The Interplay between Ghrelin and Microglia in Neuroinflammation: Implications for Obesity and Neurodegenerative Diseases
Abstract
:1. Introduction
2. Expression and Functions of Ghre and Ghre Receptor
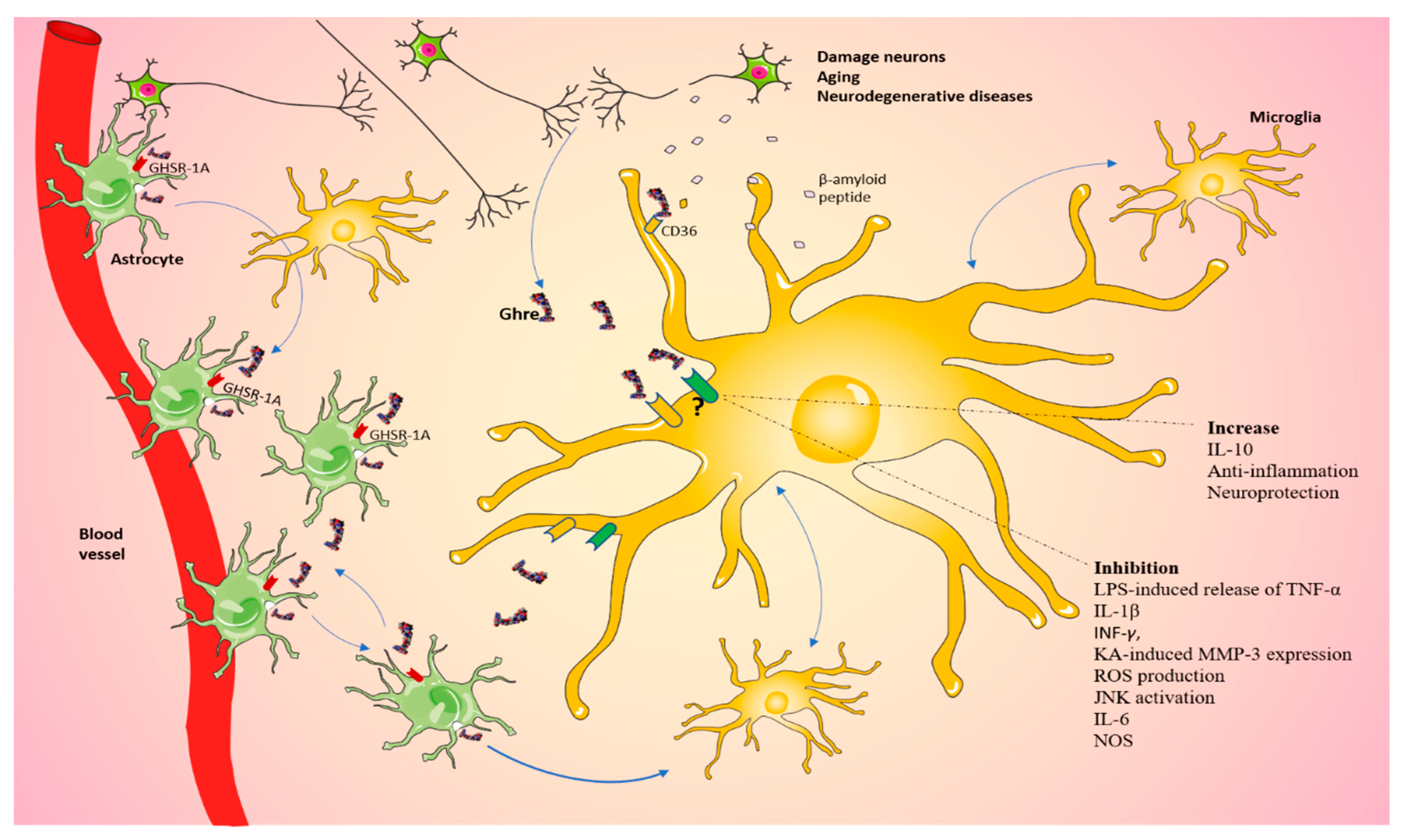
3. Role of Ghre in Neuroinflammation and Neurometabolism
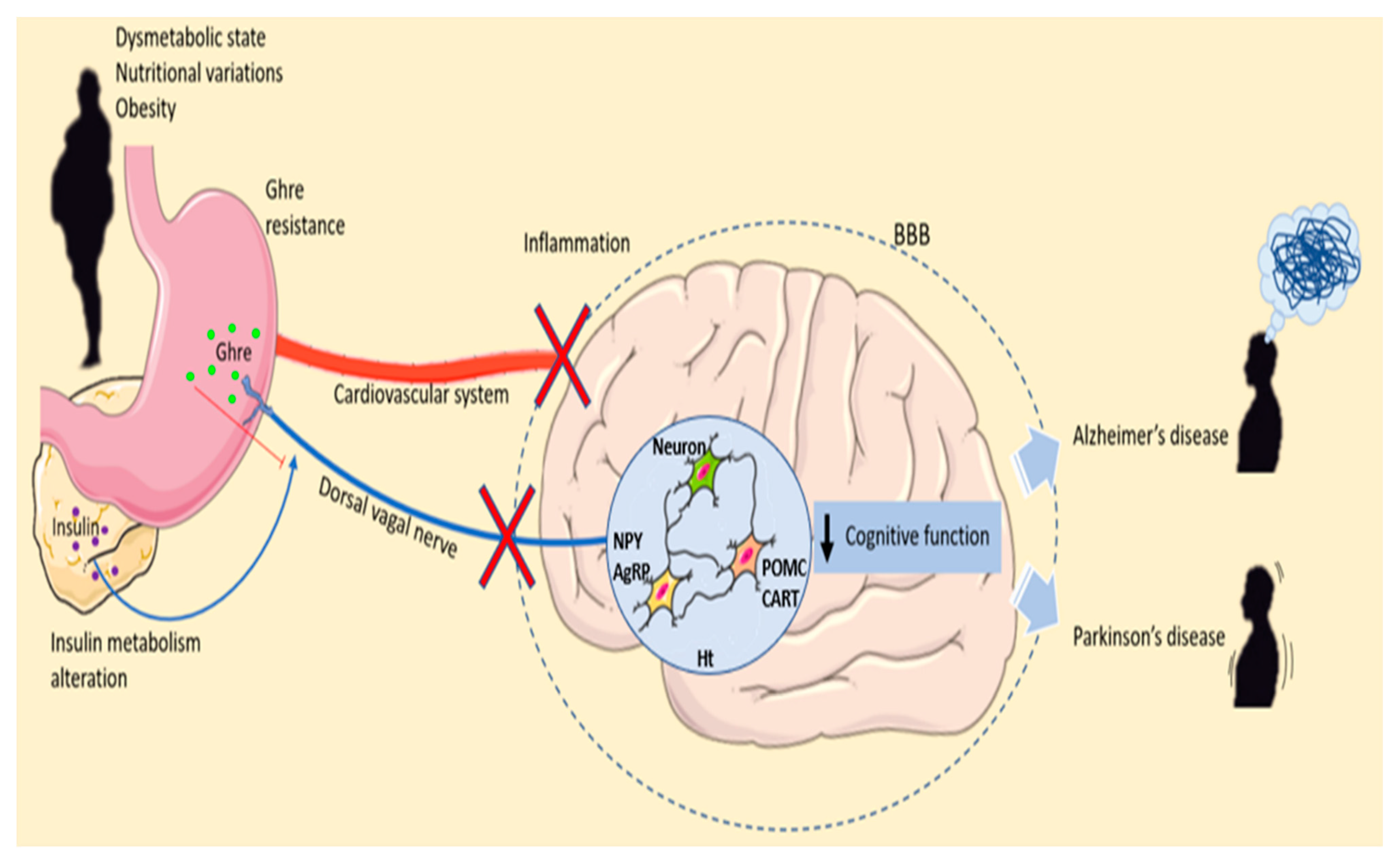
4. Ghre and Obesity
5. Microglia: Friends and Foes of the CNS
6. Ghre, Microglia and Food Intake
7. Obesity and Neurodegenerative Diseases
8. Microglia, Ghre and Neurodegenerative Diseases
9. Microglia and Ghre in AD
10. PD, Microglia and Ghre
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Cope, E.C.; LaMarca, E.A.; Monari, P.K.; Olson, L.B.; Martinez, S.; Zych, A.D.; Katchur, N.J.; Gould, E. Microglia Play an Active Role in Obesity-Associated Cognitive Decline. J. Neurosci. 2018, 38, 8889–8904. [Google Scholar] [CrossRef] [Green Version]
- Hattori, N.; Saito, T.; Yagyu, T.; Jiang, B.H.; Kitagawa, K.; Inagaki, C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J. Clin. Endocrinol. Metab. 2001, 86, 4284–4291. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, N.; Hölscher, C. Acylated Ghrelin as a Multi-Targeted Therapy for Alzheimer’s and Parkinson’s Disease. Front. Neurosci. 2020, 14, 614828. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Aman, Y.; Ahmed, I.; Chetelat, G.; Landeau, B.; Ray Chaudhuri, K.; Brooks, D.J.; Edison, P. Influence of microglial activation on neuronal function in Alzheimer’s and Parkinson’s disease dementia. Alzheimers Dement. 2015, 11, 608–621.e7. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef] [Green Version]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Patanè, M.; Vicario, N.; Di Bella, V.; Cosentini, I.; Barresi, V.; Gulino, R.; Pellitteri, R.; Russo, A.; Stanzani, S. Olfactory Ensheathing Cells express both Ghrelin and Ghrelin Receptor in vitro: A new hypothesis in favor of a neurotrophic effect. Neuropeptides 2020, 79, 101997. [Google Scholar] [CrossRef]
- Kirsz, K.; Zieba, D.A. Ghrelin-mediated appetite regulation in the central nervous system. Peptides 2011, 32, 2256–2264. [Google Scholar] [CrossRef]
- Yanagi, S.; Sato, T.; Kangawa, K.; Nakazato, M. The homeostatic force of ghrelin. Cell Metab. 2018, 27, 786–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, W.A.; Tschöp, M.; Robinson, S.M.; Heiman, M.L. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J. Pharmacol. Exp. Ther. 2002, 302, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Russo, A.; Pellitteri, R.; Stanzani, S. Hippocampal Ghrelin-positive neurons directly project to arcuate hypothalamic and medial amygdaloid nuclei. Could they modulate food-intake? Neurosci. Lett. 2017, 653, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Mani, B.K.; Shankar, K.; Zigman, J.M. Ghrelin’s Relationship to Blood Glucose. Endocrinology 2019, 160, 1247–1261. [Google Scholar] [CrossRef]
- Hosoda, H.; Kojima, M.; Matsuo, H.; Kangawa, K. Ghrelin and des-acyl ghrelin: Two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000, 279, 909–913. [Google Scholar] [CrossRef]
- Ferens, D.M.; Yin, L.; Ohashi-Doi, K.; Habgood, M.; Bron, R.; Brock, J.A.; Gale, J.D.; Furness, J.B. Evidence for functional ghrelin receptors on parasympathetic preganglionic neurons of micturition control pathways in the rat. Clin. Exp. Pharm. Physiol. 2010, 37, 926–932. [Google Scholar] [CrossRef]
- Dixit, V.D.; Schaffer, E.M.; Pyle, R.S.; Collins, G.D.; Sakthivel, S.K.; Palaniappan, R.; Lillard, J.W.; Taub, D.D., Jr. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Inv. 2004, 114, 57–66. [Google Scholar] [CrossRef]
- Alvarez-Crespo, M.; Skibicka, K.P.; Farkas, I.; Molnár, C.S.; Egecioglu, E.; Hrabovszky, E.; Liposits, Z.; Dickson, S.L. The amygdala as a neurobiological target for ghrelin in rats: Neuroanatomical, electrophysiological and behavioral evidence. PLoS ONE 2012, 7, e46321. [Google Scholar] [CrossRef]
- Russo, C.; Russo, A.; Pellitteri, R.; Stanzani, S. Ghrelin-containing neurons in the olfactory bulb send collateralized projections into medial amygdaloid and arcuate hypothalamic nuclei: Neuroanatomical study. Exp. Brain Res. 2018, 236, 2223–2229. [Google Scholar] [CrossRef]
- Delporte, C. Structure and physiological actions of ghrelin. Scientifica 2013, 2013, 518909. [Google Scholar] [CrossRef]
- Taub, D.D. Novel connections between the neuroendocrine and immune systems: The ghrelin immunoregulatory network. Vitam. Horm. 2008, 77, 325–346. [Google Scholar]
- Chow, K.B.; Sun, J.; Chu, K.M.; Tai Cheung, W.; Cheng, C.H.; Wise, H. The truncated ghrelin receptor polypeptide (GHS-R1b) is localized in the endoplasmic reticulum where it forms heterodimers with ghrelin receptors (GHS-R1a) to attenuate their cell surface expression. Mol. Cell Endocrinol. 2012, 348, 247–254. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Cheyuo, C.; Wu, R.; Zhou, M.; Jacob, A.; Coppa, G.; Wang, P. Ghrelin suppresses inflammation and neuronal nitric oxide synthase in focal cerebral ischemia via the vagus nerve. Shock 2011, 35, 258–265. [Google Scholar] [CrossRef]
- Miller, A.A.; Spencer, S.J. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef]
- Sheikh, M.H.; Errede, M.; d’Amati, A.; Khan, N.Q.; Fanti, S.; Loiola, R.A.; McArthur, S.; Purvis, G.S.D.; O’Riordan, C.E.; Ferorelli, D.; et al. Impact of metabolic disorders on the structural, functional, and immunological integrity of the blood-brain barrier:Therapeutic avenues. FASEB J. 2022, 36, e22107. [Google Scholar] [CrossRef]
- Ku, J.M.; Taher, M.; Chin, K.Y.; Barsby, T.; Austin, V.; Wong, C.; Andrews, Z.B.; Spencer, S.; Miller, A.A. Protective actions of des-acylated ghrelin on brain injury and blood-brain barrier disruption after stroke in mice. Clin. Sci. 2016, 130, 1545–1558. [Google Scholar] [CrossRef] [Green Version]
- Kohno, D.; Gao, H.Z.; Muroya, S.; Kikuyama, S.; Yada, T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 2003, 52, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Moon, M.; Kim, H.G.; Hwang, L.; Seo, J.H.; Kim, S.; Hwang, S.; Kim, S.; Lee, D.; Chung, H.; Oh, M.S.; et al. Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease by blocking microglial activation. Neurotox. Res. 2009, 15, 332–347. [Google Scholar] [CrossRef]
- Deng, B.; Fang, F.; Yang, T.; Yu, Z.; Zhang, B.; Xie, X. Ghrelin inhibits AngII -induced expression of TNF-α, IL-8, MCP-1 in human umbilical vein endothelial cells. Int. J. Clin. Exp. 2015, 8, 579–588. [Google Scholar]
- Rezaeian, F.; Wettstein, R.; Scheuer, C.; Bäumker, K.; Bächle, A.; Vollmar, B.; Menger, M.D.; Harder, Y. Ghrelin protects musculocutaneous tissue from ischemic necrosis by improving microvascular perfusion. American journal of physiology. Am. J. Physiol. Heart Circ. 2012, 302, H603–H610. [Google Scholar] [CrossRef]
- Chang, L.; Niu, F.; Chen, J.; Cao, X.; Liu, Z.; Bao, X.; Xu, Y. Ghrelin improves muscle function in dystrophin-deficient mdx mice by inhibiting NLRP3 inflammasome activation. Life Sci. 2019, 232, 116654. [Google Scholar] [CrossRef]
- Chorny, A.; Anderso6-+n, P.; Gonzalez-Rey, E.; Delgado, M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J. Immunol. 2008, 180, 8369–8377. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.S.; Sendra, S.; Lloret, J.; Bosch, I. Systems and WBANs for Controlling Obesity. J. Healthc. Eng. 2018, 2018, 1564748. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. The Effects of Leptin on Glial Cells in Neurological Diseases. Front. Neurosci. 2019, 13, 828. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, T.; Wang, G.; Li, Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci. Rep. 2018, 38, BSR20181061. [Google Scholar] [CrossRef]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef]
- Tong, J.; Prigeon, R.L.; Davis, H.W.; Bidlingmaier, M.; Kahn, S.E.; Cummings, D.E.; Tschöp, M.H.; D’Alessio, D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010, 59, 2145–2151. [Google Scholar] [CrossRef] [Green Version]
- Korbonits, M.; Grossman, A.B. Ghrelin: Update on a novel hormonal system. Eur. J. Endocrinol. 2004, 151 (Suppl. S1), S67–S70. [Google Scholar] [CrossRef] [Green Version]
- Broglio, F.; Gottero, C.; Benso, A.; Prodam, F.; Destefanis, S.; Gauna, C.; Maccario, M.; Deghenghi, R.; van der Lely, A.J.; Ghigo, E. Effects of ghrelin on the insulin and glycemic responses to glucose, arginine, or free fatty acids load in humans. J. Clin. Endocrinol. Metab. 2003, 88, 4268–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delhanty, P.J.; van Kerkwijk, A.; Huisman, M.; van de Zande, B.; Verhoef-Post, M.; Gauna, C.; Hofland, L.; Themmen, A.P.; van der Lely, A.J. Unsaturated fatty acids prevent desensitization of the human growth hormone secretagogue receptor by blocking its internalization. American journal of physiology. Endocrinol. Metab. 2010, 299, E497–E505. [Google Scholar] [CrossRef]
- Steculorum, S.M.; Collden, G.; Coupe, B.; Croizier, S.; Lockie, S.; Andrews, Z.B.; Jarosch, F.; Klussmann, S.; Bouret, S.G. Neonatal ghrelin programs development of hypothalamic feeding circuits. J. Clin. Investig. 2015, 125, 846–858. [Google Scholar] [CrossRef] [Green Version]
- Briggs, D.I.; Enriori, P.J.; Lemus, M.B.; Cowley, M.A.; Andrews, Z.B. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 2010, 151, 4745–4755. [Google Scholar] [CrossRef] [Green Version]
- Erdmann, J.; Lippl, F.; Wagenpfeil, S.; Schusdziarra, V. Differential association of basal and postprandial plasma ghrelin with leptin, insulin, and type 2 diabetes. Diabetes 2005, 54, 1371–1378. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Tiwari, H.K.; Lin, W.Y.; Allison, D.B.; Chung, W.K.; Leibel, R.L.; Yi, N.; Liu, N. Genetic association analysis of 30 genes related to obesity in a European American population. Int. J. Obes. 2014, 38, 724–729. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, V.V.; Maday, S. Compartment-specific dynamics and functions of autophagy in neurons. Dev. Neurobiol. 2018, 78, 298–310. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. The Function of Autophagy in Neurodegenerative Diseases. Int. J. Mol. Sci. 2015, 16, 26797–26812. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Dou, S.; Zhu, J.; Shao, Z.; Wang, C.; Cheng, B. Ghrelin protects dopaminergic neurons against MPTP neurotoxicity through promoting autophagy and inhibiting endoplasmic reticulum mediated apoptosis. Brain Res. 2020, 1746, 147023. [Google Scholar] [CrossRef]
- Scrivo, A.; Bourdenx, M.; Pampliega, O.; Cuervo, A.M. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 2018, 17, 802–815. [Google Scholar] [CrossRef]
- Ferreira-Marques, M.; Carvalho, A.; Cavadas, C.; Aveleira, C.A. PI3K/AKT/MTOR and ERK1/2-MAPK signaling pathways are involved in autophagy stimulation induced by caloric restriction or caloric restriction mimetics in cortical neurons. Aging 2021, 13, 7872–7882. [Google Scholar] [CrossRef]
- Chung, H.; Choi, J.; Park, S. Ghrelin protects adult rat hippocampal neural stem cells from excessive autophagy during oxygen-glucose deprivation. Endocr. J. 2018, 65, 63–73. [Google Scholar] [CrossRef]
- Cecarini, V.; Bonfili, L.; Cuccioloni, M.; Keller, J.N.; Bruce-Keller, A.J.; Eleuteri, A.M. Effects of Ghrelin on the Proteolytic Pathways of Alzheimer’s Disease Neuronal Cells. Mol. Neurobiol. 2016, 53, 3168–3178. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; López, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nature reviews. Endocrinology 2017, 13, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dou, S.; Zhu, J.; Shao, Z.; Wang, C.; Cheng, B. Regulatory effects of ghrelin on endoplasmic reticulum stress, oxidative stress, and autophagy: Therapeutic potential. Neuropeptides 2021, 85, 102112. [Google Scholar] [CrossRef]
- Zigman, J.M.; Bouret, S.G.; Andrews, Z.B. Obesity Impairs the Action of the Neuroendocrine Ghrelin System. Trends Endocrinol. Metab. 2016, 27, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Vasek, M.J.; Garber, C.; Dorsey, D.; Durrant, D.M.; Bollman, B.; Soung, A.; Yu, J.; Perez-Torres, C.; Frouin, A.; Wilton, D.K.; et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 2016, 534, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Taso, O.; Wang, R.; Bayram, S.; Graham, A.C.; Garcia-Reitboeck, P.; Mallach, A.; Andrews, W.D.; Piers, T.M.; Botia, J.A.; et al. Trem2 promotes anti-inflammatory responses in microglia and is suppressed under pro-inflammatory conditions. Hum. Mol. Genet. 2020, 29, 3224–3248. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nature reviews. Immunology 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Chhor, V.; Le Charpentier, T.; Lebon, S.; Oré, M.V.; Celador, I.L.; Josserand, J.; Degos, V.; Jacotot, E.; Hagberg, H.; Sävman, K.; et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav. Immun. 2013, 32, 70–85. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.Q.; Ma, X.T.; Hu, Z.W.; Yang, S.; Chen, M.; Bosco, D.B.; Wu, L.J.; Tian, D.S. Dual Functions of Microglia in Ischemic Stroke. Neurosci. Bull. 2019, 35, 921–933. [Google Scholar] [CrossRef]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nature reviews. Neurology 2015, 11, 56–64. [Google Scholar] [CrossRef]
- Tian, R.; Mao, G. Ghrelin reduces cerebral ischemic injury in rats by reducing M1 microglia/macrophages. Eur. J. Histochem. 2022, 66, 3350. [Google Scholar] [CrossRef]
- Lockie, S.H.; Andrews, Z.B. The hormonal signature of energy deficit: Increasing the value of food reward. Mol. Metab. 2013, 2, 329–336. [Google Scholar] [CrossRef]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 444. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Mendes, N.F.; Velloso, L.A. Perivascular macrophages in high-fat diet-induced hypothalamic inflammation. J. Neuroinflammation 2022, 19, 136. [Google Scholar] [CrossRef]
- Mendes, N.F.; Kim, Y.B.; Velloso, L.A.; Araújo, E.P. Hypothalamic Microglial Activation in Obesity: A Mini-Review. Front. Neurosci. 2018, 12, 846. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, J.; Dorfman, M.D.; Fasnacht, R.; Douglass, J.D.; Wyse-Jackson, A.C.; Barria, A.; Thaler, J.P. CX3CL1 Action on Microglia Protects from Diet-Induced Obesity by Restoring POMC Neuronal Excitability and Melanocortin System Activity Impaired by High-Fat Diet Feeding. Int. J. Mol. Sci. 2022, 23, 6380. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Miao, Y.; Gao, L.; Pan, H.; Zhu, S. Ghrelin-containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regul. Pept. 2006, 134, 126–131. [Google Scholar] [CrossRef]
- Travagli, R.A.; Hermann, G.E.; Browning, K.N.; Rogers, R.C. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 2006, 68, 279–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naznin, F.; Toshinai, K.; Waise, T.M.; NamKoong, C.; Md Moin, A.S.; Sakoda, H.; Nakazato, M. Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J. Endocrinol. 2015, 226, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, S.N.; Sominsky, L.; Soch, A.; Wang, H.; Ziko, I.; Rank, M.M.; Spencer, S.J. Conditional microglial depletion in rats leads to reversible anorexia and weight loss by disrupting gustatory circuitry. Brain Behav. Immun. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimer’s Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet. Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Thomas, K.; Beyer, F.; Lewe, G.; Zhang, R.; Schindler, S.; Schönknecht, P.; Stumvoll, M.; Villringer, A.; Witte, A.V. Higher body mass index is linked to altered hypothalamic microstructure. Sci. Rep. 2019, 9, 17373. [Google Scholar] [CrossRef] [Green Version]
- Stillman, C.M.; Weinstein, A.M.; Marsland, A.L.; Gianaros, P.J.; Erickson, K.I. Body-Brain Connections: The Effects of Obesity and Behavioral Interventions on Neurocognitive Aging. Front. Aging Neurosci. 2017, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Uranga, R.M.; Keller, J.N. The Complex Interactions Between Obesity, Metabolism and the Brain. Frontiers in neuroscience. Front. Neurosci. 2019, 13, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Perry, G.; Siedlak, S.L.; Richey, P.; Kawai, M.; Cras, P.; Kalaria, R.N.; Galloway, P.G.; Scardina, J.M.; Cordell, B.; Greenberg, B.D. Association of heparan sulfate proteoglycan with the neurofibrillary tangles of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 1991, 11, 3679–3683. [Google Scholar] [CrossRef] [Green Version]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef] [Green Version]
- Beach, T.G.; Adler, C.H.; Zhang, N.; Serrano, G.E.; Sue, L.I.; Driver-Dunckley, E.; Mehta, S.H.; Zamrini, E.E.; Sabbagh, M.N.; Shill, H.A.; et al. Severe hyposmia distinguishes neuropathologically confirmed dementia with Lewy bodies from Alzheimer’s disease dementia. PLoS ONE 2020, 15, e0231720. [Google Scholar] [CrossRef] [Green Version]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Neher, J.J.; Cunningham, C. Priming Microglia for Innate Immune Memory in the Brain. Trends Immunol. 2019, 40, 358–374. [Google Scholar] [CrossRef]
- Patel, K.; Taub, D.D. Role of neuropeptides, hormones, and growth factors in regulating thymopoiesis in middle to old age. F1000 Biol. Rep. 2009, 1, 42. [Google Scholar] [CrossRef]
- Jiao, L.; Du, X.; Jia, F.; Li, Y.; Zhu, D.; Tang, T.; Jiao, Q.; Jiang, H. Early low-dose ghrelin intervention via miniosmotic pumps could protect against the progressive dopaminergic neuron loss in Parkinson’s disease mice. Neurobiol. Aging 2021, 101, 70–78. [Google Scholar] [CrossRef]
- Ding, S.; Xu, S.; Ma, Y.; Liu, G.; Jang, H.; Fang, J. Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules 2019, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Meng, X.F.; Zhang, C. NLRP3 Inflammasome in Metabolic-Associated Kidney Diseases: An Update. Front. Immunol. 2021, 12, 714340. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.; Kong, C.; Su, X.; Zhang, X.; Bai, W.; He, X. MiR-124 Enriched Exosomes Promoted the M2 Polarization of Microglia and Enhanced Hippocampus Neurogenesis After Traumatic Brain Injury by Inhibiting TLR4 Pathway. Neurochem. Res. 2019, 44, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Baatar, D.; Patel, K.; Taub, D.D. The effects of ghrelin on inflammation and the immune system. Mol. Cell. Endocrinol. 2011, 340, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, P.; Li, P.; Feng, L.; Ren, Q.; Xie, X.; Xu, J. Ghrelin protects the heart against ischemia/reperfusion injury via inhibition of TLR4/NLRP3 inflammasome pathway. Life Sci. 2017, 186, 50–58. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; He, X.; Yu, H.; Feng, J. Ghrelin Attenuates Neuroinflammation and Demyelination in Experimental Autoimmune Encephalomyelitis Involving NLRP3 Inflammasome Signaling Pathway and Pyroptosis. Front. Pharmacol. 2019, 10, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Jin, M.Z.; Yang, Z.Y.; Jin, W.L. Microglia in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 270–280. [Google Scholar] [CrossRef]
- Burguillos, M.A.; Deierborg, T.; Kavanagh, E.; Persson, A.; Hajji, N.; Garcia-Quintanilla, A.; Cano, J.; Brundin, P.; Englund, E.; Venero, J.L.; et al. Caspase signalling controls microglia activation and neurotoxicity. Nature 2011, 472, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Xu, J.; Tang, S.Q.; Li, H.Y.; Jiang, Q.Y.; Zou, X.T. Ghrelin and its biological effects on pigs. Peptides 2009, 30, 1203–1211. [Google Scholar] [CrossRef]
- de Sousa, V.L.; Araújo, S.B.; Antonio, L.M.; Silva-Queiroz, M.; Colodeti, L.C.; Soares, C.; Barros-Aragão, F.; Mota-Araujo, H.P.; Alves, V.S.; Coutinho-Silva, R.; et al. Innate immune memory mediates increased susceptibility to Alzheimer’s disease-like pathology in sepsis surviving mice. Brain Behav. Immun. 2021, 95, 287–298. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Wang, X.; Sun, L.; Schultzberg, M.; Hjorth, E. Can. inflammation be resolved in Alzheimer’s disease? Ther. Adv. Neurol. Disord. 2018, 11, 1756286418791107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panza, F.; Frisardi, V.; Seripa, D.; Imbimbo, B.P.; Sancarlo, D.; D’Onofrio, G.; Addante, F.; Paris, F.; Pilotto, A.; Solfrizzi, V. Metabolic syndrome, mild cognitive impairment, and dementia. Curr. Alzheimer Res. 2011, 8, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Dinel, A.L.; André, C.; Aubert, A.; Ferreira, G.; Layé, S.; Castanon, N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS ONE 2011, 6, e24325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Ramdas, M.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15888–15893. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, J.; Krippeit-Drews, P.; Drews, G. Acyl-Ghrelin Influences Pancreatic β-Cell Function by Interference with KATP Channels. Diabetes 2021, 70, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Page, L.C.; Gastaldelli, A.; Gray, S.M.; D’Alessio, D.A.; Tong, J. Interaction of GLP-1 and Ghrelin on Glucose Tolerance in Healthy Humans. Diabetes 2018, 67, 1976–1985. [Google Scholar] [CrossRef] [Green Version]
- Lewitt, M.S. The Role of the Growth Hormone/Insulin-Like Growth Factor System in Visceral Adiposity. Biochem. Insights. 2017, 10, 1178626417703995. [Google Scholar] [CrossRef] [Green Version]
- Kunath, N.; van Groen, T.; Allison, D.B.; Kumar, A.; Dozier-Sharpe, M.; Kadish, I. Ghrelin agonist does not foster insulin resistance but improves cognition in an Alzheimer’s disease mouse model. Sci. Rep. 2015, 5, 11452. [Google Scholar] [CrossRef] [Green Version]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.C.; Decker, H.; Silverman, M.A.; Kazi, H.; Melo, H.M.; McClean, P.L.; et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J. Clin. Investig. 2012, 122, 1339–1353. [Google Scholar] [CrossRef] [Green Version]
- Pinkney, J. The role of ghrelin in metabolic regulation. Curr. Opin. Clin. Nutr. Metab. Care. 2014, 17, 497–502. [Google Scholar] [CrossRef]
- Lee, J.; Lim, E.; Kim, Y.; Li, E.; Park, S. Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J. Endocrinol. 2010, 205, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, H.Y.; Yune, T.Y. MMP-3 secreted from endothelial cells of blood vessels after spinal cord injury activates microglia, leading to oligodendrocyte cell death. Neurobiol. Dis. 2015, 82, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.D.; Zhu, Y.G.; Lin, N.; Zhang, J.; Ye, Q.Y.; Huang, H.P.; Chen, X.C. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: Implications for Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, R.F.; Touret, N.; Kuwata, H.; Tandon, N.N.; Grinstein, S.; Trimble, W.S. Uptake of oxidized low density lipoprotein by CD36 occurs by an actin-dependent pathway distinct from macropinocytosis. J. Biol. Chem. 2009, 284, 30288–30297. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Yune, T.Y. Ghrelin inhibits oligodendrocyte cell death by attenuating microglial activation. Endocrinol. Metab. 2014, 29, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Bulgarelli, I.; Tamiazzo, L.; Bresciani, E.; Rapetti, D.; Caporali, S.; Lattuada, D.; Locatelli, V.; Torsello, A. Desacyl-ghrelin and synthetic GH-secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by beta-amyloid fibrils. J. Neurosci. Res. 2009, 87, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.; Priego, T.; Martín, A.I.; Villanúa, M.A.; López-Calderón, A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E486–E492. [Google Scholar] [CrossRef] [Green Version]
- Dhurandhar, E.J.; Allison, D.B.; van Groen, T.; Kadish, I. Hunger in the absence of caloric restriction improves cognition and attenuates Alzheimer’s disease pathology in a mouse model. PLoS ONE 2013, 8, e60437. [Google Scholar] [CrossRef] [Green Version]
- Martins, I.; Gomes, S.; Costa, R.O.; Otvos, L.; Oliveira, C.R.; Resende, R.; Pereira, C.M. Leptin and ghrelin prevent hippocampal dysfunction induced by Aβ oligomers. Neuroscience 2013, 241, 41–51. [Google Scholar] [CrossRef]
- Chaney, A.; Williams, S.R.; Boutin, H. In vivo molecular imaging of neuroinflammation in Alzheimer’s disease. J. Neurochem. 2019, 149, 438–451. [Google Scholar] [CrossRef]
- Diano, S.; Farr, S.A.; Benoit, S.C.; McNay, E.C.; da Silva, I.; Horvath, B.; Gaskin, F.S.; Nonaka, N.; Jaeger, L.B.; Banks, W.A.; et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 2006, 9, 381–388. [Google Scholar] [CrossRef]
- Leung, E.; Guo, L.; Bu, J.; Maloof, M.; El Khoury, J.; Geula, C. Microglia activation mediates fibrillar amyloid-β toxicity in the aged primate cortex. Neurobiol. Aging 2011, 32, 387–397. [Google Scholar] [CrossRef] [Green Version]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Santos, V.V.; Stark, R.; Rial, D.; Silva, H.B.; Bayliss, J.A.; Lemus, M.B.; Davies, J.S.; Cunha, R.A.; Prediger, R.D.; Andrews, Z.B. Acyl ghrelin improves cognition, synaptic plasticity deficits and neuroinflammation following amyloid β (Aβ1-40) administration in mice. J. Neuroendocrinol. 2017, 29, 12476. [Google Scholar] [CrossRef] [Green Version]
- Moon, M.; Choi, J.G.; Nam, D.W.; Hong, H.S.; Choi, Y.J.; Oh, M.S.; Mook-Jung, I. Ghrelin ameliorates cognitive dysfunction and neurodegeneration in intrahippocampal amyloid-β1-42 oligomer-injected mice. J. Alzheimer’s Dis. 2011, 23, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Seminara, R.S.; Jeet, C.; Biswas, S.; Kanwal, B.; Iftikhar, W.; Sakibuzzaman, M.; Rutkofsky, I.H. The Neurocognitive Effects of Ghrelin-induced Signaling on the Hippocampus: A Promising Approach to Alzheimer’s Disease. Cureus 2018, 10, e3285. [Google Scholar] [CrossRef] [Green Version]
- Stoyanova, I.I. Ghrelin: A link between ageing, metabolism and neurodegenerative disorders. Neurobiol. Dis. 2014, 72 Pt A, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain J. Neurol. 1991, 114 Pt 5, 2283–2301. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Renaud, J.; Simola, N.; Martinoli, M.G. Diabetes, a Contemporary Risk for Parkinson’s Disease: Epidemiological and Cellular Evidences. Front. Aging Neurosci. 2019, 11, 302. [Google Scholar] [CrossRef] [Green Version]
- Pagano, G.; Polychronis, S.; Wilson, H.; Giordano, B.; Ferrara, N.; Niccolini, F.; Politis, M. Diabetes mellitus and Parkinson disease. Neurology 2018, 90, e1654–e1662. [Google Scholar] [CrossRef]
- Kam, T.I.; Hinkle, J.T.; Dawson, T.M.; Dawson, V.L. Microglia and astrocyte dysfunction in parkinson’s disease. Neurobiol. Dis. 2020, 144, 105028. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef] [Green Version]
- Fiszer, U.; Michałowska, M.; Baranowska, B.; Wolińska-Witort, E.; Jeske, W.; Jethon, M.; Piaścik-Gromada, M.; Marcinowska-Suchowierska, E. Leptin and ghrelin concentrations and weight loss in Parkinson’s disease. Acta Neurol. Scand. 2010, 121, 230–236. [Google Scholar] [CrossRef]
- Bayliss, J.A.; Lemus, M.; Santos, V.V.; Deo, M.; Elsworth, J.D.; Andrews, Z.B. Acylated but not des-acyl ghrelin is neuroprotective in an MPTP mouse model of Parkinson’s disease. J. Neurochem. 2016, 137, 460–471. [Google Scholar] [CrossRef]
- Morgan, A.H.; Rees, D.J.; Andrews, Z.B.; Davies, J.S. Ghrelin mediated neuroprotection—A possible therapy for Parkinson’s disease? Neuropharmacology 2018, 136 Pt B, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Schellekens, H.; Dinan, T.G.; Cryan, J.F. Taking two to tango: A role for ghrelin receptor heterodimerization in stress and reward. Front. Neurosci. 2013, 7, 148. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.M.; Jiang, J.; Wilson, B.; Zhang, W.; Hong, J.S.; Liu, B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: Relevance to Parkinson’s disease. J. Neurochem. 2002, 81, 1285–1297. [Google Scholar] [CrossRef]
- Dixit, V.D.; Taub, D.D. Ghrelin and immunity: A young player in an old field. Exp. Gerontol. 2005, 40, 900–910. [Google Scholar] [CrossRef]

| Study Type | Model | Effect | References |
|---|---|---|---|
| In vitro | N9 microglia cells | Desacyl-ghrelin, hexarelin and EP80317 are capable in vitro to blunt the increase in IL-1β and IL-6 mRNA induced by fAβ25–35 in N9 cells. The effects of fAβ25–35 on IL-1β mRNA levels were attenuated by desacyl-ghrelin, hexarelin and EP80317, but not ghrelin. | [118] |
| In vitro | Hippocampal neurons | Leptin and ghrelin protect hippocampal neurons from Aβ oligomer-induced toxicity. Leptin and ghrelin prevent oxidative stress and mitochondrial dysfunction. Leptin and ghrelin revert GSK3β activation induced by Aβ oligomers. Neuroprotection by leptin and ghrelin occurs in a receptor-mediated manner. | [121] |
| In vivo/In vitro | Ghrelin KO in a mouse model of AD | Ghrelin deletion affects memory performance, and acyl ghrelin treatment may delay the onset of early events of AD. | [126] |
| In vivo/In vitro | Male ICR mice | Systemic injection of ghrelin rescues cognitive impairment induced by AßO in vivo, suggesting that ghrelin-mediated restoration of behavioral performance on cognitive deficit is at least in part mediated by inhibition of microgliosis and impairment of neuronal integrity. | [127] |
| In vivo | Tg APPSwDI mouse | The ghrelin agonist impaired glucose tolerance immediately after administration, but not in the long term. The ghrelin agonist improved spatial learning in the mice, raised their activity levels and reduced their body weight and fat mass. Ghrelin might improve cognition in Alzheimer’s disease via a central nervous system mechanism involving insulin signaling | [108] |
| In vivo | Tg APPSwDI mouse | Treatment with a hunger-inducing ghrelin agonist is sufficient to reduce AD-related cognitive deficits and pathology in Tg AD model mice. | [120] |
| Study Type | Model | Effect | References |
|---|---|---|---|
| In vivo/ In vitro | C57Bl/6 mice | Administration of ghrelin significantly attenuated the loss of substantia nigra pars compacta neurons and the striatal dopaminergic fibers. Ghrelin reduced nitrotyrosine levels and improved the impairment of rotarod performance. In vitro administration of ghrelin prevented 1-methyl-4-phenylpyridinium-induced dopaminergic cell loss, MMP-3 expression, microglial activation and the subsequent release of TNF-α, IL-1β and nitrite in mesencephalic cultures. | [30] |
| In vivo | A53T and wild-type mice | Ghrelin administration in PD mice did not affect weight gain in wild-type mice but improved weight loss in PD mice. Attenuation of dopaminergic neuron loss in substantia nigra and a low level of dopamine content in the striatum occur in PD mice with ghrelin treatment. | [89] |
| In vivo | PD patients | In PD patients with weight loss, higher active ghrelin concentration in plasma was not observed nor was an increased appetite. | [137] |
| In vivo | GOAT KO and Ghre KO mice | Acylated Ghrelin is the isoform responsible for in vivo neuroprotection by attenuating dopamine cell loss and glial activation. | [138] |
| Study Type | Effect | References |
|---|---|---|
| In vitro | In N9 microglia cells, which express the CD36 receptor, 10(–7) M desacyl-ghrelin prevents the stimulation effects of fAβ (25–35) on IL-6 mRNA levels. Similarly, on IL-1β, mRNA levels are reduced by desacyl-ghrelin form. | [118] |
| In vitro | In primary cultured hippocampal neurons, Ghre reduces amyloid-β oligomer production of superoxide and mitochondrial membrane depolarization. Moreover, it improves cell survival and inhibits cell death. Ghre prevents glycogen synthase kinase 3β activation. | [121] |
| In vivo | In primary hippocampal neurons, Ghre acts on M1 microglia/macrophages, improving neurological function and reducing cerebral infarction, apoptotic cells and IL-1β and TNF-α expression. | [65] |
| In vivo | Ghre blocks kainic acid-induced MMP-3 expression in hippocampal neurons, preventing neuronal cell death induced by kainic acid, promoting astroglia and microglia inactivation through COX-2, TNF-α and IL-1β regulation. | [112] |
| In vivo | Ghre agonist reduces AD pathology and improves cognition in AD mouse model, improves the performance in the water maze and reduces levels of amyloid beta and microglial activation | [120] |
| Abbreviations = AD, Alzheimer’s disease; COX-2, cyclooxygenase-2; IL-1β: interleukin-1 beta; LPS: lipopolysaccharides; MMP-3: metalloproteinase-3, stromelysin-1; N9: cell line N9 inhibitors; TNF-α: tumor necrosis factor-alpha; |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, C.; Valle, M.S.; Russo, A.; Malaguarnera, L. The Interplay between Ghrelin and Microglia in Neuroinflammation: Implications for Obesity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 13432. https://doi.org/10.3390/ijms232113432
Russo C, Valle MS, Russo A, Malaguarnera L. The Interplay between Ghrelin and Microglia in Neuroinflammation: Implications for Obesity and Neurodegenerative Diseases. International Journal of Molecular Sciences. 2022; 23(21):13432. https://doi.org/10.3390/ijms232113432
Chicago/Turabian StyleRusso, Cristina, Maria Stella Valle, Antonella Russo, and Lucia Malaguarnera. 2022. "The Interplay between Ghrelin and Microglia in Neuroinflammation: Implications for Obesity and Neurodegenerative Diseases" International Journal of Molecular Sciences 23, no. 21: 13432. https://doi.org/10.3390/ijms232113432






