAs with Long versus Short 5′ TLs. Int. J. Mol. Sci. 2022, 23, 13369. https://doi.org/10.3390/ijms232113369
Weiss B, Jaquier-Gubler P, Curran JA. Preinitiation Complex Loading onto mRNAs with Long versus Short 5′ TLs. International Journal of Molecular Sciences. 2022; 23(21):13369. https://doi.org/10.3390/ijms232113369
Chicago/Turabian StyleWeiss, Benjamin, Pascale Jaquier-Gubler, and Joseph Alphonsus Curran. 2022. "Preinitiation Complex Loading onto mRNAs with Long versus Short 5′ TLs" International Journal of Molecular Sciences 23, no. 21: 13369. https://doi.org/10.3390/ijms232113369
APA StyleWeiss, B., Jaquier-Gubler, P., & Curran, J. A. (2022). Preinitiation Complex Loading onto mRNAs with Long versus Short 5′ TLs. International Journal of Molecular Sciences, 23(21), 13369. https://doi.org/10.3390/ijms232113369






