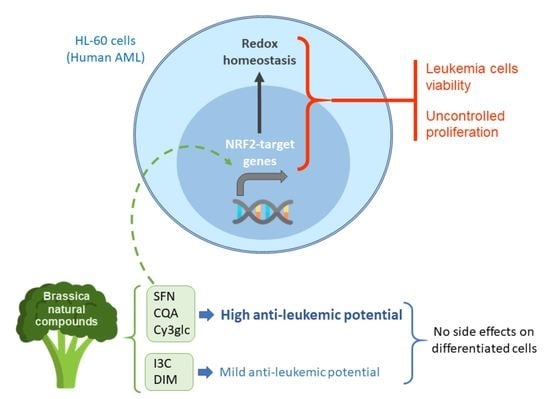
Anti-Leukemic Activity of Brassica-Derived Bioactive Compounds in HL-60 Myeloid Leukemia Cells
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Anti-Leukemic Potential of Brassica spp. Phytochemicals in HL-60 Cells
2.2. Effects of Brassica spp. Phytochemicals in the Cell Viability of Macrophage-like Differentiated HL-60 Cells
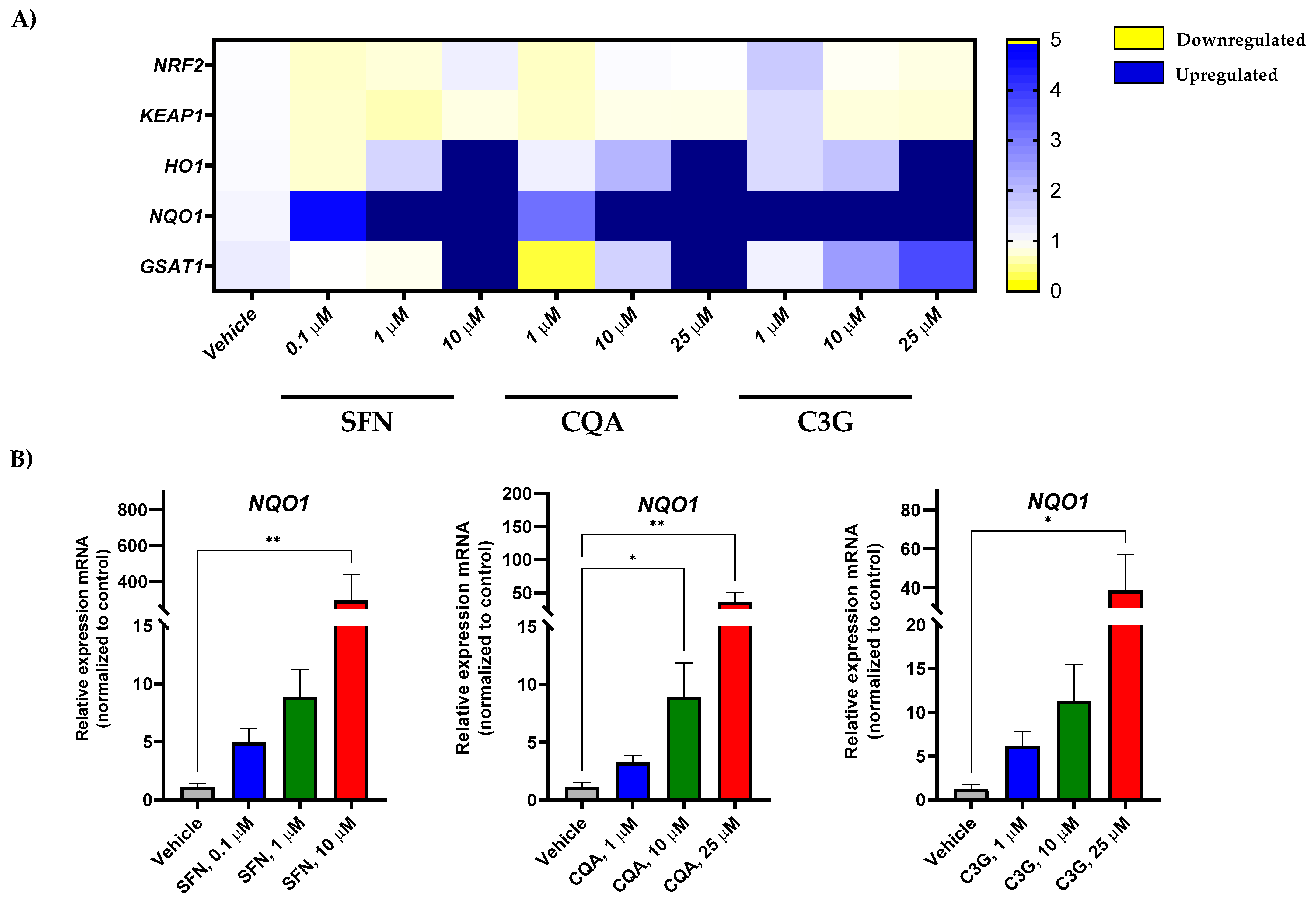
2.3. Potential of Brassica spp. Phytochemicals as NRF2 Inducers in Human Acute Myeloid Leukemia HL-60 Cells
3. Discussion
4. Materials and Methods
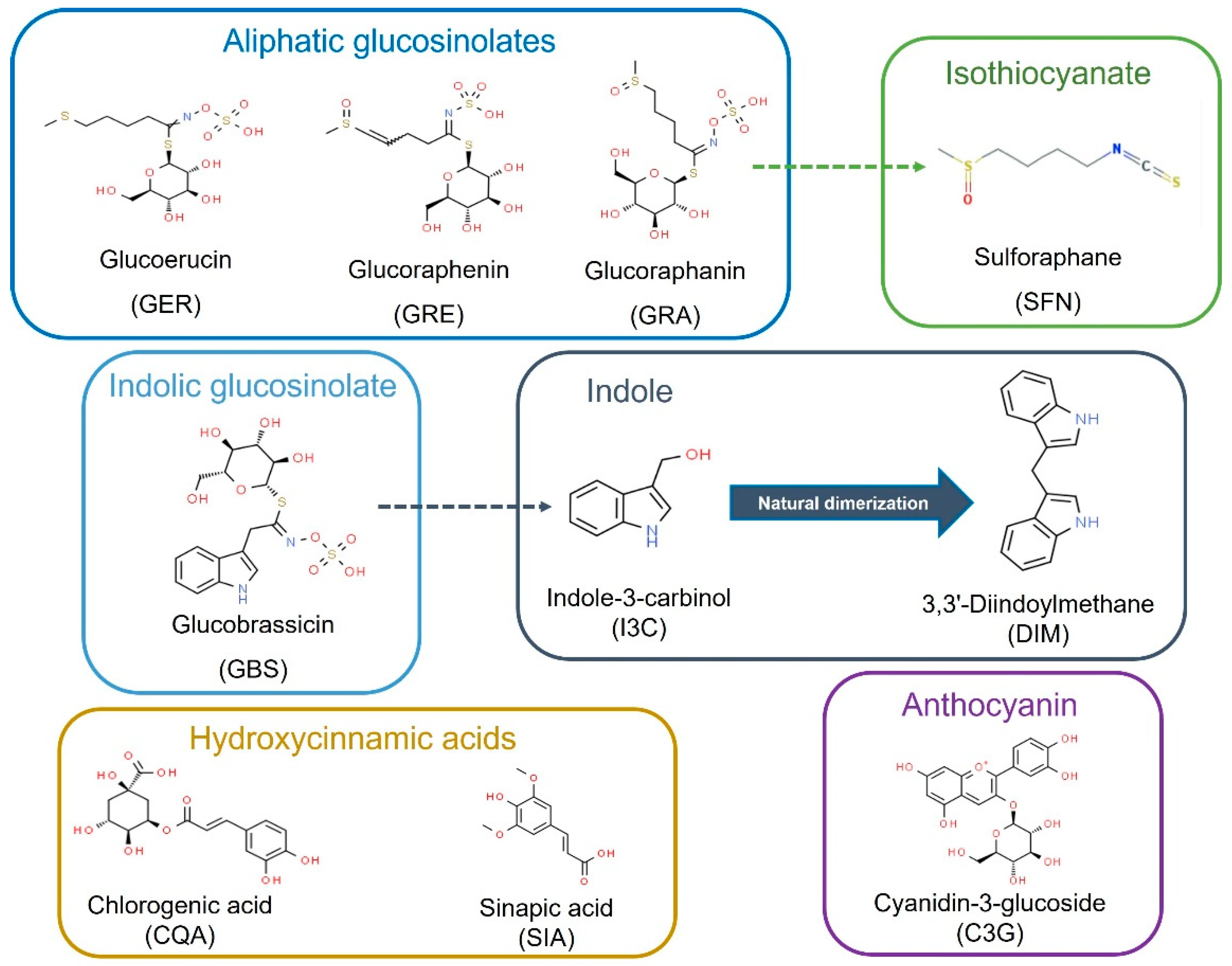
4.1. Compounds
4.2. Preparation of Compounds for In Vitro Assays
4.3. Cell Culture
4.4. In Vitro Cytotoxicity Assays
4.5. Gene Expression Assays
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kantarjian, H. Acute myeloid leukemia-Major progress over four decades and glimpses into the future. Am. J. Hematol. 2016, 91, 131–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantarjian, H.; Kadia, T.; DiNardo, C.; Daver, N.; Borthakur, G.; Jabbour, E.; Garcia-Manero, G.; Konopleva, M.; Ravandi, F. Acute myeloid leukemia: Current progress and future directions. Blood Cancer J. 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Hahn-Ast, C.; Schwab, K.; Schmidt-Wolf, I.G.H.; Brossart, P.; Glasmacher, A.; Von Lilienfeld-Toal, M. Long-term follow-up of Cladribine, high-dose Cytarabine, and Idarubicin as salvage treatment for relapsed acute myeloid leukemia and literature review. Eur. J. Haematol. 2020, 104, 538–545. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Green, S.D.; Foster, M.C.; Zeidner, J.F. Secondary AML Emerging After Therapy with Hypomethylating Agents: Outcomes, Prognostic Factors, and Treatment Options. Curr. Hematol. Malig. Rep. 2021, 16, 97–111. [Google Scholar] [CrossRef]
- van Gils, N.; Denkers, F.; Smit, L. Escape From Treatment; the Different Faces of Leukemic Stem Cells and Therapy Resistance in Acute Myeloid Leukemia. Front. Oncol. 2021, 11, 659253. [Google Scholar] [CrossRef]
- Fajardo-Orduña, G.; Ledesma-Martínez, E.; Aguiñiga-Sánchez, I.; Mora-García, M.; Weiss-Steider, B.; Santiago-Osorio, E. Inhibitors of Chemoresistance Pathways in Combination with Ara-C to Overcome Multidrug Resistance in AML. A Mini Review. Int. J. Mol. Sci. 2021, 22, 4955. [Google Scholar] [CrossRef]
- Liu, H. Emerging agents and regimens for AML. J. Hematol. Oncol. 2021, 14, 49. [Google Scholar] [CrossRef]
- Thol, F.; Heuser, M. Treatment for Relapsed/Refractory Acute Myeloid Leukemia. HemaSphere 2021, 5, e572. [Google Scholar] [CrossRef]
- Capuano, E.; Dekker, M.; Verkerk, R.; Oliviero, T. Food as Pharma? The Case of Glucosinolates. Curr. Pharm. Des. 2017, 23, 2697–2721. [Google Scholar] [CrossRef]
- Cotoraci, C.; Ciceu, A.; Sasu, A.; Miutescu, E.; Hermenean, A. The Anti-Leukemic Activity of Natural Compounds. Molecules 2021, 26, 2709. [Google Scholar] [CrossRef]
- Lenzi, M.; Cocchi, V.; Malaguti, M.; Barbalace, M.C.; Marchionni, S.; Hrelia, S.; Hrelia, P. 6-(Methylsulfonyl) hexyl isothiocyanate as potential chemopreventive agent: Molecular and cellular profile in leukaemia cell lines. Oncotarget 2017, 8, 111697–111714. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, M.; Malaguti, M.; Cocchi, V.; Hrelia, S.; Hrelia, P. Castanea sativa Mill. bark extract exhibits chemopreventive properties triggering extrinsic apoptotic pathway in Jurkat cells. BMC Complement. Altern. Med. 2017, 17, 251. [Google Scholar] [CrossRef]
- Prashar, A.; Siddiqui, F.; Singh, A.K. Synthetic and green vegetable isothiocyanates target red blood leukemia cancers. Fitoterapia 2012, 83, 255–265. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhauser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S219. [Google Scholar] [CrossRef]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [Green Version]
- Royston, K.J.; Tollefsbol, T.O. The Epigenetic Impact of Cruciferous Vegetables on Cancer Prevention. Curr. Pharmacol. Rep. 2015, 1, 46–51. [Google Scholar] [CrossRef]
- Sita, G.; Hrelia, P.; Tarozzi, A.; Morroni, F. Isothiocyanates Are Promising Compounds against Oxidative Stress, Neuroinflammation and Cell Death that May Benefit Neurodegeneration in Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteve, M. Mechanisms Underlying Biological Effects of Cruciferous Glucosinolate-Derived Isothiocyanates/Indoles: A Focus on Metabolic Syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Li, L.; Iwamoto, N.; Nakajima-Takagi, Y.; Kaneko, H.; Nakayama, Y.; Eguchi, M.; Wada, Y.; Kumagai, Y.; Yamamoto, M. The Antioxidant Defense System Keap1-Nrf2 Comprises a Multiple Sensing Mechanism for Responding to a Wide Range of Chemical Compounds. Mol. Cell. Biol. 2009, 29, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Quirante-Moya, S.; García-Ibañez, P.; Quirante-Moya, F.; Villaño, D.; Moreno, D.A. The Role of Brassica Bioactives on Human Health: Are We Studying It the Right Way? Molecules 2020, 25, 1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeger, H.; Mokhtari, R.B. Perspective on dietary isothiocyanates in the prevention, development and treatment of cancer. J. Cancer Metastasis Treat. 2020, 2020, 26. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef] [Green Version]
- Baenas, N.; Francisco, M.; Velasco, P.; Cartea, M.E.; García-Villegas, C.; Moreno, D.A. Bioactive Compounds from Brassicaceae as Health Promoters. In Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters: Part II; da Silva, L.R., Silva, B.M., Eds.; Bentham Science Publisher: Sharjah, United Arab Emirates, 2016; pp. 27–47. [Google Scholar] [CrossRef] [Green Version]
- Moreno, D.A.; Carvajal, M.; López-Berenguer, C.; García-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Wagner, A.E. Pharmacoepigenetics of Brassica-Derived Compounds. In Pharmacoepigenetics; Academic Press: Cambridge, MA, USA, 2019; pp. 847–857. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Woźniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2017, 58, 1391–1405. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Xiao, J.-H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxidative Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Mechchate, H.; de Oliveira, R.C.; Es-Safi, I.; Mourão, E.V.; Bouhrim, M.; Kyrylchuk, A.; Pontes, G.S.; Bousta, D.; Grafov, A. Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds. Pharmaceuticals 2021, 14, 770. [Google Scholar] [CrossRef]
- Zhu, W.J.; Lin, L.P.; Liu, D.; Qian, J.C.; Zhou, B.B.; Yuan, D.D.; Tan, R.X. Pharmacophore-inspired discovery of FLT3 inhibitor from kimchi. Food Chem. 2021, 361, 130139. [Google Scholar] [CrossRef]
- Guirado, A.; Sánchez, J.I.L.; Ruiz-Alcaraz, A.J.; Bautista, D.; Gálvez, J. Synthesis and biological evaluation of 4-alkoxy-6,9-dichloro[1,2,4]triazolo[4,3-a]quinoxalines as inhibitors of TNF-α and IL-6. Eur. J. Med. Chem. 2012, 54, 87–94. [Google Scholar] [CrossRef]
- Ruiz-Alcaraz, A.J.; Martínez-Sánchez, M.A.; García-Peñarrubia, P.; Martinez-Esparza, M.; Ramos-Molina, B.; Moreno, D.A. Analysis of the anti-inflammatory potential of Brassica bioactive compounds in a human macrophage-like cell model derived from HL-60 cells. Biomed. Pharmacother. 2022, 149, 112804. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Chen, J.-G.; Zarth, A.T.; Ng, D.K.; Wang, J.-B.; Kensler, K.H.; Jacobson, L.P.; Muñoz, A.; Johnson, J.L.; Groopman, J.D.; et al. Rapid and Sustainable Detoxication of Airborne Pollutants by Broccoli Sprout Beverage: Results of a Randomized Clinical Trial in China. Cancer Prev. Res. 2014, 7, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Atwell, L.L.; E Farris, P.; Ho, E.; Shannon, J. Associations between cruciferous vegetable intake and selected biomarkers among women scheduled for breast biopsies. Public Health Nutr. 2015, 19, 1288–1295. [Google Scholar] [CrossRef] [Green Version]
- Traka, M.H.; Melchini, A.; Coode-Bate, J.; Al Kadhi, O.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O’Neill, C.M.; Bernuzzi, F.; et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention-results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Crowley, E.; Rowan, N.J.; Faller, D.; Friel, A.M. Natural and Synthetic Isothiocyanates Possess Anticancer Potential Against Liver and Prostate Cancer In Vitro. Anticancer Res. 2019, 39, 3469–3485. [Google Scholar] [CrossRef]
- Ding, Y.; Paonessa, J.D.; Randall, K.L.; Argoti, D.; Chen, L.; Vouros, P.; Zhang, Y. Sulforaphane inhibits 4-aminobiphenyl-induced DNA damage in bladder cells and tissues. Carcinogenesis 2010, 31, 1999–2003. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Murata, T.; Ramos, J.W. The phytochemical p-hydroxycinnamic acid suppresses the growth and stimulates the death in human liver cancer HepG2 cells. Anti-Cancer Drugs 2021, 32, 558–566. [Google Scholar] [CrossRef]
- Wang, F.P.; Qiao, C.J.; Sun, Y.W.; Li, X.Y.; Huang, X.Y.; Zhang, W.R.; Wang, X.; Wang, M.Y. Effect and Mechanism of Sulforaphane on G2/M Phase Arrest of Acute Myeloid Leukemia KG1a and KG1 Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.-S.; Shih, Y.-L.; Lee, C.-H.; Hsueh, S.-C.; Liu, J.-Y.; Liao, N.-C.; Chen, Y.-L.; Huang, Y.-P.; Lu, H.-F.; Chung, J.-G. Sulforaphane-induced apoptosis in human leukemia HL-60 cells through extrinsic and intrinsic signal pathways and altering associated genes expression assayed by cDNA microarray. Environ. Toxicol. 2016, 32, 311–328. [Google Scholar] [CrossRef]
- Steverding, D.; Michaels, S.; Read, K.D. In Vitro and In Vivo Studies of Trypanocidal Activity of Dietary Isothiocyanates. Planta Medica 2014, 80, 183–186. [Google Scholar] [CrossRef]
- Fimognari, C.; Berti, F.; Nüsse, M.; Cantelli-Forti, G.; Hrelia, P. Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanidin-3-O-β-glucopyranoside. Biochem. Pharmacol. 2004, 67, 2047–2056. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zhou, C.-Y.; Qiu, C.-H.; Lu, X.-M.; Wang, Y.-T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL-60 cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Hernandes, L.C.; Machado, A.R.T.; Tuttis, K.; Ribeiro, D.L.; Aissa, A.F.; Dévoz, P.P.; Antunes, L.M.G. Caffeic acid and chlorogenic acid cytotoxicity, genotoxicity and impact on global DNA methylation in human leukemic cell lines. Genet. Mol. Biol. 2020, 43, e20190347. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [Green Version]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, D.; Ma, K.; Wu, X.; Hao, H.; Jiang, S. NAD(P)H:Quinone Oxidoreductase 1 (NQO1) as a Therapeutic and Diagnostic Target in Cancer. J. Med. Chem. 2018, 61, 6983–7003. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Garate, M.; Wani, A.A.; Li, G. The NAD(P)H:Quinone Oxidoreductase 1 induces cell cycle progression and proliferation of melanoma cells. Free Radic. Biol. Med. 2010, 48, 1601–1609. [Google Scholar] [CrossRef]
- Oh, E.-T.; Kim, J.-W.; Kim, J.M.; Kim, S.J.; Lee, J.-S.; Hong, S.-S.; Goodwin, J.; Ruthenborg, R.J.; Jung, M.G.; Lee, H.-J.; et al. NQO1 inhibits proteasome-mediated degradation of HIF-1α. Nat. Commun. 2016, 7, 13593. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Li, Y.; Deng, C.; Wang, Y.; Piao, J.; Lin, Z.; Chen, L. Significant association of PKM2 and NQO1 proteins with poor prognosis in breast cancer. Pathol. Res. Pract. 2020, 216, 153173. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates From Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef]

| IC50 (μM) | ||
|---|---|---|
| Compound | Treatment Time | |
| 48 h | 72 h | |
| SFN | 5.9 ± 0.3 | 11.3 ± 0.5 |
| CQA | 17.4 ± 0.7 | 28.9 ± 0.7 |
| C3G | 7.0 ± 1.0 | 21.9 ± 0.3 |
| Viability (%) | |||
|---|---|---|---|
| Compound | Dose (µM) | ||
| 10 | 25 | 50 | |
| SFN | 98.2 ± 12.1 | 87.6 ± 6.8 | 70.0 ± 17.5 * |
| CQA | 113.5 ± 19.2 | 114.4 ± 20.6 | 108.7 ± 11.8 |
| C3G | 113.7 ± 24.4 | 108.1 ± 7.4 | 107.2 ± 12.8 |
| I3C | 93.6 ± 8.0 | 99.5 ± 5.4 | 95.1 ± 10.4 |
| DIM | 101.5 ± 22.7 | 117.7 ± 27.2 | 104.8 ± 13.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Sánchez, M.Á.; Martínez-Sánchez, M.A.; Verdejo-Sánchez, M.; García-Ibáñez, P.; Oliva Bolarín, A.; Ramos-Molina, B.; Moreno, D.A.; Ruiz-Alcaraz, A.J. Anti-Leukemic Activity of Brassica-Derived Bioactive Compounds in HL-60 Myeloid Leukemia Cells. Int. J. Mol. Sci. 2022, 23, 13400. https://doi.org/10.3390/ijms232113400
Núñez-Sánchez MÁ, Martínez-Sánchez MA, Verdejo-Sánchez M, García-Ibáñez P, Oliva Bolarín A, Ramos-Molina B, Moreno DA, Ruiz-Alcaraz AJ. Anti-Leukemic Activity of Brassica-Derived Bioactive Compounds in HL-60 Myeloid Leukemia Cells. International Journal of Molecular Sciences. 2022; 23(21):13400. https://doi.org/10.3390/ijms232113400
Chicago/Turabian StyleNúñez-Sánchez, María Ángeles, María Antonia Martínez-Sánchez, Marina Verdejo-Sánchez, Paula García-Ibáñez, Alba Oliva Bolarín, Bruno Ramos-Molina, Diego A. Moreno, and Antonio J. Ruiz-Alcaraz. 2022. "Anti-Leukemic Activity of Brassica-Derived Bioactive Compounds in HL-60 Myeloid Leukemia Cells" International Journal of Molecular Sciences 23, no. 21: 13400. https://doi.org/10.3390/ijms232113400
APA StyleNúñez-Sánchez, M. Á., Martínez-Sánchez, M. A., Verdejo-Sánchez, M., García-Ibáñez, P., Oliva Bolarín, A., Ramos-Molina, B., Moreno, D. A., & Ruiz-Alcaraz, A. J. (2022). Anti-Leukemic Activity of Brassica-Derived Bioactive Compounds in HL-60 Myeloid Leukemia Cells. International Journal of Molecular Sciences, 23(21), 13400. https://doi.org/10.3390/ijms232113400