Tamalin Function Is Required for the Survival of Neurons and Oligodendrocytes in the CNS
Abstract
:1. Introduction
2. Results
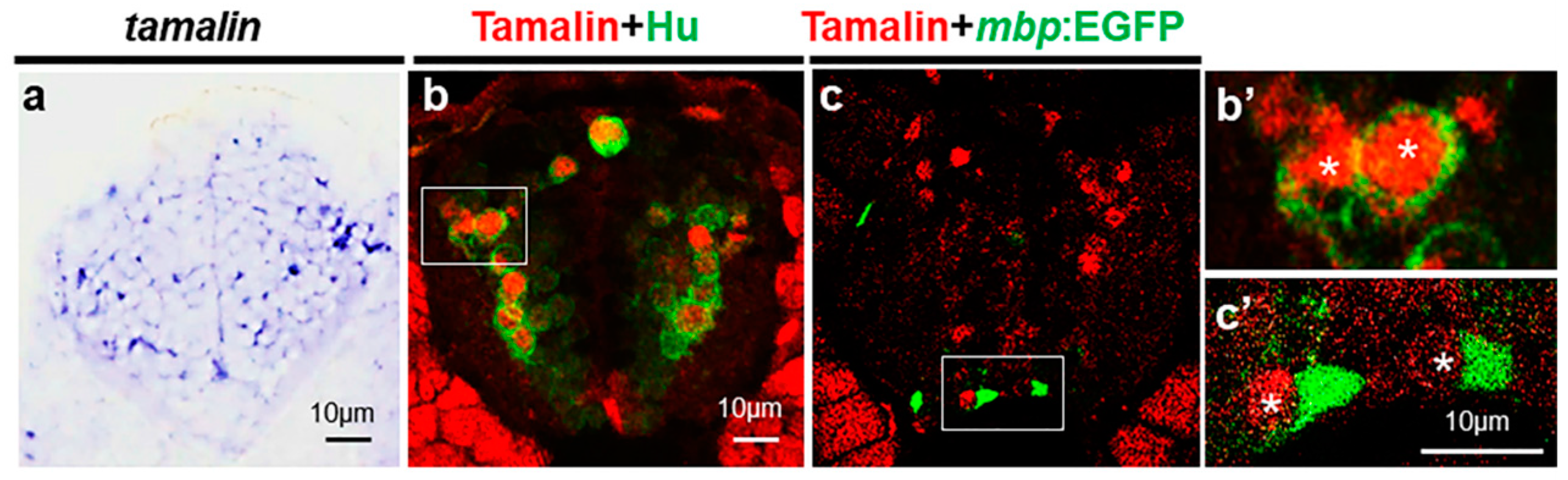
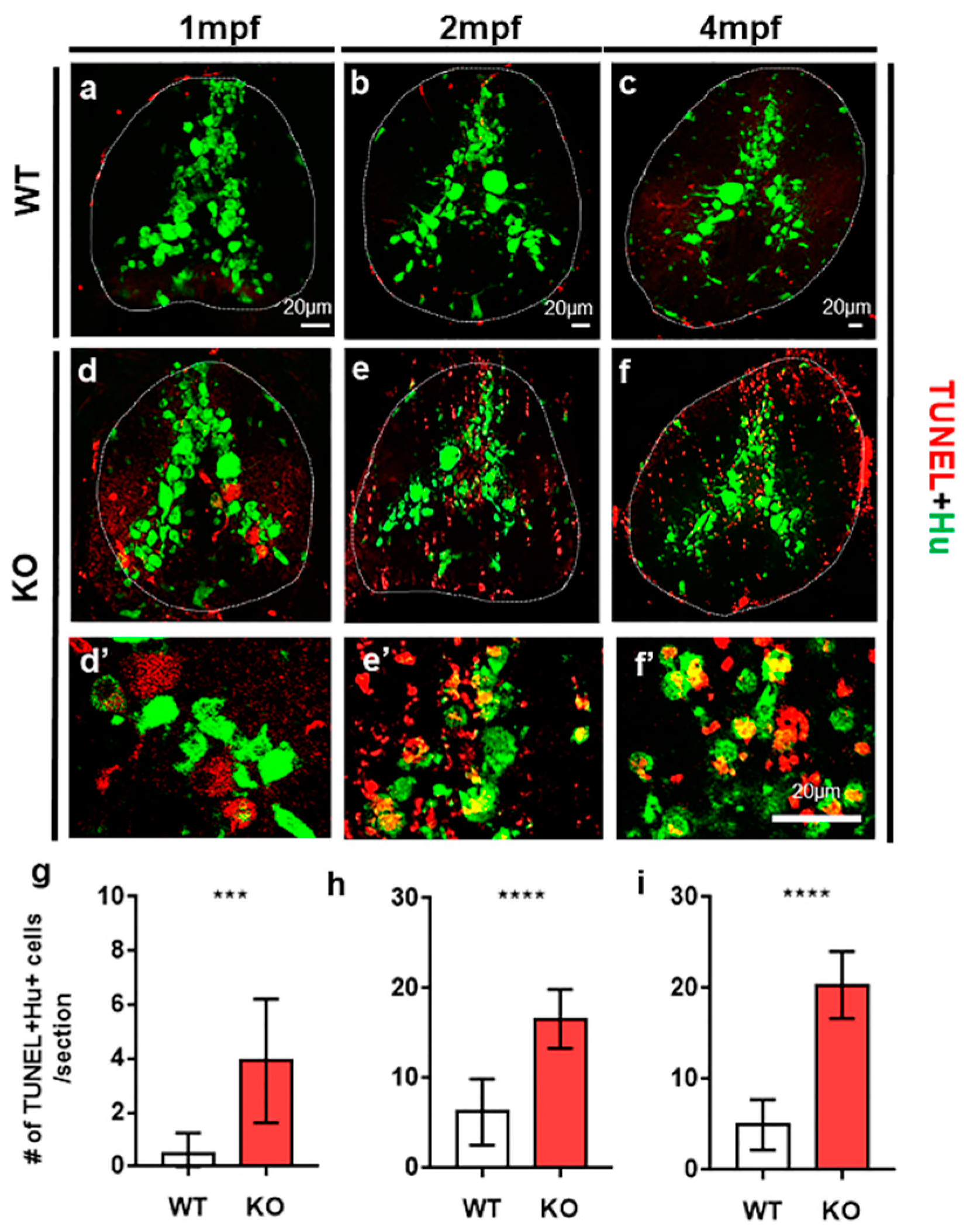
2.1. Tamalin Is Required for the Survival of Neurons in the Postembryonic CNS
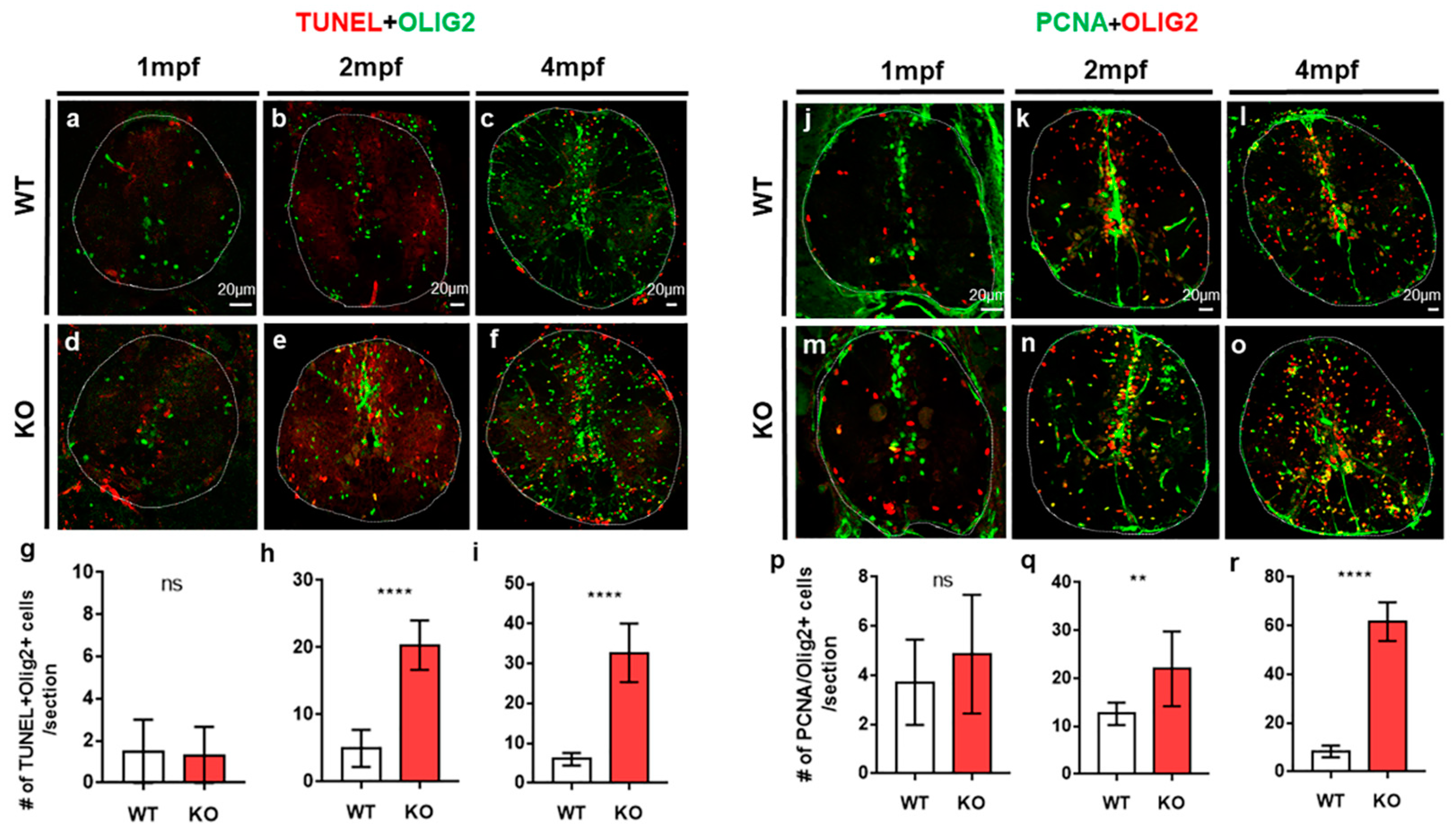
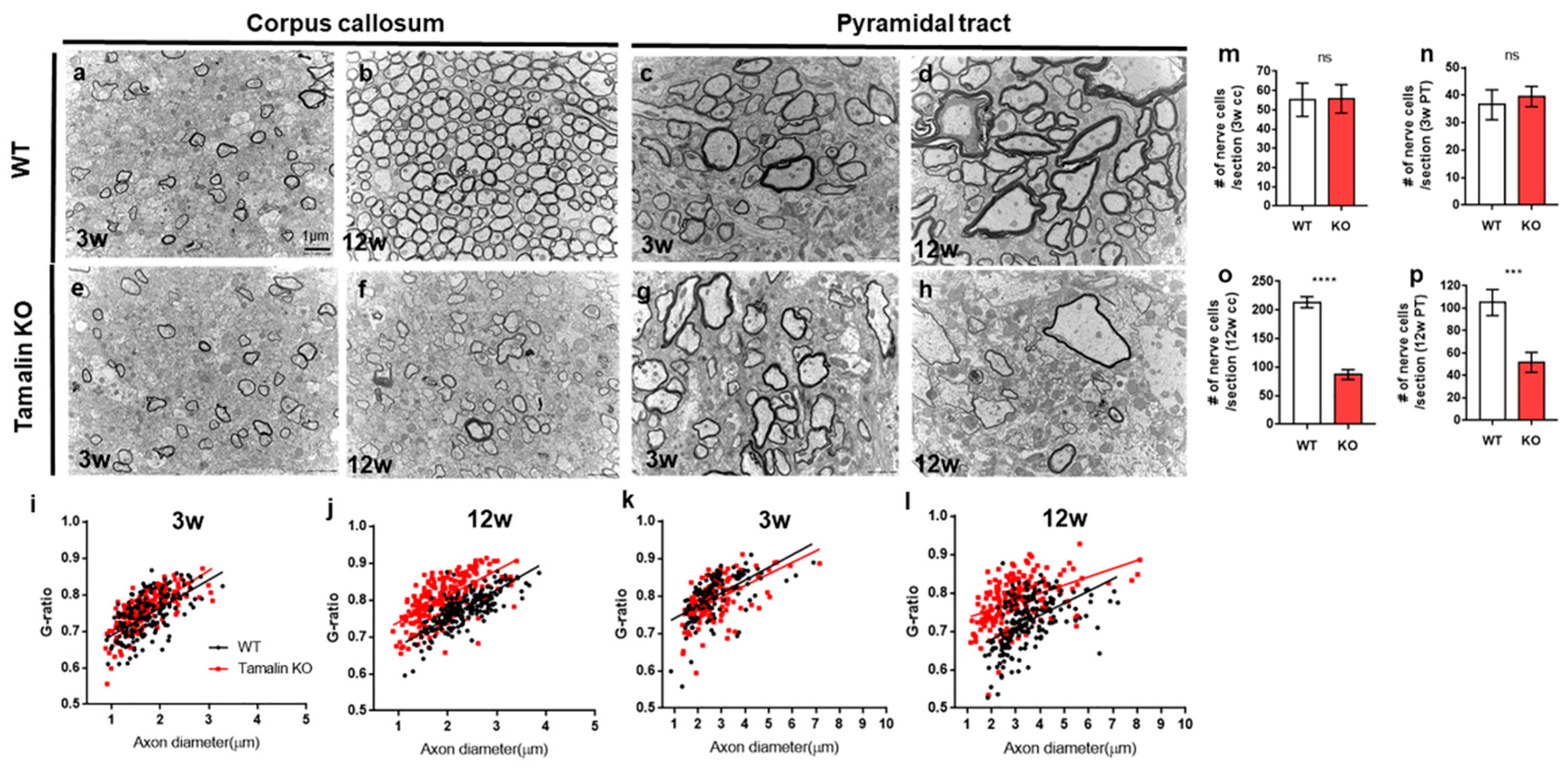
2.2. The Loss of Tamalin Function Caused Oligodendrocyte Cell Death and Hypomyelination
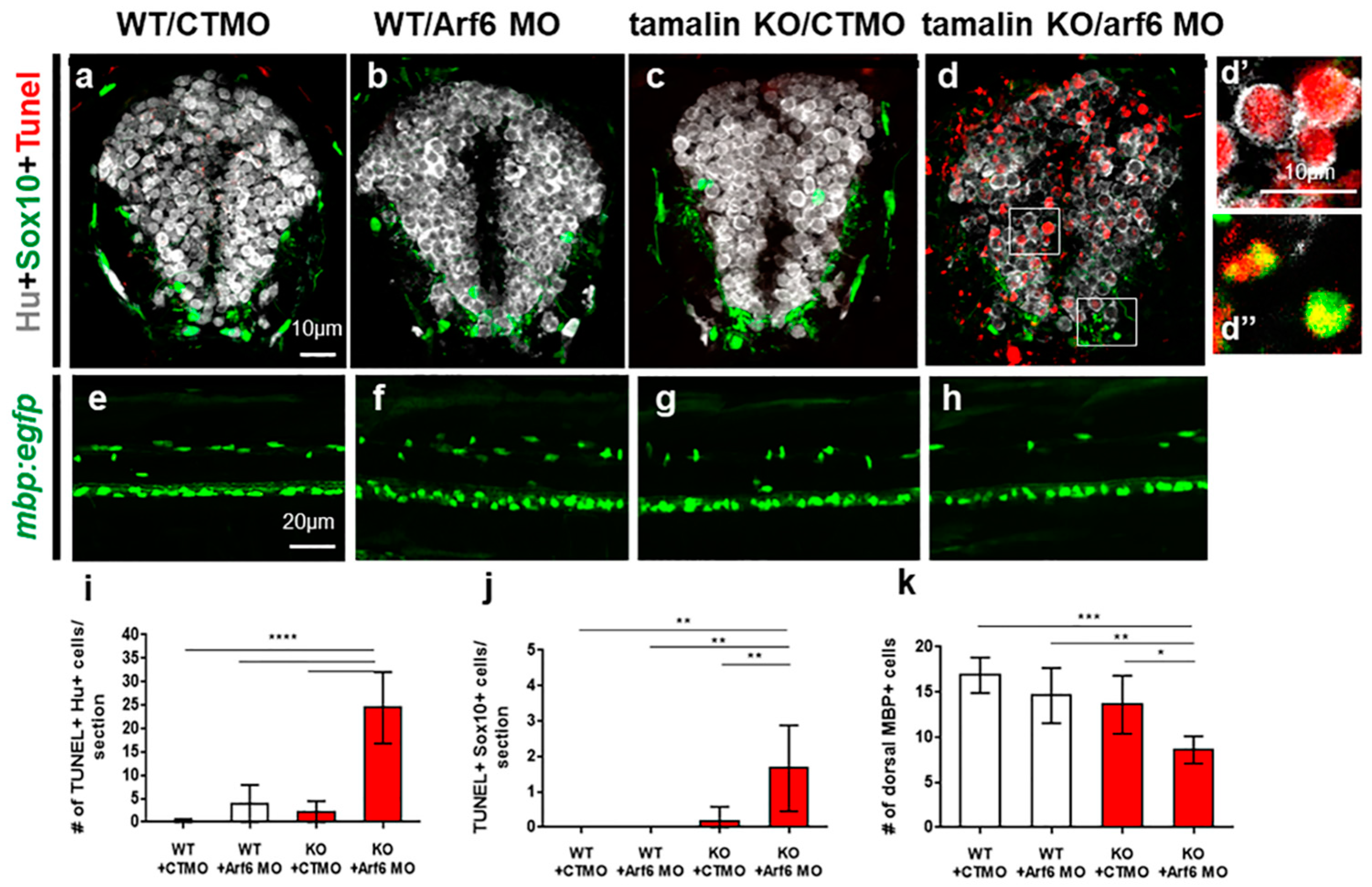
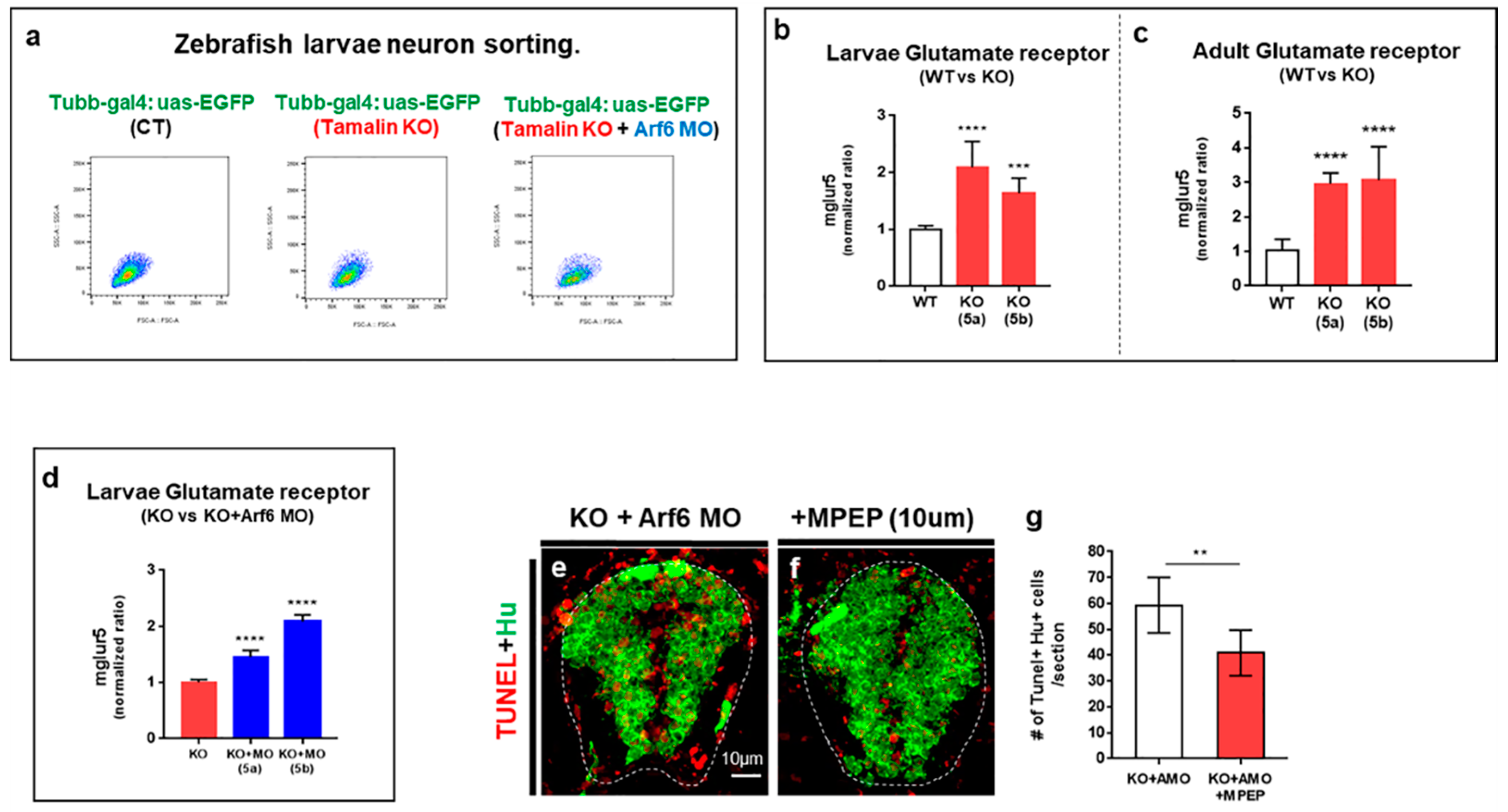
2.3. The Loss of Tamalin and Arf6 Synergistically Induced Neurodegeneration through Glutamate Toxicity
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Fish Lines and Mouse Lines
4.3. Immunohistochemistry and Wholemount In Situ RNA Hybridization
4.4. TUNEL Assay
4.5. The Generation of CRISPR/Cas9 Mediated KO Zebrafish and Genotyping
4.6. Transmission Electron Microscopic Analysis for Zebrafish
4.7. Transmission Electron Microscopic Analysis for Mouse
4.8. Behavioral Analysis with Elevated plus Maze (EPM)
4.9. MPEP Treatments
4.10. Quantitative Real-Time PCR
4.11. Morpholino Injection and Heat-Inducible Plasmid Construction
4.12. Embryo Dissociation and FACS Sorting
4.13. Adult Spinal Cord Dissection
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitano, J.; Kimura, K.; Yamazaki, Y.; Soda, T.; Shigemoto, R.; Nakajima, Y.; Nakanishi, S. Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J. Neurosci. 2002, 22, 1280–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban, P.F.; Yoon, H.Y.; Becker, J.; Dorsey, S.G.; Caprari, P.; Palko, M.E.; Coppola, V.; Saragovi, H.U.; Randazzo, P.A.; Tessarollo, L. A kinase-deficient TrkC receptor isoform activates Arf6-Rac1 signaling through the scaffold protein tamalin. J. Cell Biol. 2006, 173, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Hirose, M.; Kitano, J.; Nakajima, Y.; Moriyoshi, K.; Yanagi, S.; Yamamura, H.; Muto, T.; Jingami, H.; Nakanishi, S. Phosphorylation and recruitment of Syk by immunoreceptor tyrosine-based activation motif-based phosphorylation of tamalin. J. Biol. Chem. 2004, 279, 32308–32315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitano, J.; Yamazaki, Y.; Kimura, K.; Masukado, T.; Nakajima, Y.; Nakanishi, S. Tamalin is a scaffold protein that interacts with multiple neuronal proteins in distinct modes of protein-protein association. J. Biol. Chem. 2003, 278, 14762–14768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, J.; Choi, S.; Ahn, P.G.; Sun, W.; Lee, H.W.; Kim, H. PDZ-scaffold protein, Tamalin promotes dendritic outgrowth and arborization in rat hippocampal neuron. Biochem. Biophys. Res. Commun. 2012, 422, 250–255. [Google Scholar] [CrossRef]
- Ogawa, M.; Miyakawa, T.; Nakamura, K.; Kitano, J.; Furushima, K.; Kiyonari, H.; Nakayama, R.; Nakao, K.; Moriyoshi, K.; Nakanishi, S. Altered sensitivities to morphine and cocaine in scaffold protein tamalin knockout mice. Proc. Natl. Acad. Sci. USA 2007, 104, 14789–14794. [Google Scholar] [CrossRef] [Green Version]
- Yanpallewar, S.U.; Barrick, C.A.; Palko, M.E.; Fulgenzi, G.; Tessarollo, L. Tamalin is a critical mediator of electroconvulsive shock-induced adult neuroplasticity. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 2252–2262. [Google Scholar] [CrossRef] [Green Version]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflug. Arch. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Wang, H.; Rosenberg, P.A.; Volpe, J.J.; Jensen, F.E. Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 7751–7756. [Google Scholar] [CrossRef]
- Newcombe, J.; Uddin, A.; Dove, R.; Patel, B.; Turski, L.; Nishizawa, Y.; Smith, T. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 2008, 18, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.; Wolswijk, G.; Bo, L.; van der Valk, P.; Polman, C.H.; Troost, D.; Aronica, E. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain 2003, 126, 1755–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.; Ramsakha, N.; Sharma, R.; Gulia, R.; Ojha, P.; Lu, W.; Bhattacharyya, S. The post-synaptic scaffolding protein tamalin regulates ligand-mediated trafficking of metabotropic glutamate receptors. J. Biol. Chem. 2020, 295, 8575–8588. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kim, S.; Chung, A.Y.; Kim, H.T.; So, J.H.; Ryu, J.; Park, H.C.; Kim, C.H. Visualization of myelination in GFP-transgenic zebrafish. Dev. Dyn. 2010, 239, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Mehta, A.; Richardson, J.S.; Appel, B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev. Biol. 2002, 248, 356–368. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, D.M.; Movsesyan, V.; Vicini, S.; Faden, A.I. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br. J. Pharmacol. 2000, 131, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Nevrivy, D.J.; Peterson, V.J.; Avram, D.; Ishmael, J.E.; Hansen, S.G.; Dowell, P.; Hruby, D.E.; Dawson, M.I.; Leid, M. Interaction of GRASP, a protein encoded by a novel retinoic acid-induced gene, with members of the cytohesin family of guanine nucleotide exchange factors. J. Biol. Chem. 2000, 275, 16827–16836. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elrahman, K.S.; Ferguson, S.S.G. Noncanonical Metabotropic Glutamate Receptor 5 Signaling in Alzheimer’s Disease. Ann. Rev. Pharmacol. Toxicol. 2022, 62, 235–254. [Google Scholar] [CrossRef]
- Battaglia, G.; Bruno, V. Metabotropic glutamate receptor involvement in the pathophysiology of amyotrophic lateral sclerosis: New potential drug targets for therapeutic applications. Curr. Opin. Pharmacol. 2018, 38, 65–71. [Google Scholar] [CrossRef]
- Kalinowska, M.; Francesconi, A. Group I Metabotropic Glutamate Receptor Interacting Proteins: Fine-Tuning Receptor Functions in Health and Disease. Curr. Neuropharmacol. 2016, 14, 494–503. [Google Scholar] [CrossRef]
- Caraci, F.; Battaglia, G.; Sortino, M.A.; Spampinato, S.; Molinaro, G.; Copani, A.; Nicoletti, F.; Bruno, V. Metabotropic glutamate receptors in neurodegeneration/neuroprotection: Still a hot topic? Neurochem. Int. 2012, 61, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giribaldi, F.; Milanese, M.; Bonifacino, T.; Anna Rossi, P.I.; Di Prisco, S.; Pittaluga, A.; Tacchetti, C.; Puliti, A.; Usai, C.; Bonanno, G. Group I metabotropic glutamate autoreceptors induce abnormal glutamate exocytosis in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology 2013, 66, 253–263. [Google Scholar] [CrossRef]
- Milanese, M.; Giribaldi, F.; Melone, M.; Bonifacino, T.; Musante, I.; Carminati, E.; Rossi, P.I.; Vergani, L.; Voci, A.; Conti, F.; et al. Knocking down metabotropic glutamate receptor 1 improves survival and disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2014, 64, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Neyman, S.; Braunewell, K.H.; O’Connell, K.E.; Dev, K.K.; Manahan-Vaughan, D. Inhibition of the Interaction Between Group I Metabotropic Glutamate Receptors and PDZ-Domain Proteins Prevents Hippocampal Long-Term Depression, but Not Long-Term Potentiation. Front. Synaptic Neurosci. 2019, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matosin, N.; Fernandez-Enright, F.; Lum, J.S.; Andrews, J.L.; Engel, M.; Huang, X.F.; Newell, K.A. Metabotropic glutamate receptor 5, and its trafficking molecules Norbin and Tamalin, are increased in the CA1 hippocampal region of subjects with schizophrenia. Schizophr. Res. 2015, 166, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.Y.; MacDonald, M.L.; Borgmann-Winter, K.E.; Banerjee, A.; Sleiman, P.; Tom, A.; Khan, A.; Lee, K.C.; Roussos, P.; Siegel, S.J.; et al. mGluR5 hypofunction is integral to glutamatergic dysregulation in schizophrenia. Mol. Psychiatry 2020, 25, 750–760. [Google Scholar] [CrossRef]
- Matosin, N.; Fernandez-Enright, F.; Fung, S.J.; Lum, J.S.; Engel, M.; Andrews, J.L.; Huang, X.F.; Weickert, C.S.; Newell, K.A. Alterations of mGluR5 and its endogenous regulators Norbin, Tamalin and Preso1 in schizophrenia: Towards a model of mGluR5 dysregulation. Acta Neuropathol. 2015, 130, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Fukaya, M.; Sugawara, T.; Hara, Y.; Okamoto, H.; Yamauchi, J.; Sakagami, H. Cytohesin-2 mediates group I metabotropic glutamate receptor-dependent mechanical allodynia through the activation of ADP ribosylation factor 6 in the spinal cord. Neurobiol. Dis. 2021, 159, 105466. [Google Scholar] [CrossRef]
- Akiyama, M.; Hasegawa, H.; Hongu, T.; Frohman, M.A.; Harada, A.; Sakagami, H.; Kanaho, Y. Trans-regulation of oligodendrocyte myelination by neurons through small GTPase Arf6-regulated secretion of fibroblast growth factor-2. Nat. Commun. 2014, 5, 4744. [Google Scholar] [CrossRef]
- Torii, T.; Miyamoto, Y.; Onami, N.; Tsumura, H.; Nemoto, N.; Kawahara, K.; Kato, M.; Kotera, J.; Nakamura, K.; Tanoue, A.; et al. In vivo expression of the Arf6 Guanine-nucleotide exchange factor cytohesin-1 in mice exhibits enhanced myelin thickness in nerves. J. Mol. Neurosci. 2013, 51, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Torii, T.; Miyamoto, Y.; Yamamoto, M.; Ohbuchi, K.; Tsumura, H.; Kawahara, K.; Tanoue, A.; Sakagami, H.; Yamauchi, J. Arf6 mediates Schwann cell differentiation and myelination. Biochem. Biophys. Res. Commun. 2015, 465, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Torii, T.; Ohno, N.; Miyamoto, Y.; Kawahara, K.; Saitoh, Y.; Nakamura, K.; Takashima, S.; Sakagami, H.; Tanoue, A.; Yamauchi, J. Arf6 guanine-nucleotide exchange factor cytohesin-2 regulates myelination in nerves. Biochem. Biophys. Res. Commun. 2015, 460, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Munzel, E.J.; Schaefer, K.; Obirei, B.; Kremmer, E.; Burton, E.A.; Kuscha, V.; Becker, C.G.; Brosamle, C.; Williams, A.; Becker, T. Claudin k is specifically expressed in cells that form myelin during development of the nervous system and regeneration of the optic nerve in adult zebrafish. Glia 2012, 60, 253–270. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, K.; Kawakami, K. Targeted gene expression by the Gal4-UAS system in zebrafish. Dev. Growth Differ. 2008, 50, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Brend, T.; Holley, S.A. Zebrafish whole mount high-resolution double fluorescent in situ hybridization. J. Vis. Exp. 2009, 25, 1229. [Google Scholar] [CrossRef] [Green Version]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef]
- Jao, L.E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.T.; Kim, I.H.; Lee, K.J.; Lee, J.R.; Park, S.K.; Chun, Y.H.; Kim, H.; Rhyu, I.J. Specific plasticity of parallel fiber/Purkinje cell spine synapses by motor skill learning. Neuroreport 2002, 13, 1607–1610. [Google Scholar] [CrossRef]
- Brock, A.J.; Goody, S.M.G.; Mead, A.N.; Sudwarts, A.; Parker, M.O.; Brennan, C.H. Assessing the Value of the Zebrafish Conditioned Place Preference Model for Predicting Human Abuse Potential. J. Pharmacol. Exp. Ther. 2017, 363, 66–79. [Google Scholar] [CrossRef]
- Lambaerts, K.; Van Dyck, S.; Mortier, E.; Ivarsson, Y.; Degeest, G.; Luyten, A.; Vermeiren, E.; Peers, B.; David, G.; Zimmermann, P. Syntenin, a syndecan adaptor and an Arf6 phosphatidylinositol 4,5-bisphosphate effector, is essential for epiboly and gastrulation cell movements in zebrafish. J. Cell Sci. 2012, 125, 1129–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosacak, M.I.; Bhattarai, P.; Kizil, C. Protocol for Dissection and Dissociation of Zebrafish Telencephalon for Single-Cell Sequencing. STAR Protoc. 2020, 1, 100042. [Google Scholar] [CrossRef] [PubMed]
- Samsa, L.A.; Fleming, N.; Magness, S.; Qian, L.; Liu, J. Isolation and Characterization of Single Cells from Zebrafish Embryos. J. Vis. Exp. 2016, 12, 53877. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.; Mo, S.; Kim, S.; Kim, H.; Park, H.-C. Tamalin Function Is Required for the Survival of Neurons and Oligodendrocytes in the CNS. Int. J. Mol. Sci. 2022, 23, 13395. https://doi.org/10.3390/ijms232113395
Seo Y, Mo S, Kim S, Kim H, Park H-C. Tamalin Function Is Required for the Survival of Neurons and Oligodendrocytes in the CNS. International Journal of Molecular Sciences. 2022; 23(21):13395. https://doi.org/10.3390/ijms232113395
Chicago/Turabian StyleSeo, Yongbo, Seojung Mo, Suhyun Kim, Hyun Kim, and Hae-Chul Park. 2022. "Tamalin Function Is Required for the Survival of Neurons and Oligodendrocytes in the CNS" International Journal of Molecular Sciences 23, no. 21: 13395. https://doi.org/10.3390/ijms232113395
APA StyleSeo, Y., Mo, S., Kim, S., Kim, H., & Park, H.-C. (2022). Tamalin Function Is Required for the Survival of Neurons and Oligodendrocytes in the CNS. International Journal of Molecular Sciences, 23(21), 13395. https://doi.org/10.3390/ijms232113395



