Giant Triton Snail Charonia tritonis Macrophage-Expressed Gene 1 Protein Ct-Mpeg1: Molecular Identification, Expression Analysis, and Antimicrobial Activity
Abstract
:1. Introduction
2. Results
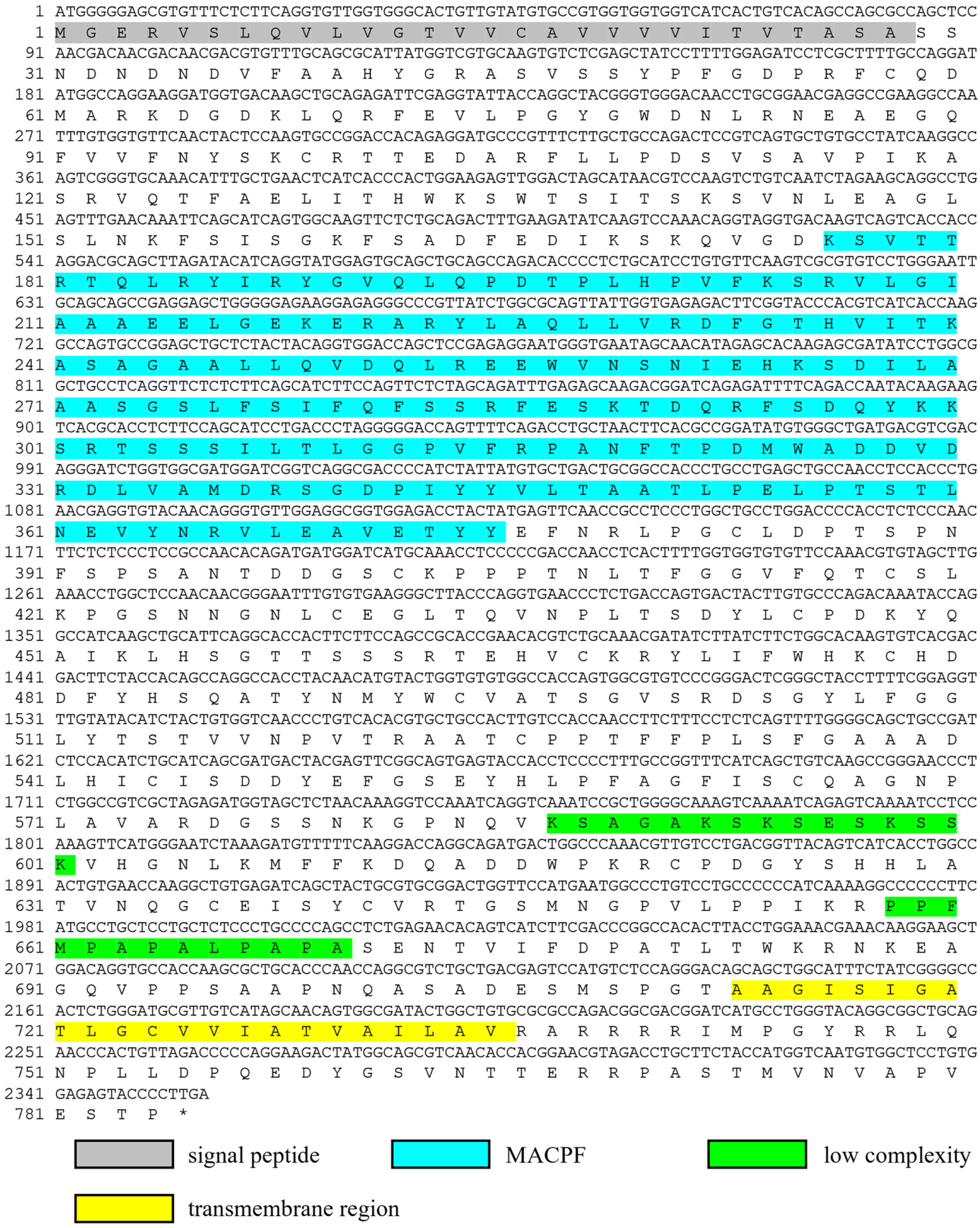
2.1. Molecular Cloning and Bioinformatic Analysis of Ct-Mgep1 cDNA
2.2. Expression Profiles of Ct-Mpeg1 mRNA in Different Tissues
2.3. Prokaryotic Expression and Purification of rCt-Mpeg1
2.4. The Antibacterial Activity of rCt-Mgep1 Protein
2.5. rCt-Mgep1 Has a Significant Impact on the Transcript Abundance of Genes Invloved in TCA Cycle and Outer Membrane Protein of V. alginolyticus
3. Discussion
4. Materials and Methods
4.1. Animals and Tissue Collection
4.2. Molecular Cloning and Bioinformatics Analysis of Ct-Mgep1
4.3. RNA Isolation and Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
4.4. Construction of Prokaryotic Expression Vector
4.5. Over-Expression and Purification of Recombinant Ct-Mgep1 Protein
4.6. Antimicrobial Activity Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayly-Jones, C.; Pang, S.S.; Spicer, B.A.; Whisstock, J.C.; Dunstone, M.A. Ancient but Not Forgotten: New Insights Into MPEG1, a Macrophage Perforin-Like Immune Effector. Front. Immunol. 2020, 11, 581906. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.; Mullereberhard, H.J.; Podack, E.R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein-c9. Nature 1982, 298, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Law, R.H.P.; Lukoyanova, N.; Voskoboinik, I.; Caradoc-Davies, T.T.; Baran, K.; Dunstone, M.A.; D’Angelo, M.E.; Orlova, E.V.; Coulibaly, F.; Verschoor, S.; et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 2010, 468, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Reiter, Y.; Ciobotariu, A.; Jones, J.; Morgan, B.P.; Fishelson, Z. Complement membrane attack complex, perforin, and bacterial exotoxins induce in k562 cells calcium-dependent cross-protection from lysis. J. Immunol. 1995, 155, 2203–2210. [Google Scholar]
- Rosado, C.J.; Buckle, A.M.; Law, R.H.P.; Butcher, R.E.; Kan, W.T.; Bird, C.H.; Ung, K.; Browne, K.A.; Baran, K.; Bashtannyk-Puhalovich, T.A.; et al. A common fold mediates vertebrate defense and bacterial attack. Science 2007, 317, 1548–1551. [Google Scholar] [CrossRef] [Green Version]
- McCormack, R.; de Armas, L.; Shiratsuchi, M.; Podack, E.R. Killing machines: Three pore-forming proteins of the immune system. Immunol. Res. 2013, 57, 268–278. [Google Scholar] [CrossRef] [Green Version]
- McCormack, R.M.; de Armas, L.R.; Shiratsuchi, M.; Fiorentino, D.G.; Olsson, M.L.; Lichtenheld, M.G.; Morales, A.; Lyapichev, K.; Gonzalez, L.E.; Strbo, N.; et al. Perforin-2 is essential for intracellular defense of parenchymal cells and phagocytes against pathogenic bacteria. Elife 2015, 4, e06508. [Google Scholar] [CrossRef]
- McCormack, R.; Podack, E.R. Perforin-2/Mpeg1 and other pore-forming proteins throughout evolution. J. Leukoc. Biol. 2015, 98, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Wiens, M.; Korzhev, M.; Krasko, A.; Thakur, N.L.; Perovic-Ottstadt, S.; Breter, H.J.; Ushijima, H.; Diehl-Seifert, R.; Muller, I.M.; Muller, W.E.G. Innate immune defense of the sponge Suberites domuncula against bacteria involves a MyD88-dependent signaling pathway—Induction of a perforin-like molecule. J. Biol. Chem. 2005, 280, 27949–27959. [Google Scholar] [CrossRef] [Green Version]
- Thakur, N.L.; Hentschel, U.; Krasko, A.; Pabel, C.T.; Anil, A.C.; Muller, W.E.G. Antibacterial activity of the sponge Suberites domuncula and its primmorphs: Potential basis for epibacterial chemical defense. Aquat. Microb. Ecol. 2003, 31, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Mah, S.A.; Moy, G.W.; Swanson, W.J.; Vacquier, V.D. A perforin-like protein from a marine mollusk. Biochem. Biophys. Res. Commun. 2004, 316, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.D.; Zhang, K.F.; Zhang, Z.P.; Zou, Z.H.; Jia, X.W.; Wang, S.H.; Lin, P.; Wang, Y.L. Molecular cloning and responsive expression of macrophage expressed gene from small abalone Haliotis diversicolor supertexta. Fish Shellfish Immunol. 2008, 24, 346–359. [Google Scholar] [CrossRef] [PubMed]
- He, X.C.; Zhang, Y.; Yu, Z.N. An Mpeg (macrophage expressed gene) from the Pacific oyster Crassostrea gigas: Molecular characterization and gene expression. Fish Shellfish Immunol. 2011, 30, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Kemp, I.K.; Coyne, V.E. Identification and characterisation of the Mpeg1 homologue in the South African abalone, Haliotis midae. Fish Shellfish Immunol. 2011, 31, 754–764. [Google Scholar] [CrossRef]
- Bathige, S.; Umasuthan, N.; Whang, I.; Lim, B.S.; Won, S.H.; Lee, J. Antibacterial activity and immune responses of a molluscan macrophage expressed gene-1 from disk abalone, Haliotis discus discus. Fish Shellfish Immunol. 2014, 39, 263–272. [Google Scholar] [CrossRef]
- Green, T.J.; Montagnani, C.; Benkendorff, K.; Robinson, N.; Speck, P. Ontogeny and water temperature influences the antiviral response of the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2014, 36, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.G.; Xu, M.; Zhang, C.L.; Jia, H.X.; Zhang, H.; He, M.X.; Liu, W.G. Comparative Transcriptomic and Expression Profiles Between the Foot Muscle and Mantle Tissues in the Giant Triton Snail Charonia tritonis. Front. Physiol. 2021, 12, 632518. [Google Scholar] [CrossRef]
- Spilsbury, K.; Omara, M.A.; Wu, W.M.; Rowe, P.B.; Symonds, G.; Takayama, Y. Isolation of a novel macrophage-specific gene by differential cDNA analysis. Blood 1995, 85, 1620–1629. [Google Scholar] [CrossRef]
- Fields, K.A.; McCormack, R.; de Armas, L.R.; Podack, E.R. Perforin-2 Restricts Growth of Chlamydia trachomatis in Macrophages. Infect. Immun. 2013, 81, 3045–3054. [Google Scholar] [CrossRef] [Green Version]
- Rougeot, J.; Zakrzewska, A.; Kanwal, Z.; Jansen, H.J.; Spaink, H.P.; Meijer, A.H. RNA sequencing of FACS-sorted immune cell populations from zebrafish infection models to identify cell specific responses to intracellular pathogens. Methods Mol. Biol. 2014, 1197, 261–274. [Google Scholar] [CrossRef]
- Benard, E.L.; Racz, P.I.; Rougeot, J.; Nezhinsky, A.E.; Verbeek, F.J.; Spaink, H.P.; Meijer, A.H. Macrophage-Expressed Perforins Mpeg1 and Mpeg1.2 Have an Anti-Bacterial Function in Zebrafish. J. Innate Immun. 2015, 7, 136–152. [Google Scholar] [CrossRef]
- Ni, L.Y.; Han, Q.; Chen, H.P.; Luo, X.C.; Li, A.X.; Dan, X.M.; Li, Y.W. Grouper (Epinephelus coioides) Mpeg1s: Molecular identification, expression analysis, and antimicrobial activity. Fish Shellfish Immunol. 2019, 92, 690–697. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Q.; Zhang, X.; Chen, H.; Zhang, Y.; Sun, Y.; Yang, S.; Chen, C. CsrA Regulates Swarming Motility and Carbohydrate and Amino Acid Metabolism in Vibrio alginolyticus. Microorganisms 2021, 9, 2383. [Google Scholar] [CrossRef]
- McCormack, R.; de Armas, L.R.; Shiratsuchi, M.; Ramos, J.E.; Podack, E.R. Inhibition of Intracellular Bacterial Replication in Fibroblasts Is Dependent on the Perforin-Like Protein (Perforin-2) Encoded by Macrophage-Expressed Gene 1. J. Innate Immun. 2013, 5, 185–194. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, B.; Fang, J.; Chen, H.; Sun, Y.; Yang, S.; Gao, Q.; Zhang, Y.; Chen, C. GcvB Regulon Revealed by Transcriptomic and Proteomic Analysis in Vibrio alginolyticus. Int. J. Mol. Sci. 2022, 23, 9399. [Google Scholar] [CrossRef]






| Primers | Sequence (5′-3′) |
|---|---|
| For ORF cloning | |
| Ct-Mgep1-F | GAGAAAGTCGCACGAAAGTTG |
| Ct-Mgep1-R | GGATCGTCAATCATAACAATTC |
| For qPCR | |
| QCt-Mgep1-F | CTCCGCCAACACAGATGATG |
| QCt-Mgep1-R | TGGAAGAAGTGGTGCCTGAA |
| Q18S-F | ATGGTCAGAACTACGACGGTAT |
| Q18S-R | GTATTGCGGTGTTAGAGGTGAA |
| Q-aceE-F | TTTCCAGTCTTCTGCTGCGT |
| Q-aceE-R | CATCAGTTTAGGGTGCGGGT |
| Q-acnB-F | TTCAACGCGTAACTTCCCGA |
| Q-acnB-R | CGTAGCGTCGATTTGCTTCG |
| Q-sucC-F | AGCATCCTACACCACGCAAA |
| Q-sucC-R | AGTCTACAGAGTGCAACGCC |
| Q-sucA-F | ACCAAACTGACGCTAACGGT |
| Q-sucA-R | GGCGTTTCTTCCGCAACTTT |
| Q-sdhA-F | ATGCGTTGGGAAAACAGCAC |
| Q-sdhA-R | AGACTTGTCCGCTAGATGCG |
| Q-ompA-F | ACGTTGGTGGTAAGATGGGT |
| Q-ompA-R | TGGCCACCGAAAGAAGTTTG |
| Q-ompT-F | CAAGCGAGCGTACAGATGAC |
| Q-ompT-R | ATCGTTGTTCCAAGCGTGAC |
| Q-ompV-F | TGGAACGTAGAAGCTGGTGT |
| Q-ompV-R | TTTTCGCTGTATCACCGTCG |
| Q-ompN1-F | CCTTGCTGCGGTTTATGGTT |
| Q-ompN1-R | GTGCCATTTCTTCACGAGCA |
| Q-ompW-F | TAGCAGCAACACCATTCAGC |
| Q-ompW-R | TGAGACCTGCACCAACGTAT |
| Q16S-F | CTGGAACTGAGACACGGTCC |
| Q16S-R | CTCGCACCCTCCGTATTACC |
| For Ct-Mgep1 recombinant protein construction | |
| pET28b-F | CACCACCACCACCACCAC |
| pET28b-R | GGTATATCTCCTTCTTAAAGTTAAACAAAATTATTTC |
| Ct-Mgep1-orf_F | ctttaagaaggagatatacATGAGCTCCAACGACAACG |
| Ct-Mgep1-orf_F | cagtggtggtggtggtggtgCTCGTTCAGGGTGGAGGTTG |
| pET28b-check-F | AAGTGGCGAGCCCGATCTTC |
| pET28b-check-R | CTAGGGCGCTGGCAAGTGTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Liu, B.; Zhang, G.; Yao, G.; Zhang, Y.; Cen, X.; He, M. Giant Triton Snail Charonia tritonis Macrophage-Expressed Gene 1 Protein Ct-Mpeg1: Molecular Identification, Expression Analysis, and Antimicrobial Activity. Int. J. Mol. Sci. 2022, 23, 13415. https://doi.org/10.3390/ijms232113415
Liu W, Liu B, Zhang G, Yao G, Zhang Y, Cen X, He M. Giant Triton Snail Charonia tritonis Macrophage-Expressed Gene 1 Protein Ct-Mpeg1: Molecular Identification, Expression Analysis, and Antimicrobial Activity. International Journal of Molecular Sciences. 2022; 23(21):13415. https://doi.org/10.3390/ijms232113415
Chicago/Turabian StyleLiu, Wenguang, Bing Liu, Gege Zhang, Gaoyou Yao, Yang Zhang, Xitong Cen, and Maoxian He. 2022. "Giant Triton Snail Charonia tritonis Macrophage-Expressed Gene 1 Protein Ct-Mpeg1: Molecular Identification, Expression Analysis, and Antimicrobial Activity" International Journal of Molecular Sciences 23, no. 21: 13415. https://doi.org/10.3390/ijms232113415
APA StyleLiu, W., Liu, B., Zhang, G., Yao, G., Zhang, Y., Cen, X., & He, M. (2022). Giant Triton Snail Charonia tritonis Macrophage-Expressed Gene 1 Protein Ct-Mpeg1: Molecular Identification, Expression Analysis, and Antimicrobial Activity. International Journal of Molecular Sciences, 23(21), 13415. https://doi.org/10.3390/ijms232113415







