Immunothrombotic Mechanisms Induced by Ingenol Mebutate Lead to Rapid Necrosis and Clearance of Anogenital Warts
Abstract
:1. Introduction
2. Results
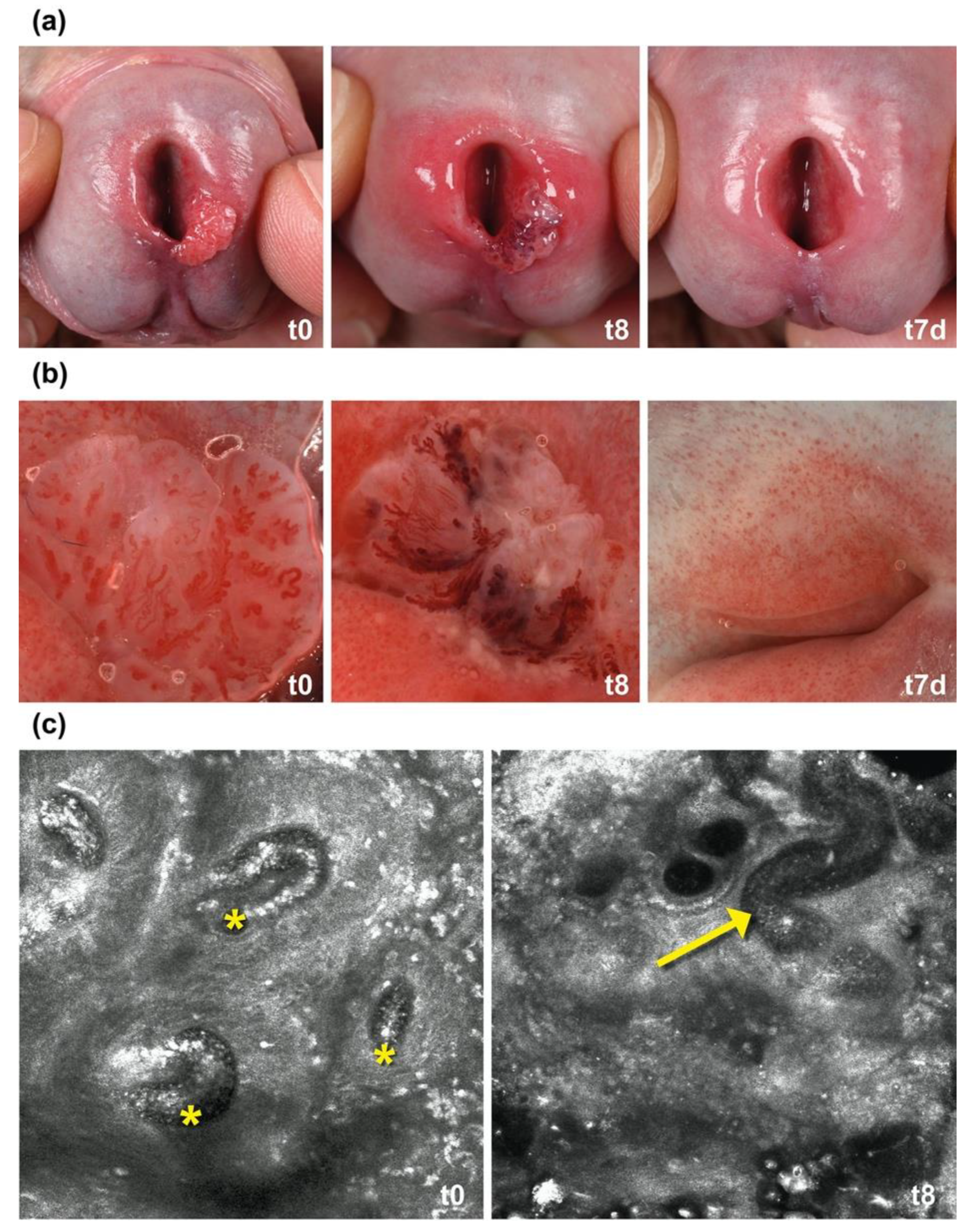
2.1. IM Induces Thrombosis in Capillaries in AGW
2.2. Histopathology Confirmed Microthrombosis with Neutrophils
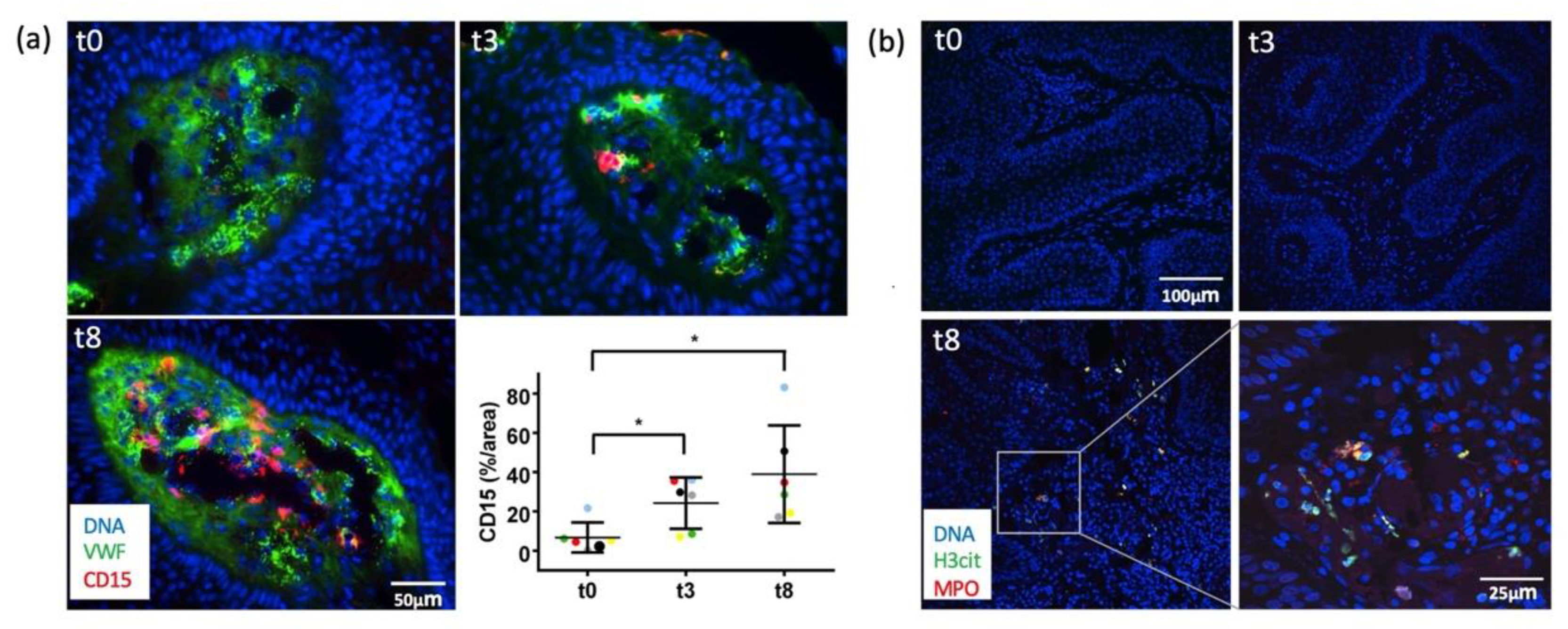
2.3. Immunofluorescence Analyses Demonstrated Early VWF Secretion and Formation of NETs
2.4. In Vitro Analyses Confirmed Endothelial Cell Activation and VWF Secretion after IM Stimulation
2.5. IM Superinduces IL-8 (CXCL8) Expression in HPV-E6/E7 Transfected HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Dermoscopic and In Vivo Confocal Laser Microscopy Evaluation of AGW
4.2. Biopsies of IM-Treated Anogenital Warts
4.3. Histomorphology, Immunohistochemistry, and Immunofluorescence
4.4. Cell Culture
4.5. ELISA
4.6. Transfection
4.7. RT-qPCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansen, M.H.E.; Kessels, J.; Nelemans, P.J.; Kouloubis, N.; Arits, A.; van Pelt, H.P.A.; Quaedvlieg, P.J.F.; Essers, B.A.B.; Steijlen, P.M.; Kelleners-Smeets, N.W.J.; et al. Randomized Trial of Four Treatment Approaches for Actinic Keratosis. N. Engl. J. Med. 2019, 380, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Swanson, N.; Anderson, L.L.; Melgaard, A.; Xu, Z.; Berman, B. Ingenol mebutate gel for actinic keratosis. N. Engl. J. Med. 2012, 366, 1010–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalfe, Y.; Rosen, T. Ingenol Mebutate: Expanded Utility. J. Drugs Dermatol. 2020, 19, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, S.A.; Jansen, T.M.; Homey, B.; Gerber, P.A. Successful therapy of condylomata acuminata with ingenol mebutate. Hautarzt 2015, 66, 223–225. [Google Scholar] [CrossRef]
- Braun, S.A.; Gerber, P.A. Successful treatment of perianal condylomata acuminata with ingenol mebutate gel. J. Dtschl. Dermatol. Ges. 2016, 14, 616–617. [Google Scholar] [CrossRef]
- Schopf, R.E. Ingenol mebutate gel is effective against anogenital warts—A case series in 17 patients. J. Eur. Acad. Dermatol. Venereol. 2015, 30, 1041–1043. [Google Scholar] [CrossRef]
- Braun, S.A.; Gerber, P.A. Ingenol mebutate for the management of genital warts in sensitive anatomic locations. J. Am. Acad. Dermatol. 2017, 77, e9–e10. [Google Scholar] [CrossRef] [Green Version]
- Braun, S.A.; Barsch, M.; Gerber, P.A. Fast Ablation of Anogenital Warts of the Urinary Meatus by Low-dose Ingenol Mebutate Gel. Sex Transm Dis 2018, 45, e80–e82. [Google Scholar] [CrossRef]
- Larsen, H.K.; Banzhaf, C.A.; Thomsen, S.F.; Gormsen, M.; Schopf, R.E.; Haedersdal, M. An exploratory, prospective, open-label trial of ingenol mebutate gel 0.05% for the treatment of external anogenital warts. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 825–831. [Google Scholar] [CrossRef]
- Magdaleno-Tapial, J.; Valenzuela-Onate, C.; Giacaman-von der Weth, M.; Ferrer-Guillen, B.; Garcia-Legaz Martinez, M.; Martinez-Domenech, A.; Hernandez-Bel, P. Ingenol mebutate for the treatment of anogenital condylomata acuminata. Clin. Exp. Dermatol. 2019, 44, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Domenech, A.; Magdaleno-Tapial, J.; Garcia-Legaz Martinez, M.; Hernandez-Bel, P. Successful treatment of condylomata acuminata at the urethral meatus with high-dose ingenol mebutate gel: Report of two cases. Int. J. STD AIDS 2019, 30, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Shahidi Dadras, M.; Bizaval, Z.; Hoormand, M.; Mozafari, N. Ingenol Mebutate Gel 0.05% in the Treatment of Anogenital Warts: A Prospective Controlled Trial Comparing It With Topical Podophyllin Solution 25. Sex. Transm. Dis. 2020, 47, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Shahidi Dadras, M.; Hoormand, M.; Bizaval, Z.; Mozafari, N. Ingenol mebutate for the management of cryotherapy-resistant anogenital warts. Dermatol. Ther. 2020, 33, e13937. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.H.; Gupta, A.K.; Tyring, S.K. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: Rapid lesion necrosis followed by lesion-specific immune response. J. Am. Acad. Dermatol. 2012, 66, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Emmert, S.; Haenssle, H.A.; Zibert, J.R.; Schon, M.; Hald, A.; Hansen, M.H.; Litman, T.; Schon, M.P. Tumor-Preferential Induction of Immune Responses and Epidermal Cell Death in Actinic Keratoses by Ingenol Mebutate. PLoS ONE 2016, 11, e0160096. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, S.K.; Zhang, X.D.; Hersey, P. Ingenol 3-angelate induces dual modes of cell death and differentially regulates tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in melanoma cells. Mol. Cancer Ther. 2004, 3, 1651–1658. [Google Scholar] [CrossRef]
- Ogbourne, S.M.; Suhrbier, A.; Jones, B.; Cozzi, S.J.; Boyle, G.M.; Morris, M.; McAlpine, D.; Johns, J.; Scott, T.M.; Sutherland, K.P.; et al. Antitumor activity of 3-ingenyl angelate: Plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004, 64, 2833–2839. [Google Scholar] [CrossRef] [Green Version]
- Stahlhut, M.; Bertelsen, M.; Hoyer-Hansen, M.; Svendsen, N.; Eriksson, A.H.; Lord, J.M.; Scheel-Toellner, D.; Young, S.P.; Zibert, J.R. Ingenol mebutate: Induced cell death patterns in normal and cancer epithelial cells. J. Drugs Dermatol. 2012, 11, 1181–1192. [Google Scholar]
- Lim, P.S.; Sutton, C.R.; Rao, S. Protein kinase C in the immune system: From signalling to chromatin regulation. Immunology 2015, 146, 508–522. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Czerwinski, P.; Hortmann, M.; Sohn, H.Y.; Forstermann, U.; Li, H. Protein kinase C alpha promotes angiogenic activity of human endothelial cells via induction of vascular endothelial growth factor. Cardiovasc. Res. 2008, 78, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Kedei, N.; Lundberg, D.J.; Toth, A.; Welburn, P.; Garfield, S.H.; Blumberg, P.M. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004, 64, 3243–3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampson, P.; Chahal, H.; Khanim, F.; Hayden, R.; Mulder, A.; Assi, L.K.; Bunce, C.M.; Lord, J.M. PEP005, a selective small-molecule activator of protein kinase C, has potent antileukemic activity mediated via the delta isoform of PKC. Blood 2005, 106, 1362–1368. [Google Scholar] [CrossRef] [Green Version]
- Challacombe, J.M.; Suhrbier, A.; Parsons, P.G.; Jones, B.; Hampson, P.; Kavanagh, D.; Rainger, G.E.; Morris, M.; Lord, J.M.; Le, T.T.; et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J. Immunol. 2006, 177, 8123–8132. [Google Scholar] [CrossRef] [Green Version]
- Hampson, P.; Kavanagh, D.; Smith, E.; Wang, K.; Lord, J.M.; Ed Rainger, G. The anti-tumor agent, ingenol-3-angelate (PEP005), promotes the recruitment of cytotoxic neutrophils by activation of vascular endothelial cells in a PKC-delta dependent manner. Cancer Immunol. Immunother. 2008, 57, 1241–1251. [Google Scholar] [CrossRef]
- Braun, S.A.; Baran, J.; Schrumpf, H.; Buhren, B.A.; Bolke, E.; Homey, B.; Gerber, P.A. Ingenol mebutate induces a tumor cell-directed inflammatory response and antimicrobial peptides thereby promoting rapid tumor destruction and wound healing. Eur. J. Med. Res. 2018, 23, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, S.A.; Bunemann, E.; Baran, J.; Homey, B.; Gerber, P.A. Time-lapse imaging points towards a non-toxic, mainly immune-driven mode of action of ingenol mebutate in the treatment of anogenital warts. Exp. Dermatol. 2018, 27, 675–677. [Google Scholar] [CrossRef]
- Schneider, S.W.; Nuschele, S.; Wixforth, A.; Gorzelanny, C.; Alexander-Katz, A.; Netz, R.R.; Schneider, M.F. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl. Acad. Sci. USA 2007, 104, 7899–7903. [Google Scholar] [CrossRef] [Green Version]
- Pendu, R.; Terraube, V.; Christophe, O.D.; Gahmberg, C.G.; de Groot, P.G.; Lenting, P.J.; Denis, C.V. P-selectin glycoprotein ligand 1 and beta2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood 2006, 108, 3746–3752. [Google Scholar] [CrossRef]
- Petri, B.; Broermann, A.; Li, H.; Khandoga, A.G.; Zarbock, A.; Krombach, F.; Goerge, T.; Schneider, S.W.; Jones, C.; Nieswandt, B.; et al. von Willebrand factor promotes leukocyte extravasation. Blood 2010, 116, 4712–4719. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.T.; Suckau, J.; Frank, K.; Desch, A.; Goertz, L.; Wagner, A.H.; Hecker, M.; Goerge, T.; Umansky, L.; Beckhove, P.; et al. von Willebrand factor fibers promote cancer-associated platelet aggregation in malignant melanoma of mice and humans. Blood 2015, 125, 3153–3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luster, A.D. Chemokines--chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998, 338, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Ott, I.; Marx, N.; Luther, T.; Kenngott, S.; Gawaz, M.; Kotzsch, M.; Schomig, A. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- van Aken, B.E.; Reitsma, P.H.; Rosendaal, F.R. Interleukin 8 and venous thrombosis: Evidence for a role of inflammation in thrombosis. Br. J. Haematol. 2002, 116, 173–177. [Google Scholar] [CrossRef]
- Li, L.; Shukla, S.; Lee, A.; Garfield, S.H.; Maloney, D.J.; Ambudkar, S.V.; Yuspa, S.H. The skin cancer chemotherapeutic agent ingenol-3-angelate (PEP005) is a substrate for the epidermal multidrug transporter (ABCB1) and targets tumor vasculature. Cancer Res. 2010, 70, 4509–4519. [Google Scholar] [CrossRef] [Green Version]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Desch, A.; Strozyk, E.A.; Bauer, A.T.; Huck, V.; Niemeyer, V.; Wieland, T.; Schneider, S.W. Highly invasive melanoma cells activate the vascular endothelium via an MMP-2/integrin alphavbeta5-induced secretion of VEGF-A. Am. J. Pathol. 2012, 181, 693–705. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Goertz, L.; Schneider, S.W.; Desch, A.; Mayer, F.T.; Koett, J.; Nowak, K.; Karampinis, I.; Bohlmann, M.K.; Umansky, V.; Bauer, A.T. Heparins that block VEGF-A-mediated von Willebrand factor fiber generation are potent inhibitors of hematogenous but not lymphatic metastasis. Oncotarget 2016, 7, 68527–68545. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Garagiola, I.; Baronciani, L. Role of von Willebrand factor in the haemostasis. Blood Transfus. 2011, 9 (Suppl. 2), s3–s8. [Google Scholar] [CrossRef]
- Lorenzi, O.; Frieden, M.; Villemin, P.; Fournier, M.; Foti, M.; Vischer, U.M. Protein kinase C-delta mediates von Willebrand factor secretion from endothelial cells in response to vascular endothelial growth factor (VEGF) but not histamine. J. Thromb. Haemost. 2008, 6, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Leunig, A.; Pekayvaz, K.; Popp, O.; Joppich, M.; Polewka, V.; Escaig, R.; Anjum, A.; Hoffknecht, M.L.; Gold, C.; et al. Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19. JCI Insight 2021, 6, e150862. [Google Scholar] [CrossRef]
- Bardoel, B.W.; Kenny, E.F.; Sollberger, G.; Zychlinsky, A. The balancing act of neutrophils. Cell Host Microbe 2014, 15, 526–536. [Google Scholar] [CrossRef] [Green Version]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated with Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Pellegrini, D.; Kawakami, R.; Guagliumi, G.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Nasr, A.; Kutys, R.; Guo, L.; Cornelissen, A.; et al. Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study. Circulation 2021, 143, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Al-Eitan, L.N.; Tarkhan, A.H.; Alghamdi, M.A.; Al-Qarqaz, F.A.; Al-Kofahi, H.S. Transcriptome analysis of HPV-induced warts and healthy skin in humans. BMC Med. Genom. 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigalli, J.P.; Reichel, M.; Tocchetti, G.N.; Reuter, T.; Dyckhoff, G.; Herold-Mende, C.; Weiss, J. Human papilloma virus (HPV) 18 proteins E6 and E7 up-regulate ABC transporters in oropharyngeal carcinoma. Involvement of the nonsense-mediated decay (NMD) pathway. Cancer Lett. 2018, 428, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Alkharsah, K.R. VEGF Upregulation in Viral Infections and Its Possible Therapeutic Implications. Int. J. Mol. Sci. 2018, 19, 1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, S.A.; Silling, S.; Schloer, S.M.; Hofmann, S.C.; Fritzen, B.; Oellig, F.; Lehmann, P.; Homey, B.; Assaf, C.; Emmert, S.; et al. Human Papillomavirus-type distribution in anogenital lesions of prepubertal children. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Kerk, N.; Strozyk, E.A.; Poppelmann, B.; Schneider, S.W. The mechanism of melanoma-associated thrombin activity and von Willebrand factor release from endothelial cells. J. Investig. Dermatol. 2010, 130, 2259–2268. [Google Scholar] [CrossRef]


| Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 | Patient #6 | Patient #7 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timepoint [h] | 0 | 3 | 8 | 0 | 3 | 8 | 0 | 3 | 8 | 0 | 3 | 8 | 0 | 3 | 8 | 0 | 3 | 8 | 0 | 3 | 8 |
| Hyperkeratosis | o | o | +++ | + | + | + | + | + | + | + | ++ | + | + | + | o | ++ | ++ | ++ | ++ | + | + |
| Parakeratosis | o | o | o | o | o | o | o | o | o | o | + | o | o | o | o | o | + | + | + | + | + |
| Spongiosis | o | o | + | o | o | o | o | o | ++ | o | o | ++ | o | o | o | o | o | + | o | + | + |
| Pallor of the epidermis | o | o | + | + | ++ | ++ | o | o | ++ | o | + | ++ | o | + | ++ | o | + | + | o | + | + |
| Necrotic, apoptotic keratinocytes | o | o | o | o | + | + | o | o | + | o | o | + | o | o | + | o | + | + | o | + | + |
| Ballooning degeneration | o | o | o | + | o | ++ | o | o | ++ | o | o | ++ | o | o | +++ | o | o | + | o | + | + |
| Reticulated degeneration | o | o | o | o | o | o | o | o | o | o | ++ | o | o | +++ | o | o | + | o | o | + | |
| Extravasated erythrocytes around capillaries | o | o | + | o | + | ++ | o | o | +++ | o | o | +++ | o | o | ++ | o | o | + | o | o | o |
| Thrombus in capillaries | o | o | o | o | o | o | o | o | + | o | o | + | o | o | + | o | o | + | o | + | + |
| Thrombus in postcapillary venules | o | o | o | o | o | x | o | x | + | o | o | + | o | o | x | o | o | + | o | x | x |
| Fibrinoid degeneration of vessel walls | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o |
| Fibrin perivascular | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o | o | + | o | o | o |
| Extravasated erythrocytes around postcapillary venules | o | o | o | o | o | x | o | x | ++ | o | o | o | o | + | x | o | o | + | o | x | x |
| Diapedesis of neutrophils | o | o | o | o | o | x | o | x | ++ | o | o | + | o | o | x | o | o | +++ | o | x | x |
| Leukocytoclasia | o | o | o | o | o | + | o | o | ++ | o | o | o | o | o | + | o | o | + | o | o | o |
| Intraepidermal neutrophils (MPO) | o | o | o | o | o | o | o | o | + | o | o | o | o | o | + | o | o | + | o | o | + |
| Intravascular neutrophils (MPO) | o | o | + | + | ++ | ++ | o | + | +++ | o | o | +++ | o | ++ | ++ | o | + | +++ | o | ++ | +++ |
| Perivascular neutrophils (MPO) | o | + | + | o | ++ | ++ | o | + | +++ | o | + | +++ | + | ++ | ++ | o | + | +++ | + | ++ | +++ |
| Diapedesis neutrophils (MPO) | o | + | o | o | + | + | o | o | ++ | o | o | +++ | o | + | ++ | o | o | +++ | 0 | + | +++ |
| Dermal T helper cells (CD4) | + | + | ++ | + | + | + | + | + | + | + | + | ++ | ++ | ++ | + | ++ | ++ | ++ | + | ++ | ++ |
| Dermal cytotoxic T cells (CD8) | + | + | ++ | + | + | + | + | + | +++ | + | + | ++ | ++ | ++ | + | ++ | ++ | +++ | + | ++ | +++ |
| Dermal macrophages (CD163) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ++ | ++ | ++ | + | ++ | ++ |
| Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 | Patient #6 | Patient #7 | |
|---|---|---|---|---|---|---|---|
| Gender | M | W | M | M | M | M | M |
| Age | 27 | 22 | 22 | 28 | 50 | 62 | 64 |
| History of warts | 24 months | 7 months | 6 months | 10 months | 12 months | 2 months | 12 months |
| Location | Mons pubis, penile shaft | Labia maiora | Mons pubis | Mons pubis, penile shaft | Penile shaft | Penile shaft | Penile shaft |
| Previous treatments | Cryotherapy Podophyllo-toxin | - | - | - | Cryotherapy | - | Cryotherapy |
| Comorbidities | - | - | Gastro-esophageal reflux disease | - | - | Arterial hypertension, peripheral artery disease | Arterial hypertension |
| HPV type | HPV 6 | HPV 6 | HPV 6 | HPV 6 | HPV 6 | HPV 6 | HPV 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, S.A.; Bauer, A.T.; Németh, C.; Rózsa, A.; Rusch, L.; Erpenbeck, L.; Schloer, S.; Silling, S.; Metze, D.; Gerber, P.A.; et al. Immunothrombotic Mechanisms Induced by Ingenol Mebutate Lead to Rapid Necrosis and Clearance of Anogenital Warts. Int. J. Mol. Sci. 2022, 23, 13377. https://doi.org/10.3390/ijms232113377
Braun SA, Bauer AT, Németh C, Rózsa A, Rusch L, Erpenbeck L, Schloer S, Silling S, Metze D, Gerber PA, et al. Immunothrombotic Mechanisms Induced by Ingenol Mebutate Lead to Rapid Necrosis and Clearance of Anogenital Warts. International Journal of Molecular Sciences. 2022; 23(21):13377. https://doi.org/10.3390/ijms232113377
Chicago/Turabian StyleBraun, Stephan A., Alexander T. Bauer, Csongor Németh, Annamária Rózsa, Louisa Rusch, Luise Erpenbeck, Sebastian Schloer, Steffi Silling, Dieter Metze, Peter A. Gerber, and et al. 2022. "Immunothrombotic Mechanisms Induced by Ingenol Mebutate Lead to Rapid Necrosis and Clearance of Anogenital Warts" International Journal of Molecular Sciences 23, no. 21: 13377. https://doi.org/10.3390/ijms232113377





