Abstract
The mitochondrial large-conductance calcium-activated potassium channel (mitoBKCa) is located in the inner mitochondrial membrane and seems to play a crucial role in cytoprotection. The mitoBKCa channel is regulated by many modulators, including activators, such as calcium ions and inhibitors, such as heme and its oxidized form hemin. Heme/hemin binds to the heme-binding motif (CXXCH) located between two RCK domains present in the mitochondrial matrix. In the present study, we used the patch-clamp technique in the outside-out configuration to record the activity of mitoBKCa channels. This allowed for the application of channel modulators to the intermembrane-space side of the mitoBKCa. We found that hemin applied in this configuration inhibits the activity of mitoBKCa. In addition, we proved that the observed hemin effect is specific and it is not due to its interaction with the inner mitochondrial membrane. Our data suggest the existence of a new potential heme/hemin binding site in the structure of the mitoBKCa channel located on the mitochondrial intermembrane space side, which could constitute a new way for the regulation of mitoBKCa channel activity.
1. Introduction
Potassium channels are the most common ion channels found in the cell membranes of many organisms—from viruses, bacteria, and plants to humans [1,2,3,4,5]. They are found not only in the cell membrane but also in the membrane surrounding the cell nucleus, endoplasmic reticulum, lysosomes, Golgi apparatus, and inner mitochondrial membrane [6]. The large-conductance calcium-activated potassium (BKCa) channel is found in most types of mammalian cells [7,8,9,10] and plays a key role in a variety of physiological processes. Briefly, BKCa channels play a role in the regulation of vascular blood pressure [11], bladder function [12], release of neurotransmitters [13,14], proper functioning of the circadian clock [15], and many other processes.
The BKCa channel is a protein consisting of four α subunits that form the channel pore with a conductivity from 200–300 pS [16]. Each α subunit contains seven transmembrane segments, a short N-terminus located on the extracellular side, and a large C-terminus with two regulatory domains of K+ conductance (RCK1 and RCK2) [17,18] located in the cytoplasm. BKCa channel activity is regulated by various stimuli, including voltage [19], membrane tension [20,21,22], and Ca2+ [23,24,25,26]. It was shown that two binding sites for Ca2+ are present in the structure of the BKCa channel, and both of them are located on the cytoplasmic part of the channel [27,28,29,30].
BKCa channel activity is regulated by several activators and inhibitors. The known endogenous activators of the BKCa channel, apart from Ca2+, include phosphatidylinositol 4,5-bisphosphate (PIP2) [31] and polyunsaturated fatty acids [32] or corticosterone [33]. Additionally, many synthetic activators have been developed, such as the potassium channel openers NS1619 [34], NS11021 [35], and CGS7184 [36]. Naturally occurring inhibitors of the BKCa channel include the indole alkaloid paxillin (Pax) [37], iberiotoxin (IbTx) [38], and charybdotoxin (ChbTx) [39]. The binding sites for IbTx and ChbTx in the BKCa channel are located extracellularly [40,41]. Additionally, heme and its oxidized form, hemin, inhibit the activity of the plasmalemmal BKCa channel by binding with the heme-binding motif (HBM) located in the linker between the RCK1 and RCK2 domains [42,43]. HBM is composed of two cysteines (C612, C615) and one histidine (H616) in the CXXCH motif [42,43]. The same motif is present in cytochrome c [44,45,46]; however, despite this, the mechanisms of heme-binding within the BKCa channel and cytochrome c are slightly different. Heme c is attached to cytochrome c by two thioether bonds formed between the heme vinyl groups and cysteine sulfur atoms. In addition, the iron ion of heme c is ligated by two amino acid side chains from the protein, where the proximal ligand is histidine from the HBM, and the distal ligand is provided elsewhere on the polypeptide [44,47]. However, heme b is only transiently bound in the BKCa channel, supposedly by iron ion coordination with the imidazole ring of histidine [42,48]. It was also shown that cysteine (C615) is important for the inhibition of BKCa channel activity by heme; however, details of this interaction are not known [42].
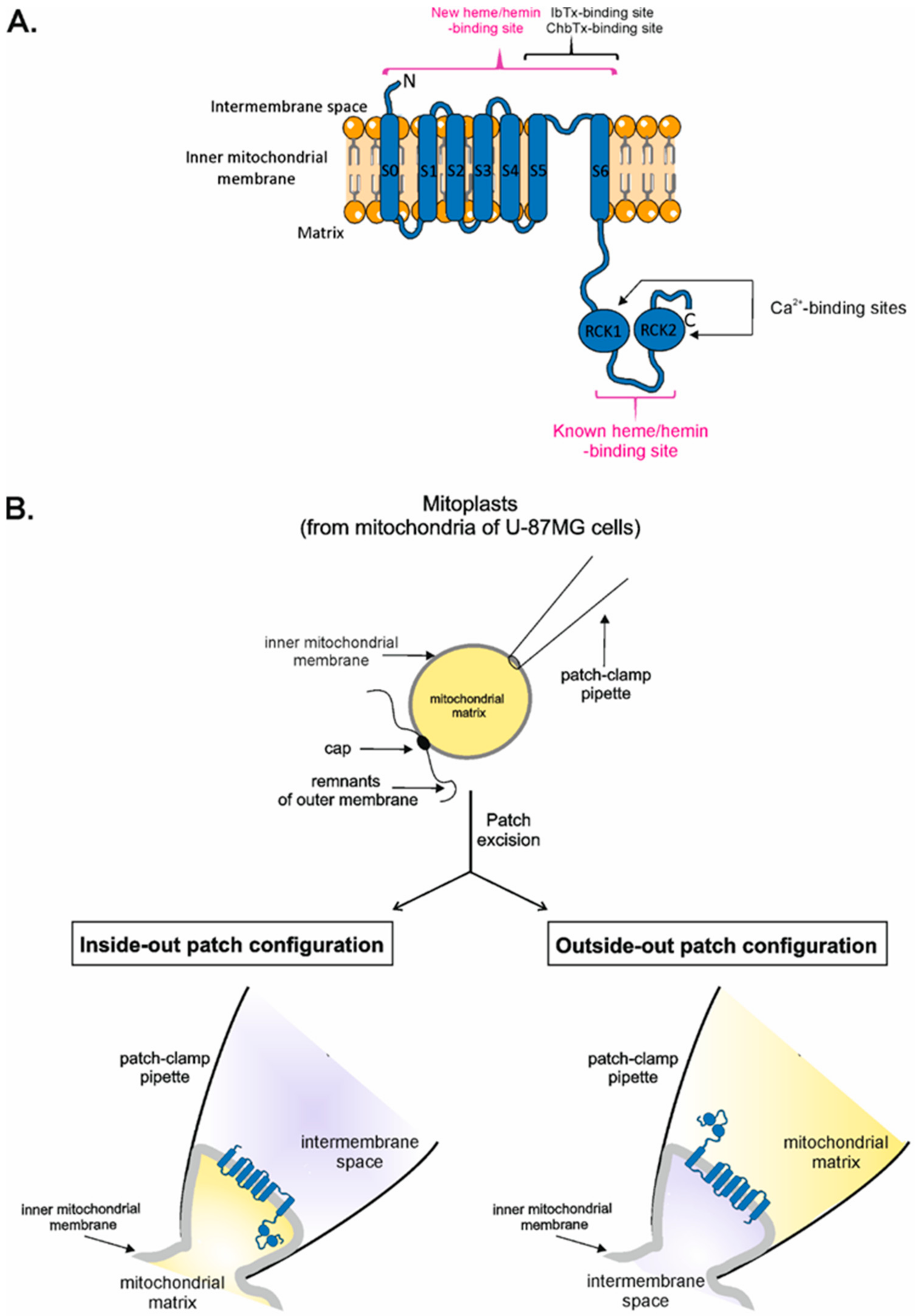
In addition to the cell membrane, the BKCa channel is also present in the inner mitochondrial membrane, i.e., mitoBKCa [49,50,51]. Several studies indicate that mitoBKCa channels play a central role in protecting the heart from ischemia. Pharmacological activation of these channels affects the generation of reactive oxygen species and mitochondrial Ca2+, thus, preventing cell death, likely by hindering the unchecked opening of the mitochondrial transition pore (reviewed in [52,53,54,55]). The mitoBKCa channel is formed by a DEC splice variant encoded by the KCNMA1 gene [56]. MitoBKCa seems to play a crucial role in cytoprotection [57,58,59,60]. Patch-clamp experiments on mitoplasts indicate that intramitochondrial (matrix) Ca2+ regulates mitoBKCa activity; therefore, the C-terminus of the mitoBKCa channel with RCK domains faces the mitochondrial matrix, while the N-terminus is located in the intermembrane space [54] (Figure 1A). Similar to plasmalemmal BKCa channels, mitoBKCa is also inhibited by Pax [61,62,63], IbTx [50,64], ChbTx [57,65,66], and hemin [61,67], which proves the existence of an HBM in the C-terminus of the mitoBKCa channel (Figure 1A). However, there are no data regarding the regulation of the activity of the mitoBKCa channel by external (cytosolic) heme/hemin.

Figure 1.
Topology of the mitoBKCa channel α subunit and scheme of patch-clamp experiments in different configurations. (A) Schematic topology of mitoBKCa α subunit with marked binding sites for selected modulators of the mitoBKCa channel activity. Binding sites for Ca2+ in the mitoBKCa channel are located on the mitochondrial matrix side, while iberiotoxin- and charybdotoxin-binding sites are present on the external, intermembrane space side of the channel. The known heme-binding site (CXXCH) is located in the linker between RCK domains in the C-terminus, while a new hypothetical external heme-binding site is located on the intermembrane space side of the mitoBKCa channel. (B) Schematic representation of patching of the mitoplast and patch-clamp experiments in the inside-out and outside-out modes. For details of patch-clamp measurements see the “Materials and Methods” section.
An important source of hemin in the human body is intracerebral hemorrhages, during which hemin penetrates the interstitial space and surrounds neurons, astrocytes, and other cells in the brain [68]. The inhibition of BKCa channels by hemin may be a potential mechanism for the neurotoxicity associated with intracerebral hemorrhages, e.g., by elimination of the cytoprotective properties of the mitoBKCa channel.
In this work, we applied hemin to the mitochondrial intermembrane space side and observed the inhibition of mitoBKCa channel activity. In addition, we investigated whether the mitoBKCa channel inhibited by hemin applied to the mitochondrial intermembrane space side is reactivated by NaHS, used as a hydrogen sulfide (H2S) donor, similar to results obtained for mitoBKCa channel regulation from the matrix side [69]. Our data suggest the existence of a new potential heme/hemin binding site in the structure of the mitoBKCa channel located on the mitochondrial intermembrane space side.
2. Results
2.1. Definitions
Inside-out configuration of the mitoBKCa channel—the mitochondrial matrix side of the channel is exposed to the recording chamber, where modulators of the channel activity are directly applied, while the mitochondrial intermembrane space side of the channel is facing the recording pipette (Figure 1B).
Outside-out configuration of the mitoBKCa channel—the mitochondrial intermembrane space side of the channel is exposed to the recording chamber, where modulators of the channel activity are directly applied, while the mitochondrial matrix side of the channel is facing the recording pipette (Figure 1B).
External hemin is applied from the mitochondrial intermembrane space side of the channel.
2.2. Vectorial Properties of mitoBKCa Channels
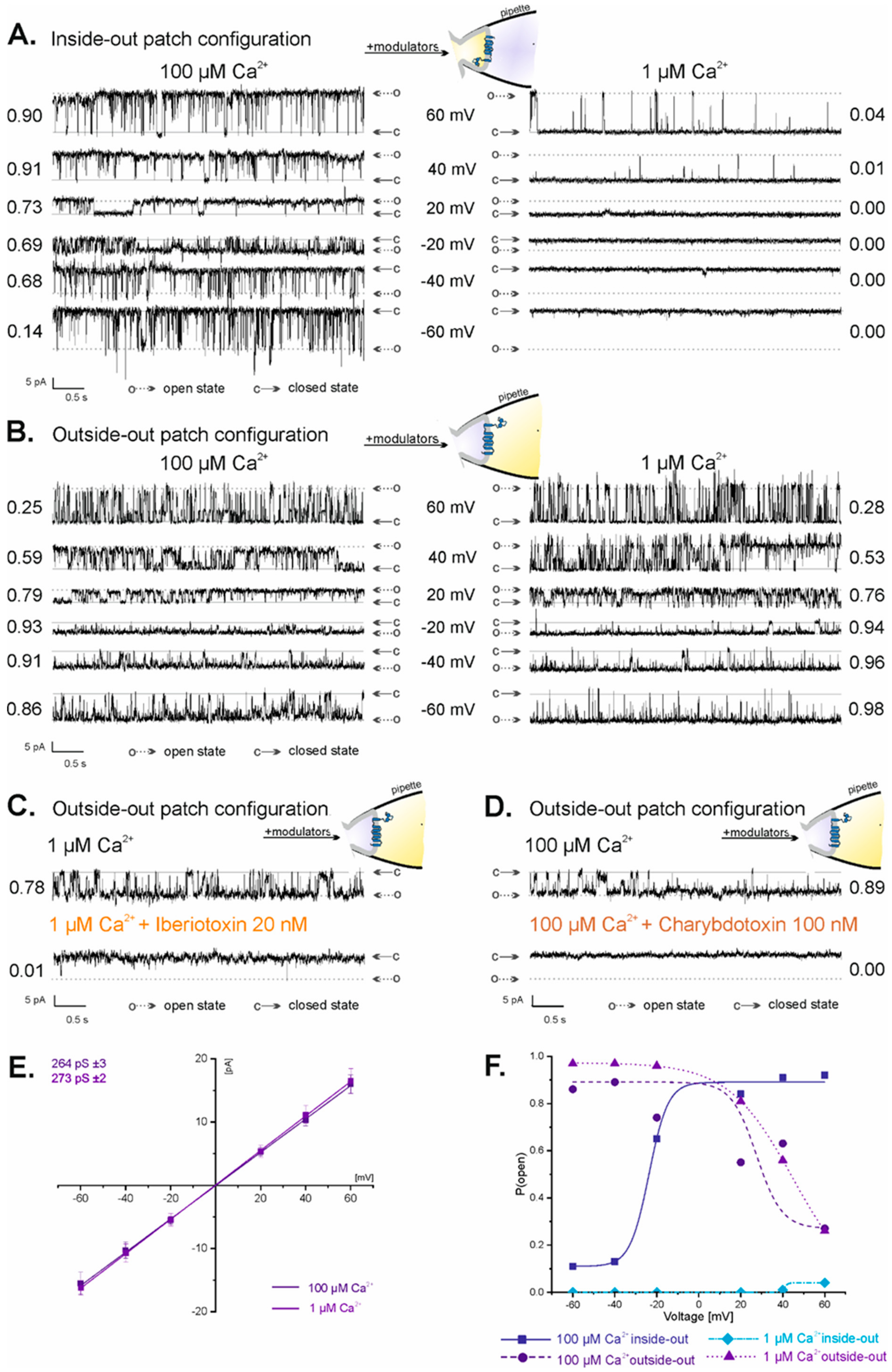
Two possible orientations of membrane patches derived from the inner mitochondrial membranes were observed in our patch-clamp experiments: inside-out and outside-out. They occurred randomly; however, the outside-out configuration was observed much less frequently (<20% of all experiments) than the inside-out configuration. Substances known to modulate mitoBKCa channel activity in a specific manner, for which localization of binding sites is defined, were used to distinguish between inside-out and outside-out mitoBKCa channel orientation. The mitoBKCa channels in the inside-out configuration were sensitive to calcium ions applied to the recording chamber—in 100 µM Ca2+, P(o) ranged from 0.14 at −60 mV to 0.90 at 60 mV (Figure 2A, left panel), while in 1 µM Ca2+, the channel was almost inactive at all voltages (P(o) = 0 at −60 mV to 40 mV and P(o) = 0.04 at 60 mV) (Figure 2A, right panel). In the outside-out configuration, where the Ca2+ binding sites located on the mitochondrial matrix side of the channel were inside the recording pipette, the mitoBKCa channel was not affected by changes in the concentration of calcium ions applied to the recording chamber (Figure 2B). In both 100 µM Ca2+ and 1 µM Ca2+, the activity of the mitoBKCa channel in the outside-out configuration was similar: P(o) in 100 µM Ca2+ ranged from 0.86 at −60 mV to 0.25 at 60 mV (Figure 2B, left panel), while in 1 µM Ca2+, it ranged between 0.98 and 0.28 at −60 mV and at 60 mV, respectively (Figure 2B, right panel). To additionally prove the outside-out configuration of the mitoBKCa channel, 20 nM IbTx (Figure 2C) and 100 nM ChbTx (Figure 2D), whose binding sites are located on the mitochondrial intermembrane space side of the channel, were applied. Both substances applied to the recording chamber completely inhibited the activity of the mitoBKCa channel (Figure 2C,D), proving that the intermembrane space side of the channel was exposed to the recording chamber. The conductance of the mitoBKCa channels in the outside-out configuration was similar to the previously reported conductance of the mitoBKCa channels in the inside-out configuration from the U-87MG cell line [70]. The channel conductance in the outside-out configuration was estimated to be 264 ± 3 pS in 100 µM Ca2+ (n = 10) and 273 ± 2 pS in 1 µM Ca2+ (n = 10) (Figure 2E). A comparison of the dependence of P(o) of the channel on voltages between inside-out and outside-out configurations in high and low Ca2+ concentrations is shown in Figure 2F. The dependence of P(o) of the mitoBKCa channel on voltages is opposite for inside-out (in 100 µM Ca2+: low values of P(o) from −60 to −40 mV, high values at voltages from −20 mV to 60 mV) and outside-out configurations (in both 100 µM Ca2+ and 1 µM Ca2+: high values of P(o) at voltages from −60 mV to 40 mV, low values at −60 mV) (Figure 2F). Altogether, the above results prove that the mitoBKCa channels observed in a fraction of the patch-clamp experiments were in the outside-out orientation.

Figure 2.
Properties of the mitoBKCa channel in the inside-out and outside-out configuration. (A) Regulation of the mitoBKCa channel in the inside-out configuration by batch Ca2+. Representative recordings of single-mitoBKCa channel activity at different voltages in high (100 µM Ca2+; left panel) and low (1 µM Ca2+; right panel) calcium solutions. (B) Lack of the regulation of the mitoBKCa channel in the outside-out configuration by bath Ca2+. Representative recordings of single-mitoBKCa channel activity at different voltages in high (100 µM Ca2+; left panel) and low (1 µM Ca2+; right panel) calcium solutions. (C,D) Inhibition of the activity of the mitoBKCa channel in the outside-out configuration by iberiotoxin and charybdotoxin. Channel activity was recorded at −40 mV. “c” denotes the closed state of the channel; “o” denotes the open state of the channel. (E) Current–voltage characteristics of the single-channel events in high and low calcium solutions. The conductance of the channel was equal to 264 ± 3 pS in 100 µM Ca2+ and 273 ± 2 pS in 1 µM Ca2+. (F) Analysis of the P(o) of the mitoBKCa channel at different voltages in inside-out and outside-out configurations in 100 µM Ca2+ and 1 µM Ca2+ solutions.
2.3. Regulation of mitoBKCa Channels by External Hemin
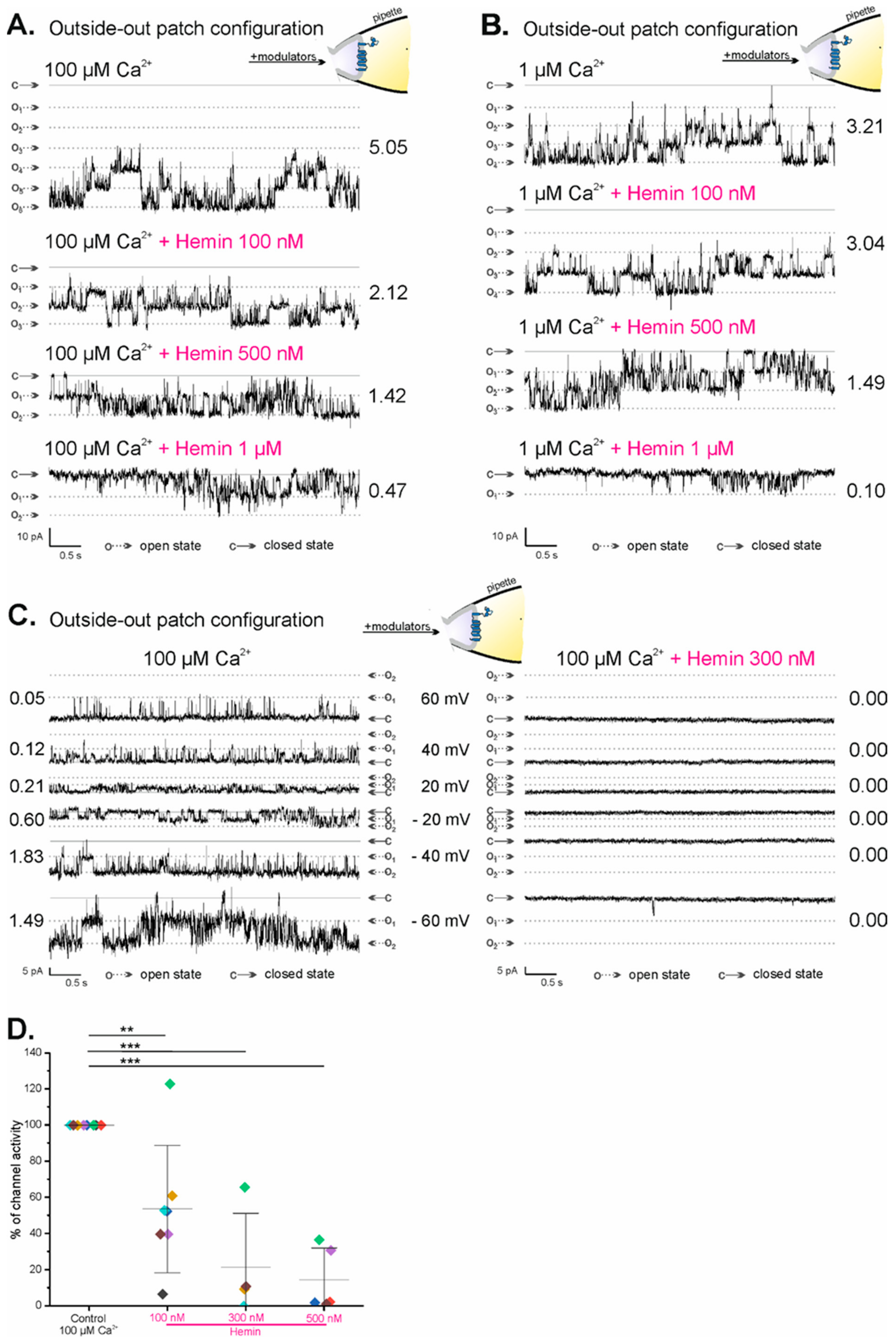
Inhibition of the activity of the mitoBKCa channel by external hemin was observed in patch-clamp experiments in which mitoBKCa channels were active in the outside-out patch configuration. Figure 3A,B show representative recordings of mitoBKCa channels under control conditions—100 µM Ca2+ and 1 µM Ca2+, respectively—and after the addition of increasing hemin concentrations (100 nM, 500 nM, and 1 µM). NP(o) of the mitoBKCa channel in the outside-out configuration decreased from 5.05 (in 100 µM Ca2+) to 2.12 and 1.42 after the application of 100 nM and 500 nM hemin, respectively, to ultimately achieve a value of 0.47 in 1 µM hemin (Figure 3A). Similarly, NP(o) of the mitoBKCa channel decreased from 3.21 (in 1 µM Ca2+) to 3.04, 1.49, and 0.10 after the application of 100 nM, 500 nM, and 1 µM hemin, respectively, applied from the mitochondrial intermembrane space side of the channel (Figure 3B). In addition, it was shown that 300 nM hemin (Figure 3C, right panel) applied from the intermembrane space side on the mitoBKCa channel in control conditions (Figure 3C, left panel) inhibited its activity at all voltages, from −60 mV to 60 mV (Figure 3C, right panel). Figure 3D shows the statistical analysis of changes in the percent activity of the mitoBKCa channel in the outside-out configuration after the application of increasing hemin concentrations in comparison to control conditions. Dose-dependent inhibition of the mitoBKCa channel with 100 nM, 300 nM, and 500 nM hemin was observed, with corresponding mean percent channel activity values of 53.60% (±35.23, n = 7; p < 0.05 **), 21.47% (±29.84, n = 4; p < 0.05 ***), and 14.56% (±17.61, n = 5; p < 0.001 ***), respectively. The above results indicate that the mitoBKCa channel is inhibited by external hemin.

Figure 3.
Regulation of the activity of the mitoBKCa channel in outside-out configuration by hemin. (A,B) Changes in the open probability of the mitoBKCa channel in multichannel patch during continuous experiments recorded at −40 mV. The gradual decrease in channel open probability after the application of increasing concentrations of hemin, both in 100 µM Ca2+ and 1 µM Ca2+. (C) Representative recordings of mitoBKCa channel activity in outside-out configuration at different voltages in the control conditions (100 µM Ca2+) and in the presence of 300 nM hemin; “c” denotes the closed state of the channel; “on” denotes the open state of the channel with “n” indicating the number of open channels. (D) Statistical analysis of mitoBKCa channel activity in the outside-out configuration at −40 mV after the application of different hemin concentrations. The data are presented as the percentage of the channel activity with respect to the control conditions (mean ± SD with points representing a given repetition). A one-way ANOVA with Tukey means a comparisons test was used to identify any significant differences (p = 0.01–0.001 (**), p < 0.001 (***). The P(o) for the statistical analysis of channel activity was calculated for a one-minute-long recording starting two or more minutes after hemin application (unless stated otherwise).
2.4. Regulation of mitoBKCa Channel Activity in the Outside-Out Configuration by NaHS and PPIX
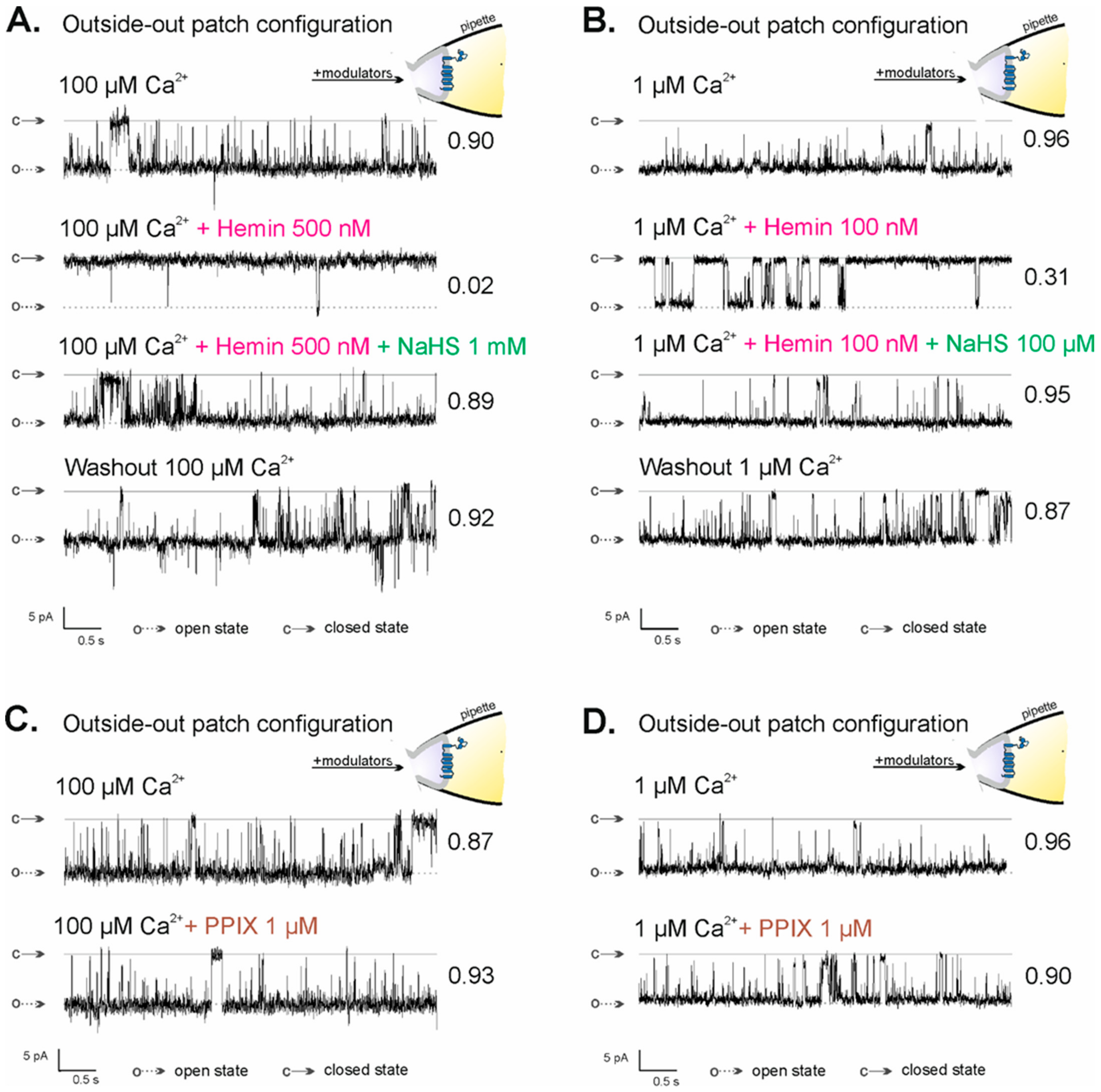
Previously, it was shown that hemin-inhibited mitoBKCa channels in the inside-out configuration were reactivated by NaHS, an H2S donor [69]. Because of the lack of a direct effect of H2S on the activity of the mitoBKCa channel and known H2S reactivity with iron ions, it was postulated that H2S binds to the iron cation of hemin [69]. To prove that the observed effect of hemin action on the activity of the mitoBKCa channel in the outside-out configuration is hemin-specific and results from the direct interaction of hemin’s iron ion with the mitoBKCa channel, analogous experiments were performed. NaHS activated hemin-inhibited mitoBKCa channels in the outside-out configuration. The effects of NaHS were similar at both low and high calcium ion concentrations: P(o) of the mitoBKCa channels increased from 0.02 (in 100 µM Ca2+ with 300 nM hemin) to P(o) = 0.89 after 1 mM NaHS administration (Figure 4A) and from 0.31 (in 1 µM Ca2+ with 100 nM hemin) to P(o) = 0.95 (Figure 4B). The activity of the mitoBKCa channel after the washout of both modulators remained at a high level: P(o) = 0.92 and P(o) = 0.87 after washout with 100 µM Ca2+ and 1 µM Ca2+, respectively. Porphyrins, including hemin, can bind lipid membranes, thereby changing their properties [71,72]. To exclude the effects of hemin due to its interaction with the membrane, experiments with protoporphyrin IX (PPIX) were performed (Figure 4C,D). PPIX is a precursor of heme/hemin that lacks a central iron ion, which is crucial in virtually all specific interactions of heme/hemin with proteins. Burton et al. showed that PPIX did not result in any change in the KATP currents, while heme increases whole-cell KATP currents. They concluded that the increases in current are specific to heme and are not a consequence of the porphyrin ring [73]. In the previous study, we have shown that PPIX applied in the inside-out configuration did not impact the activity of the mitoBKCa channel [69]. Here, we showed that 1 µM PPIX did not change the activity of the mitoBKCa channel, also in the outside-out configuration: P(o) in control conditions was 0.87 in 100 µM Ca2+ (Figure 4C) and 0.96 in 1 µM Ca2+ (Figure 4D), while after 1 µM PPIX administration, the values were 0.93 (Figure 4C) and 0.90 (Figure 4D), respectively. The above results indicate that the observed hemin effect is specific and it is not due to its interaction with the inner mitochondrial membrane, which suggests the existence of a new potential heme/hemin-binding site in the structure of the mitoBKCa channel located on the mitochondrial intermembrane space side.

Figure 4.
Regulation of the activity of the mitoBKCa channel in the outside-out configuration by NaHS and PPIX. (A,B) NaHS activates mitoBKCa channels in the outside-out configuration inhibited by external hemin. Representative recordings of single-mitoBKCa channel activity in outside-out configuration at −40 mV in control conditions ((A) 100 µM Ca2+ and (B) 1 µM Ca2+) and after the application of hemin followed by NaHS addition and washout step. (C,D) Lack of the effect of PPIX on the activity of the mitoBKCa channel in the outside-out configuration. Representative recordings of single-mitoBKCa channel activity in the outside-out configuration at −40 mV in control conditions ((C) 100 µM Ca2+ and (D) 1 µM Ca2+) and after the application of 1 µM PPIX; “c” denotes the closed state of the channel; “o” denotes the open state of the channel.
3. Discussion
In general, the biophysical and pharmacological properties of mitochondrial potassium channels are considered to be similar to those present in the plasma membrane. However, localization in the inner mitochondrial membrane indicates an additional unique role of these channels. Numerous studies using activators and inhibitors indicate that the mitoBKCa channel participates in the cytoprotection of cardiac and neuronal cells [57,59,62,74,75,76]. For example, it was shown that an opener of BKCa—NS1619—protected hearts against ischemic injury [57,59], and a natural flavonoid—quercetin, known as a mitoBKCa channel opener—also shows cardioprotective effects [77]. Interestingly, it is important to note that the activation of BKCa by specific K+ channel openers triggered the death of human glioma cells [78,79], suggesting the involvement of BKCa channels in cancer. Although direct involvement of mitoBKCa in cancer has not been shown, yet this is probable since the role of another mitochondrial channel—mitoKv1.3—in cancer is already established [80]. Together, the search for new activators, inhibitors, and mechanisms of the regulation of the activity of the BKCa channels might provide more possibilities for the development of therapeutic strategies. Despite that the properties of plasmalemmal and mitochondrial BKCa channels are very similar, differences in their regulation by hypoxia were observed previously [65]. In this study, we added to the list of differences in the modulation by hemin (see below).
Heme, a small organic molecule with a central iron ion, is partially synthesized in mitochondria [81] and plays a crucial role in numerous organismal processes [82,83]. Heme-containing proteins form a large and biologically important group, and they are found in all living species and carry out a wide variety of functions, for example, in oxygen transport (the globins), electron transfer (the cytochromes), and various heme-dependent catalytic processes (e.g., in the cytochrome P450s, nitric oxide synthases, peroxidases, and dioxygenases). In many heme proteins, e.g., soluble guanylyl cyclase or cytochrome c, heme is bound or coordinated in part by an amino acid sequence typically containing a histidine or cysteine residue, which acts as an axial fifth ligand (in addition to the four bonds provided by the nitrogen atoms of the protoporphyrin-IX ring to the iron center) to the redox-sensitive iron center, and water or a bound gas molecule acts as the sixth ligand [84]. Recent studies revealed a novel role of heme and its oxidized form, hemin, as modulators of potassium channel activity.
Except for the BKCa channel from the plasma membrane, which contains the conserved heme-binding amino-acid sequence motif CXXCH located between two RCK domains in the C-terminus [42], other potassium channels are also regulated by heme through its interaction with heme-binding motifs. One of them is the cardiac ATP-sensitive K+ channel (KATP channel), a hetero-octameric complex consisting of four pore-forming K+ channel subunits of the inward rectifier family (Kir6.2) and four regulatory sulfonylurea receptor subunits (SUR2A). It was shown that the cytoplasmic part of SUR2A contains the heme-binding motif CXXHX16H. Mutagenesis together with quantitative and spectroscopic analyses of heme-binding and single-channel experiments identified Cys628 and His648 as important for heme-binding [73]. Another potassium channel regulated by free intracellular heme is the Kv1.4 channel (voltage-gated K+), whose inactivation is mediated, among others, by the N-terminal protein structure [85,86]. Inspection of the Kv1.4 primary structure did not reveal classic heme-binding motifs, such as those found in cytochrome c (CXXCH). However, the N-terminal ball structure possesses a residue of His16 close to Cys13, forming a putative heme-responsive CXXH motif with an additional histidine residue at position 35. Sahoo et al. showed that in the Kv1.4 channel, heme is ligated by the side chain of C13 [87].
In all the above-mentioned channels, heme interacts with its cytoplasmic domains to modulate channel activity, and in each case, heme is suggested to bind a flexible region with a distinct motif. Although there are similarities in the modes of heme-binding across different channels, the functional consequences are not the same in each case, which is a clear indication of the potential versatility of heme-binding processes in ion channel control.
Until now, there were no data regarding heme-binding motifs in the extracellular parts of plasmalemmal potassium channels or in the intermembrane space side of the mitochondrial channels. In addition, Tang et al. indicated that hemin did not show any noticeable effect when applied to BKCa channels from the extracellular side [42]. Our studies showed, for the first time, that hemin inhibits the activity of the mitoBKCa channel when applied from the intermembrane space side. Differences in the above observations may result from the presence of different isoforms of the BKCa channel in the inner membrane of mitochondria and in the plasma membrane. There are known examples of different effects of the same modulator on BKCa and mitoBKCa channel activity. It was shown that H2S potentiated BKCa currents in human uterine artery smooth muscle cells [88]. H2S also increased the activity of BKCa channels in rat pituitary tumor cells [89]. In contrast, BKCa channels were inhibited by H2S in colonic smooth muscle cells [90] and human-induced pluripotent stem cell-derived mesenchymal stromal cells [91]. On the other hand, the activity of the mitoBKCa channel is not directly regulated by H2S [69]. Therefore, some differences in the isoforms, membrane composition, or local microenvironment may result in different sensitivities of BKCa and mitoBKCa channels to external hemin. However, the discovery of the differentiating factors requires further studies.
In summary, we provide single-channel functional data suggesting the presence of an additional new hemin-binding site in the mitoBKCa channel located in the mitochondrial intermembrane space. The binding of heme/hemin to this site may contribute to the overall response of mitochondria during the cytoprotection phenomenon.
4. Materials and Methods
4.1. Cell Culture
Astrocytoma U-87MG cells were cultured in DMEM (Laboratory of General Chemistry, Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland) with 2 mM L-glutamine (Gibco, Carlsbad, CA, USA), 10% FBS (Gibco, Carlsbad, CA, USA), 100 U/mL penicillin (Sigma-Aldrich, St. Louis, MO, USA), and 100 μg/mL streptomycin (Sigma-Aldrich, St. Louis, MO, USA). Cells were cultured at 37 °C in a humidified atmosphere with 5% CO2. The cells were fed and reseeded, usually every fourth day.
4.2. Mitochondria Isolation
Mitochondria from the U-87MG cell line were isolated as previously described with modifications [69,92,93]. Briefly, the cells were washed with PBS (Laboratory of General Chemistry, Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland), harvested by scraping, collected in PBS, and centrifuged at 800× g for 10 min. The cell pellet was resuspended and homogenized in isolation buffer (250 mM sucrose, 1 mM EGTA, 5 mM HEPES, pH = 7.2). The homogenate was transferred into an Eppendorf tube and centrifuged at 9200× g for 10 min. Next, the pellet was resuspended in isolation buffer and centrifuged at 780× g for 10 min. The supernatant containing mitochondria was moved to a new Eppendorf tube and centrifuged at 9200× g for 10 min. The mitochondrial pellet obtained after the last centrifugation was resuspended in a small volume of isolation buffer (20–100 µL). All manipulations were performed on ice, while all centrifugation steps were performed at 4 °C. Isolated mitochondria were kept on ice and used for patch-clamp experiments 4–6 h after isolation.
4.3. Mitoplast Preparation
Mitoplasts were prepared according to a previously described protocol [70]. Briefly, mitochondria (1–2 µL) isolated from the U-87MG cell line were incubated in 40 µL of hypotonic solution (5 mM HEPES, 100 μM CaCl2, pH = 7.2) for approximately 2 min to induce swelling and breakage of the outer membrane, followed by the addition of 10 µL of hypertonic solution (1.5 M sucrose, 30 mM HEPES, 100 μM CaCl2, pH = 7.2) to restore the isotonicity of the medium.
4.4. Patch-Clamp Experiments
Patch-clamp experiments were performed according to published protocols [70,93]. Briefly, the experiments were carried out in the inside-out or outside-out modes. In the inside-out mode, the matrix side of the channel was exposed to the recording chamber, while the intermembrane space side was facing the recording pipette. In the outside-out mode, the channel’s orientation was the opposite; the intermembrane space side of the channel was exposed to the recording chamber, while the matrix side of the channel was facing the recording pipette (Figure 1B). Freshly prepared mitoplasts (0.5–2 µL) were added by mixing directly within the recording chamber filled with a high Ca2+ solution (150 mM KCl, 10 mM HEPES, and 100 μM CaCl2 at pH = 7.2). Modulators of the channel activity were added directly to the recording chamber (in the inside-out and outside-out modes). The patch-clamp pipettes, made of borosilicate glass (Harvard Apparatus GC150–10, Holliston, MA, USA) with a resistance of ~15 MΩ, were filled with a high calcium solution. After the mitoplast caught and established the giga-ohm seal, the membrane patch was excised by tapping the pipette holder. The current was recorded using a patch-clamp amplifier (Axopatch 200B, Molecular Devices Corporation, San Jose, CA, USA), low-pass filtered at 1 kHz, and sampled at a frequency of 2.5 kHz. Clampfit 10.7 software in the single-channel search mode was used to calculate the probability of mitoBKCa channel opening (P(o)). Two types of patch-clamp recordings were performed: ten-second recordings at different voltages (from −60 mV to 60 mV, 20 mV intervals) and continuous recordings at −40 mV and 40 mV with different lengths depending on the type of experiment. Stock solutions of modulators of the activity of the mitoBKCa channel used in patch-clamp experiments were prepared in the concentrations mentioned below and then diluted to the desired concentrations. Hemin (Sigma-Aldrich, St. Louis, MO, USA) and protoporphyrin IX (PPIX, LifeTein LLC, Somerset, NJ, USA) were diluted in DMSO to a 10 mM concentration and stored at −20 °C. NaHS (Fluorochem Ltd., Hadfield, United Kingdom) was diluted in water to a 1 M concentration. NaHS stock solutions were prepared immediately before use. ChbTx (Alomone Labs, Jerusalem, Israel) and IbTx (Smartox Biotechnology, Saint Egrève, France) were diluted in water to 100 µM concentration and stored at −20 °C.
4.5. Statistical Analysis
One-way ANOVA with Tukey’s means a comparisons test was used to determine the statistical significance of the obtained results. A value of p < 0.05 was considered statistically significant. The following significance levels were adopted: 0.05–0.01 (*), 0.01–0.001 (**), and <0.001 (***).
Author Contributions
Conceptualization, A.W., A.S. and P.K.; methodology, A.W. and P.K.; formal analysis, A.W.; investigation, A.W.; writing—original draft preparation, A.W. and P.K.; writing—review and editing, A.W., A.S. and P.K..; visualization, A.W.; supervision, P.K.; project administration, A.W.; funding acquisition, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland (Narodowe Centrum Nauki) [Grant number 2018/31/N/NZ1/00928 to A.W.].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Bogusz Kulawiak (Nencki Institute of Experimental Biology) and Piotr Bednarczyk (Warsaw University of Life Sciences) for fruitful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dreyer, I.; Uozumi, N. Potassium Channels in Plant Cells. FEBS J. 2011, 278, 4293–4303. [Google Scholar] [CrossRef]
- Fischer, W.B.; Sansom, M.S.P. Viral Ion Channels: Structure and Function. Biochim. Biophys. Acta Biomembr. 2002, 1561, 27–45. [Google Scholar] [CrossRef]
- Koprowski, P.; Kubalski, A. Bacterial Ion Channels and Their Eukaryotic Homologues. BioEssays 2001, 23, 1148–1158. [Google Scholar] [CrossRef]
- Koszela-Piotrowska, I.; Matkovic, K.; Szewczyk, A.; Jarmuszkiewicz, W. A Large-Conductance Calcium-Activated Potassium Channel in Potato (Solanum Tuberosum) Tuber Mitochondria. Biochem. J. 2009, 424, 307–316. [Google Scholar] [CrossRef]
- Miller, C. An Overview of the Potassium Channel Family. Genome Biol. 2000, 1, reviews0004.1–reviews0004.5. [Google Scholar] [CrossRef]
- Checchetto, V.; Teardo, E.; Carraretto, L.; Leanza, L.; Szabo, I. Physiology of Intracellular Potassium Channels: A Unifying Role as Mediators of Counterion Fluxes? Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1258–1266. [Google Scholar] [CrossRef]
- Contet, C.; Goulding, S.P.; Kuljis, D.A.; Barth, A.L. BK Channels in the Central Nervous System. Int. Rev. Neurobiol. 2016, 128, 281–342. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Sansom, S.C. BK Channels in the Kidney: Role in K+ Secretion and Localization of Molecular Components. Am. J. Physiol. Renal Physiol. 2006, 291, F517–F529. [Google Scholar] [CrossRef]
- Yang, L.; Han, B.; Zhang, M.; Wang, Y.H.; Tao, K.; Zhu, M.X.; He, K.; Zhang, Z.G.; Hou, S. Activation of BK Channels Prevents Hepatic Stellate Cell Activation and Liver Fibrosis Through the Suppression of TGFβ1/SMAD3 and JAK/STAT3 Profibrotic Signaling Pathways. Front. Pharmacol. 2020, 11, 165. [Google Scholar] [CrossRef]
- Yun, J.; Park, H.; Ko, J.H.; Lee, W.; Kim, K.; Kim, T.; Shin, J.; Kim, K.; Kim, K.; Song, J.H.; et al. Expression of Ca2+-Activated K+ Channels in Human Dermal Fibroblasts and Their Roles in Apoptosis. Skin Pharmacol. Physiol. 2010, 23, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Peréz, G.J.; Bonev, A.D.; Eckman, D.M.; Kosek, J.C.; Wiler, S.W.; Patterson, A.J.; Nelson, M.T.; Aldrich, R.W. Vasoregulation by the Β1 Subunit of the Calcium-Activated Potassium Channel. Nature 2000, 407, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.L.; Thorneloe, K.S.; Werner, M.E.; Nelson, M.T.; Aldrich, R.W. Overactive Bladder and Incontinence in the Absence of the BK Large Conductance Ca2+-Activated K+ Channel. J. Biol. Chem. 2004, 279, 36746–36752. [Google Scholar] [CrossRef] [PubMed]
- Samengo, I.; Currò, D.; Barrese, V.; Taglialatela, M.; Martire, M. Large Conductance Calcium-Activated Potassium Channels: Their Expression and Modulation of Glutamate Release from Nerve Terminals Isolated from Rat Trigeminal Caudal Nucleus and Cerebral Cortex. Neurochem. Res. 2014, 39, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Griguoli, M.; Sgritta, M.; Cherubini, E. Presynaptic BK Channels Control Transmitter Release: Physiological Relevance and Potential Therapeutic Implications. J. Physiol. 2016, 594, 3489–3500. [Google Scholar] [CrossRef]
- Farajnia, S.; Meijer, J.H.; Michel, S. Age-Related Changes in Large-Conductance Calcium-Activated Potassium Channels in Mammalian Circadian Clock Neurons. Neurobiol. Aging 2015, 36, 2176–2183. [Google Scholar] [CrossRef]
- Kshatri, A.S.; Gonzalez-Hernandez, A.; Giraldez, T. Physiological Roles and Therapeutic Potential of Ca2+ Activated Potassium Channels in the Nervous System. Front. Mol. Neurosci. 2018, 11, 258. [Google Scholar] [CrossRef]
- Lee, U.S.; Cui, J. BK Channel Activation: Structural and Functional Insights. Trends Neurosci. 2010, 33, 415–423. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.; Cui, J. BK Channels: Multiple Sensors, One Activation Gate. Front. Physiol. 2015, 6, 29. [Google Scholar] [CrossRef]
- Horrigan, F.T.; Aldrich, R.W. Coupling between Voltage Sensor Activation, Ca2+ Binding and Channel Opening in Large Conductance (BK) Potassium Channels. J. Gen. Physiol. 2002, 120, 267–305. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Trybek, P.; Machura, Ł.; Dworakowska, B.; Grzywna, Z.J. Mechanosensitivity of the BK Channels in Human Glioblastoma Cells: Kinetics and Dynamical Complexity. J. Membr. Biol. 2018, 251, 667–679. [Google Scholar] [CrossRef]
- Mallouk, N.; Allard, B. Stretch-Induced Activation of Ca2+-Activated K+ Channels in Mouse Skeletal Muscle Fibers. Am. J. Physiol. Cell Physiol. 2000, 278, C473–C479. [Google Scholar] [CrossRef] [PubMed]
- Kirber, M.T.; Ordway, R.W.; Clapp, L.H.; Walsh, J.V.; Singer, J.J. Both Membrane Stretch and Fatty Acids Directly Activate Large Conductance Ca2+-Activated K+ Channels in Vascular Smooth Muscle Cells. FEBS Lett. 1992, 297, 24–28. [Google Scholar] [CrossRef]
- Pallotta, B.S.; Magleby, K.L.; Barrett, J.N. Single Channel Recordings of Ca2+-Activated K+ Currents in Rat Muscle Cell Culture. Nature 1981, 293, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.R.; Constanti, A.; Brown, D.A.; Clark, R.B. Intracellular Ca2+ Activates a Fast Voltage-Sensitive K+ Current in Vertebrate Sympathetic Neurones. Nature 1982, 296, 746–749. [Google Scholar] [CrossRef]
- Barrett, J.N.; Magleby, K.L.; Pallotta, B.S. Properties of Single Calcium-activated Potassium Channels in Cultured Rat Muscle. J. Physiol. 1982, 331, 211–230. [Google Scholar] [CrossRef]
- Latorre, R.; Castillo, K.; Carrasquel-Ursulaez, W.; Sepulveda, R.V.; Gonzalez-Nilo, F.; Gonzalez, C.; Alvarez, O. Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol. Rev. 2017, 97, 39–87. [Google Scholar] [CrossRef]
- Yusifov, T.; Savalli, N.; Gandhi, C.S.; Ottolia, M.; Olcese, R. The RCK2 Domain of the Human BKCa Channel Is a Calcium Sensor. Proc. Natl. Acad. Sci. USA 2008, 105, 376–381. [Google Scholar] [CrossRef]
- Xia, X.M.; Zeng, X.; Lingle, C.J. Multiple Regulatory Sites in Large-Conductance Calcium-Activated Potassium Channels. Nature 2002, 418, 880–884. [Google Scholar] [CrossRef]
- Schreiber, M.; Salkoff, L. A Novel Calcium-Sensing Domain in the BK Channel. Biophys. J. 1997, 73, 1355–1363. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, S.Y.; Yang, J.; Shi, J.; Yang, X.; Moller, A.; Zou, X.; Cui, J. Ion Sensing in the RCK1 Domain of BK Channels. Proc. Natl. Acad. Sci. USA 2010, 107, 18700–18705. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, Z.; Meng, X.Y.; Cui, M.; Logothetis, D.E. Structural Determinants of Phosphatidylinositol 4,5-Bisphosphate (PIP2) Regulation of BK Channel Activity through the RCK1 Ca2+ Coordination Site. J. Biol. Chem. 2014, 289, 18860–18872. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Aursnes, M.; Hansen, T.V.; Tungen, J.E.; Galpin, J.D.; Leisle, L.; Ahern, C.A.; Xu, R.; Heinemann, S.H.; Hoshi, T. Atomic Determinants of BK Channel Activation by Polyunsaturated Fatty Acids. Proc. Natl. Acad. Sci. USA 2016, 113, 13905–13910. [Google Scholar] [CrossRef] [PubMed]
- King, J.T.; Lovell, P.V.; Rishniw, M.; Kotlikoff, M.I.; Zeeman, M.L.; McCobb, D.P. Β2 and Β4 Subunits of BK Channels Confer Differential Sensitivity to Acute Modulation by Steroid Hormones. J. Neurophysiol. 2006, 95, 2878–2888. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; Ohi, Y.; Muraki, K.; Watanabe, M.; Imaizumi, Y. BK Channel Activation by NS-1619 Is Partially Mediated by Intracellular Ca2+ Release in Smooth Muscle Cells of Porcine Coronary Artery. Br. J. Pharmacol. 2001, 132, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Layne, J.J.; Nausch, B.; Olesen, S.P.; Nelson, M.T. BK Channel Activation by NS11021 Decreases Excitability and Contractility of Urinary Bladder Smooth Muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R378–R384. [Google Scholar] [CrossRef]
- Augustynek, B.; Koprowski, P.; Rotko, D.; Kunz, W.S.; Szewczyk, A.; Kulawiak, B. Mitochondrial BK Channel Openers CGS7181 and CGS7184 Exhibit Cytotoxic Properties. Int. J. Mol. Sci. 2018, 19, 353. [Google Scholar] [CrossRef]
- Zhou, Y.; Lingle, C.J. Paxilline Inhibits BK Channels by an Almost Exclusively Closed-Channel Block Mechanism. J. Gen. Physiol. 2014, 144, 415–440. [Google Scholar] [CrossRef]
- Galvez, A.; Gimenez-Gallego, G.; Reuben, J.P.; Roy-Contancin, L.; Feigenbaum, P.; Kaczorowski, G.J.; Garcia, M.L. Purification and Characterization of a Unique, Potent, Peptidyl Probe for the High Conductance Calcium-Activated Potassium Channel from Venom of the Scorpion Buthus Tamulus. J. Biol. Chem. 1990, 265, 11083–11090. [Google Scholar] [CrossRef]
- Miller, C.; Moczydlowski, E.; Latorre, R.; Phillips, M. Charybdotoxin, a Protein Inhibitor of Single Ca2+-Activated K+ Channels from Mammalian Skeletal Muscle. Nature 1985, 313, 316–318. [Google Scholar] [CrossRef]
- Giangiacomo, K.M.; Garcia, M.L.; McManus, O.B. Mechanism of Iberiotoxin Block of the Large-Conductance Calcium-Activated Potassium Channel from Bovine Aortic Smooth Muscle. Biochemistry 1992, 31, 6719–6727. [Google Scholar] [CrossRef]
- Banerjee, A.; Lee, A.; Campbell, E.; MacKinnon, R. Structure of a Pore-Blocking Toxin in Complex with a Eukaryotic Voltage-Dependent K+ Channel. eLife 2013, 2, e00594. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.D.; Xu, R.; Reynolds, M.F.; Garcia, M.L.; Heinemann, S.H.; Hoshi, T. Haem Can Bind to and Inhibit Mammalian Calcium-Dependent Slo1 BK Channels. Nature 2003, 425, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Jaggar, J.H.; Li, A.; Parfenova, H.; Liu, J.; Umstot, E.S.; Dopico, A.M.; Leffler, C.W. Heme Is a Carbon Monoxide Receptor for Large-Conductance Ca2+-Activated K+ Channels. Circ. Res. 2005, 97, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.A.; Leach, N.; Ferguson, S.J. The Histidine of the C-Type Cytochrome CXXCH Haem-Binding Motif Is Essential for Haem Attachment by the Escherichia Coli Cytochrome c Maturation (Ccm) Apparatus. Biochem. J. 2005, 389 Pt 2, 587–592. [Google Scholar] [CrossRef]
- Kim, H.J.; Khalimonchuk, O.; Smith, P.M.; Winge, D.R. Structure, Function, and Assembly of Heme Centers in Mitochondrial Respiratory Complexes. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1604–1616. [Google Scholar] [CrossRef]
- Kranz, R.G.; Richard-Fogal, C.; Taylor, J.-S.; Frawley, E.R. Cytochrome c Biogenesis: Mechanisms for Covalent Modifications and Trafficking of Heme and for Heme-Iron Redox Control. Microbiol. Mol. Biol. Rev. 2009, 73, 510–528. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.M.; Daltrop, O.; Allen, J.W.A.; Ferguson, S.J. C-Type Cytochrome Formation: Chemical and Biological Enigmas. Acc. Chem. Res. 2004, 37, 999–1007. [Google Scholar] [CrossRef]
- Yi, L.; Morgan, J.T.; Ragsdale, S.W. Identification of a Thiol/Disulfide Redox Switch in the Human BK Channel That Controls Its Affinity for Heme and CO. J. Biol. Chem. 2010, 285, 20117–20127. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Skalska, J.; Glab, M.; Kulawiak, B.; Dolowy, K.; Szewczyk, A. Large Conductance Potassium Ion Channel from Rat Brain Mitochondria. Biochim. Biophys. Acta-Bioenerg. 2006, 461, 1–12. [Google Scholar]
- Sek, A.; Kampa, R.P.; Kulawiak, B.; Szewczyk, A.; Bednarczyk, P. Identification of the Large-conductance Ca2+-regulated Potassium Channel in Mitochondria of Human Bronchial Epithelial Cells. Molecules 2021, 26, 3233. [Google Scholar] [CrossRef]
- Siemen, D.; Loupatatzis, C.; Borecky, J.; Gulbins, E.; Lang, F. Ca2+-Activated K Channel of the BK-Type in the Inner Mitochondrial Membrane of a Human Glioma Cell Line. Biochem. Biophys. Res. Commun. 1999, 257, 549–554. [Google Scholar] [CrossRef]
- Lorigo, M.; Oliveira, N.; Cairrao, E. Clinical Importance of the Human Umbilical Artery Potassium Channels. Cells 2020, 9, 1956. [Google Scholar] [CrossRef]
- Hofmann, F. A Concise Discussion of the Regulatory Role of cGMP Kinase I in Cardiac Physiology and Pathology. Basic Res. Cardiol. 2018, 113, 31. [Google Scholar] [CrossRef]
- Balderas, E.; Zhang, J.; Stefani, E.; Toro, L. Mitochondrial BKCa Channel. Front. Physiol. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Szewczyk, A.; Bednarczyk, P.; Jędraszko, J.; Kampa, R.P.; Koprowski, P.; Krajewska, M.; Kucman, S.; Kulawiak, B.; Laskowski, M.; Rotko, D.; et al. Mitochondrial Potassium Channels-an Overview. Postepy Biochem. 2018, 64, 196–212. [Google Scholar] [CrossRef]
- Singh, H.; Lu, R.; Bopassa, J.C.; Meredith, A.L.; Stefani, E.; Toro, L. MitoBKCa Is Encoded by the Kcnma1 Gene, and a Splicing Sequence Defines Its Mitochondrial Location. Proc. Natl. Acad. Sci. USA 2013, 110, 10836–10841. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Y.; Wang, S.; McDonald, T.; Van Eyk, J.E.; Sidor, A.; O’Rourke, B. Cytoprotective Role of Ca2+-Activated K+ Channels in the Cardiac Inner Mitochondrial Membrane. Science 2002, 298, 1029–1033. [Google Scholar] [CrossRef]
- Borchert, G.H.; Yang, C.; Kolář, F. Mitochondrial BKCa Channels Contribute to Protection of Cardiomyocytes Isolated from Chronically Hypoxic Rats. Am. J. Physiol. Hear. Circ. Physiol. 2011, 300, H507–H513. [Google Scholar] [CrossRef]
- Sato, T.; Saito, T.; Saegusa, N.; Nakaya, H. Mitochondrial Ca2+-Activated K+ Channels in Cardiac Myocytes: A Mechanism of the Cardioprotective Effect and Modulation by Protein Kinase A. Circulation 2005, 111, 198–203. [Google Scholar] [CrossRef]
- Bentzen, B.H.; Osadchii, O.; Jespersen, T.; Hansen, R.S.; Olesen, S.P.; Grunnet, M. Activation of Big Conductance Ca2+-Activated K+ Channels (BK) Protects the Heart against Ischemia-Reperfusion Injury. Pflugers Arch. Eur. J. Physiol. 2009, 457, 979–988. [Google Scholar] [CrossRef]
- Gałecka, S.; Kulawiak, B.; Bednarczyk, P.; Singh, H.; Szewczyk, A. Single Channel Properties of Mitochondrial Large Conductance Potassium Channel Formed by BK-VEDEC Splice Variant. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Kicinska, A.; Kampa, R.P.; Daniluk, J.; Sek, A.; Jarmuszkiewicz, W.; Szewczyk, A.; Bednarczyk, P. Regulation of the Mitochondrial BKCa Channel by the Citrus Flavonoid Naringenin as a Potential Means of Preventing Cell Damage. Molecules 2020, 25, 3010. [Google Scholar] [CrossRef]
- Kampa, R.P.; Kicinska, A.; Jarmuszkiewicz, W.; Pasikowska-Piwko, M.; Dolegowska, B.; Debowska, R.; Szewczyk, A.; Bednarczyk, P. Naringenin as an Opener of Mitochondrial Potassium Channels in Dermal Fibroblasts. Exp. Dermatol. 2019, 28, 543–550. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Koziel, A.; Jarmuszkiewicz, W.; Szewczyk, A. Large-Conductance Ca2+-Activated Potassium Channel in Mitochondria of Endothelial EA.Hy926 Cells. Am. J. Physiol. Hear. Circ. Physiol. 2013, 304, H1415–H1427. [Google Scholar] [CrossRef]
- Gu, X.Q.; Pamenter, M.E.; Siemen, D.; Sun, X.; Haddad, G.G. Mitochondrial but Not Plasmalemmal BK Channels Are Hypoxia-Sensitive in Human Glioma. Glia 2014, 62, 504–513. [Google Scholar] [CrossRef]
- Skalska, J.; Bednarczyk, P.; Piwońska, M.; Kulawiak, B.; Wilczynski, G.; Dołowy, K.; Kudin, A.P.; Kunz, W.S.; Szewczyk, A. Calcium Ions Regulate K+ Uptake into Brain Mitochondria: The Evidence for a Novel Potassium Channel. Int. J. Mol. Sci. 2009, 10, 1104–1120. [Google Scholar] [CrossRef]
- Augustynek, B.; Kudin, A.P.; Bednarczyk, P.; Szewczyk, A.; Kunz, W.S. Hemin Inhibits the Large Conductance Potassium Channel in Brain Mitochondria: A Putative Novel Mechanism of Neurodegeneration. Exp. Neurol. 2014, 257, 70–75. [Google Scholar] [CrossRef]
- Robinson, S.R.; Dang, T.N.; Dringen, R.; Bishop, G.M. Hemin Toxicity: A Preventable Source of Brain Damage Following Hemorrhagic Stroke. Redox Rep. 2009, 14, 228–235. [Google Scholar] [CrossRef]
- Walewska, A.; Szewczyk, A.; Krajewska, M.; Koprowski, P. Targeting Mitochondrial Large-Conductance Calcium-Activated Potassium Channel by Hydrogen Sulfide via Heme-Binding Site. J. Pharmacol. Exp. Ther. 2022, 381, 137–150. [Google Scholar] [CrossRef]
- Walewska, A.; Kulawiak, B.; Szewczyk, A.; Koprowski, P. Mechanosensitivity of Mitochondrial Large-Conductance Calcium-Activated Potassium Channels. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 797–805. [Google Scholar] [CrossRef]
- Lavi, A.; Weitman, H.; Holmes, R.T.; Smith, K.M.; Ehrenberg, B. The Depth of Porphyrin in a Membrane and the Membrane’s Physical Properties Affect the Photosensitizing Efficiency. Biophys J. 2002, 82, 2101–2110. [Google Scholar] [CrossRef]
- Schmitt, T.H.; Frezzatti, W.A.; Schreier, S. Hemin-induced lipid membrane disorder and increased permeability: A molecular model for the mechanism of cell lysis. Arch Biochem Biophys. 1993, 307, 96–103. [Google Scholar] [CrossRef]
- Burton, M.J.; Kapetanaki, S.M.; Chernova, T.; Jamieson, A.G.; Dorlet, P.; Santolini, J.; Moody, P.C.E.; Mitcheson, J.S.; Davies, N.W.; Schmid, R.; et al. A Heme-Binding Domain Controls Regulation of ATP-Dependent Potassium Channels. Proc. Natl. Acad. Sci. USA 2016, 113, 3785–3790. [Google Scholar] [CrossRef]
- Borchert, G.H.; Hlaváčková, M.; Kolář, F. Pharmacological Activation of Mitochondrial BKCa Channels Protects Isolated Cardiomyocytes against Simulated Reperfusion-Induced Injury. Exp. Biol. Med. 2013, 238, 233–241. [Google Scholar] [CrossRef]
- Soltysinska, E.; Bentzen, B.H.; Barthmes, M.; Hattel, H.; Thrush, A.B.; Harper, M.E.; Qvortrup, K.; Larsen, F.J.; Schiffer, T.A.; Losa-Reyna, J.; et al. KCNMA1 Encoded Cardiac BK Channels Afford Protection against Ischemia-Reperfusion Injury. PLoS ONE 2014, 9, e103402. [Google Scholar] [CrossRef]
- Frankenreiter, S.; Bednarczyk, P.; Kniess, A.; Bork, N.I.; Straubinger, J.; Koprowski, P.; Wrzosek, A.; Mohr, E.; Logan, A.; Murphy, M.P.; et al. CGMP-Elevating Compounds and Ischemic Conditioning Provide Cardioprotection Against Ischemia and Reperfusion Injury via Cardiomyocyte-Specific BK Channels. Circulation 2017, 136, 2337–2355. [Google Scholar] [CrossRef]
- Kampa, R.P.; Sęk, A.; Szewczyk, A.; Bednarczyk, P. Cytoprotective Effects of the Flavonoid Quercetin by Activating Mitochondrial BKCa Channels in Endothelial Cells. Biomed. Pharmacother. 2021, 142, 112039. [Google Scholar] [CrossRef]
- Debska-Vielhaber, G.; Godlewski, M.M.; Kicinska, A.; Skalska, J.; Kulawiak, B.; Piwonska, M.; Zablocki, K.; Kunz, W.S.; Szewczyk, A.; Motyl, T. Large-Conductance K+ Channel Openers Induce Death of Human Glioma Cells. J. Physiol. Pharmacol. 2009, 60, 27–36. [Google Scholar]
- Bury, M.; Girault, A.; Megalizzi, V.; Spiegl-Kreinecker, S.; Mathieu, V.; Berger, W.; Evidente, A.; Kornienko, A.; Gailly, P.; Vandier, C.; et al. Ophiobolin A Induces Paraptosis-like Cell Death in Human Glioblastoma Cells by Decreasing BKCa Channel Activity. Cell Death Dis. 2013, 4, e561. [Google Scholar] [CrossRef]
- Leanza, L.; Venturini, E.; Kadow, S.; Carpinteiro, A.; Gulbins, E.; Becker, K.A. Targeting a Mitochondrial Potassium Channel to Fight Cancer. Cell Calcium. 2015, 58, 131–138. [Google Scholar] [CrossRef]
- Nilsson, R.; Schultz, I.J.; Pierce, E.L.; Soltis, K.A.; Naranuntarat, A.; Ward, D.M.; Baughman, J.M.; Paradkar, P.N.; Kingsley, P.D.; Culotta, V.C.; et al. Discovery of Genes Essential for Heme Biosynthesis through Large-Scale Gene Expression Analysis. Cell Metab. 2009, 10, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, K.T.; Chang, H.C.; Ardehali, H. Role of Heme in Cardiovascular Physiology and Disease. J. Am. Heart Assoc. 2015, 4, e001138. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Lengalova, A.; Martínek, V.; Martínková, M. Heme: Emergent Roles of Heme in Signal Transduction, Functional Regulation and as Catalytic Centres. Chem. Soc. Rev. 2019, 48, 5624–5657. [Google Scholar] [CrossRef]
- Tsiftsoglou, A.S.; Tsamadou, A.I.; Papadopoulou, L.C. Heme as Key Regulator of Major Mammalian Cellular Functions: Molecular, Cellular, and Pharmacological Aspects. Pharmacol. Ther. 2006, 111, 327–345. [Google Scholar] [CrossRef]
- Hoshi, T.; Zagotta, W.N.; Aldrich, R.W. Biophysical and Molecular Mechanisms of Shaker Potassium Channel Inactivation. Science 1990, 250, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Zagotta, W.N.; Aldrich, R.W. Two Types of Inactivation in Shaker K+ Channels: Effects of Alterations in the Carboxy-Terminal Region. Neuron 1991, 7, 547–556. [Google Scholar] [CrossRef]
- Sahoo, N.; Goradia, N.; Ohlenschläger, O.; Schönherr, R.; Friedrich, M.; Plass, W.; Kappl, R.; Hoshi, T.; Heinemann, S.H. Heme Impairs the Ball-and-Chain Inactivation of Potassium Channels. Proc. Natl. Acad. Sci. USA 2013, 110, E4036–E4044. [Google Scholar] [CrossRef]
- Li, Y.; Bai, J.; Yang, Y.H.; Hoshi, N.; Chen, D.B. Hydrogen Sulfide Relaxes Human Uterine Artery via Activating Smooth Muscle BKCa Channels. Antioxidants 2020, 9, 1127. [Google Scholar] [CrossRef]
- Sitdikova, G.F.; Weiger, T.M.; Hermann, A. Hydrogen Sulfide Increases Calcium-Activated Potassium (BK) Channel Activity of Rat Pituitary Tumor Cells. Pflugers Arch. Eur. J. Physiol. 2010, 459, 389–397. [Google Scholar] [CrossRef]
- Quan, X.; Luo, H.; Liu, Y.; Xia, H.; Chen, W.; Tang, Q. Hydrogen Sulfide Regulates the Colonic Motility by Inhibiting Both L-Type Calcium Channels and BKCa Channels in Smooth Muscle Cells of Rat Colon. PLoS ONE 2015, 10, e0121331. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, H.; Kong, G.; Shim, W.; Zhang, G. Hydrogen Sulfide Augments the Proliferation and Survival of Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells through Inhibition of BKCa. Cytotherapy 2013, 15, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Wieckowski, M.R.; Broszkiewicz, M.; Skowronek, K.; Siemen, D.; Szewczyk, A. Putative Structural and Functional Coupling of the Mitochondrial BKCa Channel to the Respiratory Chain. PLoS ONE 2013, 8, e68125. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Kampa, R.P.; Gałecka, S.; Se, A.; Walewska, A.; Koprowski, P. Patch-Clamp Recording of the Activity of Ion Channels in the Inner Mitochondrial Membrane. Methods Mol. Biol. 2021, 2276, 235–248. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).