Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise
Abstract
:1. Introduction
2. Literature Search
3. General Aspects of Sarcopenia, Metabolic Repercussions, and Organokines
3.1. Muscular Tissue, Sarcopenia, and Physical Exercises
3.2. Sarcopenia and Diabetes
3.3. Sarcopenia and Obesity
3.4. Sarcopenia and Dyslipidemia
3.5. Organokines and the Relations with Sarcopenia, DM, Sarcopenic Obesity, and Dyslipidemia
3.5.1. Myokines
Irisin
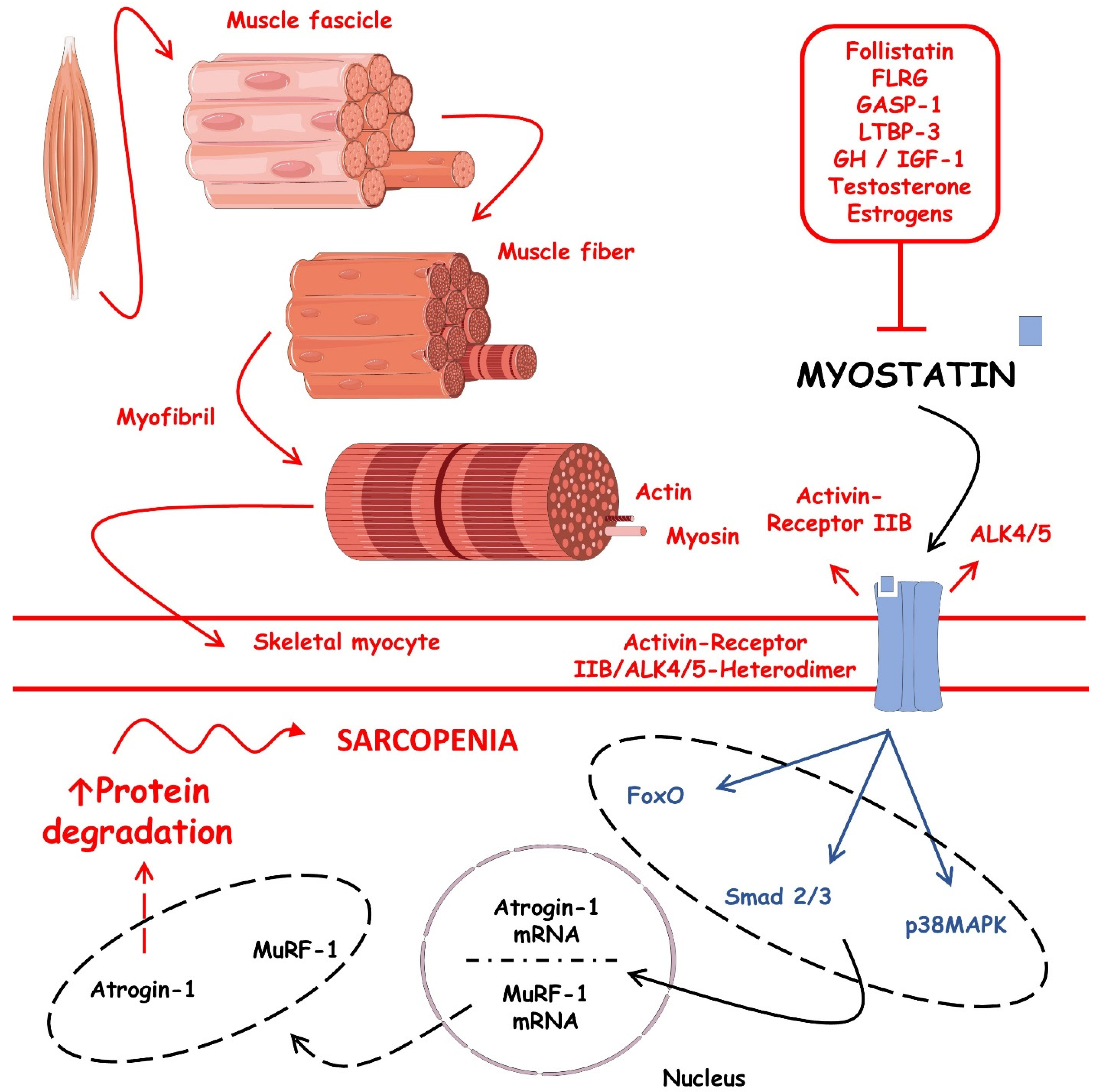
Myostatin
Sclerostin
Brain-Derived Neurotrophic Factor (BDNF)
Interleukin-6
Interleukin-15
3.5.2. Adipokines
Leptin
Lipocalin 2
Interleukin-6
Interleukin-10
Interleukin-15
Apelin
Insulin-like Growth Factor Hormone (IGF-1)
Fibroblast Growth Factor 21
Adiponectin
3.5.3. Hepatokines
Fetuin-A
Sex Hormone Binding Globulin
Leukocyte-Derived Chemotaxin-2
Insulin-like Growth Factor Hormone (IGF-1)
3.5.4. Osteokines
Osteocalcin
Irisin
Sclerostin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Ataey, A.; Jafarvand, E.; Adham, D.; Moradi-Asl, E. The Relationship Between Obesity, Overweight, and the Human Development Index in World Health Organization Eastern Mediterranean Region Countries. J. Prev. Med. Public Health 2020, 53, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef] [Green Version]
- Petropoulou, E.; Landini, L.; Athanasiadis, L.; Dialektakou, K.; Honka, M.J.; Rebelos, E. Sarcopenia and chronic illness: From diagnosis to treatment approaches. Recenti Prog. Med. 2021, 112, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Pahlavani, H. Exercise Therapy for People With Sarcopenic Obesity: Myokines and Adipokines as Effective Actors. Front. Endocrinol. (Lausanne) 2022, 13, 811751. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990s–991s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, N.; Stubbs, B.; Punzi, L.; Soysal, P.; Incalzi, R.A.; Saller, A.; Maggi, S. Effect of nutritional supplementations on physical performance and muscle strength parameters in older people: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 51, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Ha, Y.C.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Lim, S.; Park, Y.J.; Lim, J.Y.; Kim, K.W.; Park, K.S.; et al. Hyperglycemia Is Associated with Impaired Muscle Quality in Older Men with Diabetes: The Korean Longitudinal Study on Health and Aging. Diabetes Metab. J. 2016, 40, 140–146. [Google Scholar] [CrossRef]
- Mayhew, A.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.; de Souza, R.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2018, 48, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.K.; Achilla, C.; Pananastasiou, G.; Taouxidou, P.; Mitsiou, M.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. Type 2 Diabetes Mellitus is Associated with Increased Risk of Sarcopenia: A Systematic Review and Meta-analysis. Calcif. Tissue Int. 2020, 107, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.C.; Anderson, J.E. The dynamics of the nitric oxide release-transient from stretched muscle cells. Int. J. Biochem. Cell Biol. 2009, 41, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Wu, F.C.; Laurent, M.R.; Claessens, F.; Ward, K.A.; Boonen, S.; Bouillon, R.; et al. Endocrine determinants of incident sarcopenia in middle-aged and elderly European men. J. Cachexia Sarcopenia Muscle 2015, 6, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 2014, 36, 545–547. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. WIREs Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef] [Green Version]
- Hughes, B.W.; Kusner, L.L.; Kaminski, H.J. Molecular architecture of the neuromuscular junction. Muscle Nerve 2006, 33, 445–461. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef]
- Habib, S.S.; Alkahtani, S.; Alhussain, M.; Aljuhani, O. Sarcopenia Coexisting with High Adiposity Exacerbates Insulin Resistance and Dyslipidemia in Saudi Adult Men. Diabetes Metab. Syndr. Obes. 2020, 13, 3089–3097. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Picca, A.; Calvani, R.; Uchida, M.C.; Marzetti, E. If my muscle could talk: Myokines as a biomarker of frailty. Exp. Gerontol. 2019, 127, 110715. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in skeletal muscle physiology and metabolism: Recent advances and future perspectives. Acta Physiol. 2020, 228, e13367. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef] [PubMed]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bencze, M.; Negroni, E.; Vallese, D.; Yacoub-Youssef, H.; Chaouch, S.; Wolff, A.; Aamiri, A.; Di Santo, J.P.; Chazaud, B.; Butler-Browne, G.; et al. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol. Ther. 2012, 20, 2168–2179. [Google Scholar] [CrossRef] [Green Version]
- Brzeszczyńska, J.; Meyer, A.; McGregor, R.; Schilb, A.; Degen, S.; Tadini, V.; Johns, N.; Langen, R.; Schols, A.; Glass, D.J.; et al. Alterations in the in vitro and in vivo regulation of muscle regeneration in healthy ageing and the influence of sarcopenia. J. Cachexia Sarcopenia Muscle 2018, 9, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Riuzzi, F.; Sorci, G.; Arcuri, C.; Giambanco, I.; Bellezza, I.; Minelli, A.; Donato, R. Cellular and molecular mechanisms of sarcopenia: The S100B perspective. J. Cachexia Sarcopenia Muscle 2018, 9, 1255–1268. [Google Scholar] [CrossRef] [Green Version]
- Minetti, G.C.; Feige, J.N.; Rosenstiel, A.; Bombard, F.; Meier, V.; Werner, A.; Bassilana, F.; Sailer, A.W.; Kahle, P.; Lambert, C.; et al. Gαi2 signaling promotes skeletal muscle hypertrophy, myoblast differentiation, and muscle regeneration. Sci. Signal. 2011, 4, ra80. [Google Scholar] [CrossRef]
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle 2017, 8, 349–369. [Google Scholar] [CrossRef]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, F.; Segovia, G.; del Arco, A. Aging, plasticity and environmental enrichment: Structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res. Rev. 2007, 55, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Aström, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [Green Version]
- Clow, C.; Jasmin, B.J. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol. Biol. Cell 2010, 21, 2182–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, F.W.; Ruegsegger, G.N.; Toedebusch, R.G.; Yan, Z. Endurance Exercise and the Regulation of Skeletal Muscle Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 129–151. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Jones, J.D.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and inflammation: Results from the 1999-2004 National Health and Nutrition Examination Survey. Clin. Nutr. 2016, 35, 1472–1483. [Google Scholar] [CrossRef]
- Langenberg, C.; Lotta, L.A. Genomic insights into the causes of type 2 diabetes. Lancet 2018, 391, 2463–2474. [Google Scholar] [CrossRef]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Myette-Côté, É.; Durrer, C.; Neudorf, H.; Bammert, T.D.; Botezelli, J.D.; Johnson, J.D.; DeSouza, C.A.; Little, J.P. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: A randomized trial. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1210–R1219. [Google Scholar] [CrossRef]
- Davari, M.; Hashemi, R.; Mirmiran, P.; Hedayati, M.; Sahranavard, S.; Bahreini, S.; Tavakoly, R.; Talaei, B. Effects of cinnamon supplementation on expression of systemic inflammation factors, NF-kB and Sirtuin-1 (SIRT1) in type 2 diabetes: A randomized, double blind, and controlled clinical trial. Nutr. J. 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamer, M.; Molloy, G.J. Association of C-reactive protein and muscle strength in the English Longitudinal Study of Ageing. Age (Dordr) 2009, 31, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doğan, M.H.; Karadag, B.; Ozyigit, T.; Kayaoglu, S.; Ozturk, A.O.; Altuntas, Y. Correlations between sarcopenia and hypertensive target organ damage in a Turkish cohort. Acta Clin. Belg. 2012, 67, 328–332. [Google Scholar] [CrossRef]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [Green Version]
- Nezameddin, R.; Itani, L.; Kreidieh, D.; El Masri, D.; Tannir, H.; El Ghoch, M. Understanding Sarcopenic Obesity in Terms of Definition and Health Consequences: A Clinical Review. Curr. Diabetes Rev. 2020, 16, 957–961. [Google Scholar] [CrossRef]
- Biteli, P.; Barbalho, S.M.; Detregiachi, C.R.P.; Dos Santos Haber, J.F.; Chagas, E.F.B. Dyslipidemia influences the effect of physical exercise on inflammatory markers on obese women in post-menopause: A randomized clinical trial. Exp. Gerontol. 2021, 150, 111355. [Google Scholar] [CrossRef]
- Sinatora, R.V.; Chagas, E.F.B.; Mattera, F.O.P.; Mellem, L.J.; Santos, A.; Pereira, L.P.; Aranão, A.L.C.; Guiguer, E.L.; Araújo, A.C.; Haber, J.; et al. Relationship of Inflammatory Markers and Metabolic Syndrome in Postmenopausal Women. Metabolites 2022, 12, 73. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Fantin, F.; Disegna, E.; Manzato, G.; Comellato, G.; Zoico, E.; Rossi, A.P.; Mazzali, G.; Rajkumar, C.; Zamboni, M. Adipokines and Arterial Stiffness in the Elderly. Vasc. Health Risk Manag. 2020, 16, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Cobos-Palacios, L.; Ruiz-Moreno, M.I.; Vilches-Perez, A.; Vargas-Candela, A.; Muñoz-Úbeda, M.; Benítez Porres, J.; Navarro-Sanz, A.; Lopez-Carmona, M.D.; Sanz-Canovas, J.; Perez-Belmonte, L.M.; et al. Metabolically healthy obesity: Inflammatory biomarkers and adipokines in elderly population. PLoS ONE 2022, 17, e0265362. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Van Gaal, L.; Leiter, L.A.; Vijapurkar, U.; List, J.; Cuddihy, R.; Ren, J.; Davies, M.J. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism 2018, 85, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Eberhardt, M.S.; Ogden, C.; Engelgau, M.; Cadwell, B.; Hedley, A.A.; Saydah, S. Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988-1994 and 1999-2002 (Reprinted from MMWR, vol 53, pg 1066-1068, 2004). JAMA J. Am. Med. Assoc. 2005, 293, 546–547. [Google Scholar]
- Aguilar-Ballester, M.; Hurtado-Genovés, G.; Taberner-Cortés, A.; Herrero-Cervera, A.; Martínez-Hervás, S.; González-Navarro, H. Therapies for the Treatment of Cardiovascular Disease Associated with Type 2 Diabetes and Dyslipidemia. Int. J. Mol. Sci. 2021, 22, 660. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Kim, H.S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Park, J.; Ryu, S.Y.; Kim, J. The effect of menopause on the metabolic syndrome among Korean women: The Korean National Health and Nutrition Examination Survey, 2001. Diabetes Care 2007, 30, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Oguz, A.; Sahin, M.; Tuzun, D.; Kurutas, E.B.; Ulgen, C.; Bozkus, O.; Gul, K. Irisin is a predictor of sarcopenic obesity in type 2 diabetes mellitus: A cross-sectional study. Med. (Baltim.) 2021, 100, e26529. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Q.; Peng, N.; Xu, S.; Zhang, M.; Hu, Y.; Chen, Z.; Tang, K.; He, X.; Li, Y.; et al. Inverse correlation between serum irisin and cardiovascular risk factors among Chinese overweight/obese population. BMC Cardiovasc. Disord. 2021, 21, 570. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Skrzypczak-Zielińska, M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Eder, P.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. Myostatin and Follistatin-New Kids on the Block in the Diagnosis of Sarcopenia in IBD and Possible Therapeutic Implications. Biomedicines 2021, 9, 1301. [Google Scholar] [CrossRef]
- Consitt, L.A.; Clark, B.C. The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J. Frailty Aging 2018, 7, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A. Sarcopenic obesity and endocrinal adaptation with age. Int. J. Endocrinol. 2013, 2013, 204164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, H.S. Sclerostin as a Putative Myokine in Sarcopenia. Endocrinol. Metab. (Seoul) 2022, 37, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Saeki, C.; Tsubota, A. Influencing Factors and Molecular Pathogenesis of Sarcopenia and Osteosarcopenia in Chronic Liver Disease. Life 2021, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Sylvawani, M.; Setyohadi, B.; Purnamasari, D.; Abdullah, M.; Kurniawan, M.R. Comparison of insulin-like growth factor-1 and sclerostin levels between premenopausal women with and without diabetes mellitus. J. Taibah Univ. Med. Sci. 2021, 16, 719–723. [Google Scholar] [CrossRef]
- Ahn, S.H.; Jung, H.W.; Lee, E.; Baek, J.Y.; Jang, I.Y.; Park, S.J.; Lee, J.Y.; Choi, E.; Lee, Y.S.; Hong, S.; et al. Decreased Serum Level of Sclerostin in Older Adults with Sarcopenia. Endocrinol. Metab. (Seoul) 2022, 37, 487–496. [Google Scholar] [CrossRef]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, S.; Iino, N.; Koda, R.; Narita, I.; Kaneko, Y. Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr. Gerontol. Int. 2021, 21, 27–33. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.J.; Lee, D.S.; Kim, W.K.; Han, B.S.; Lee, S.C.; Bae, K.H. Metabolic Adaptation in Obesity and Type II Diabetes: Myokines, Adipokines and Hepatokines. Int. J. Mol. Sci. 2016, 18, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aryana, S. Role of Interleukin-15 in Sarcopenia: Future New Target Therapy. Int. J. Geriatr. Gerontol. 2018, 2017, 1–8. [Google Scholar] [CrossRef]
- Crane, J.D.; MacNeil, L.G.; Lally, J.S.; Ford, R.J.; Bujak, A.L.; Brar, I.K.; Kemp, B.E.; Raha, S.; Steinberg, G.R.; Tarnopolsky, M.A. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell 2015, 14, 625–634. [Google Scholar] [CrossRef]
- Shi, J.; Fan, J.; Su, Q.; Yang, Z. Cytokines and Abnormal Glucose and Lipid Metabolism. Front. Endocrinol. (Lausanne) 2019, 10, 703. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-Y.; Chen, W.-L. Examining the Association Between Serum Leptin and Sarcopenic Obesity. J. Inflamm. Res. 2021, 14, 3481–3487. [Google Scholar] [CrossRef]
- Kohara, K.; Ochi, M.; Tabara, Y.; Nagai, T.; Igase, M.; Miki, T. Leptin in sarcopenic visceral obesity: Possible link between adipocytes and myocytes. PLoS ONE 2011, 6, e24633. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Yeoh, B.S.; Vijay-Kumar, M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu. Rev. Nutr. 2017, 37, 103–130. [Google Scholar] [CrossRef]
- Jaberi, S.A.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharmacol. 2021, 142, 112002. [Google Scholar] [CrossRef]
- Choi, E.B.; Jeong, J.H.; Jang, H.M.; Ahn, Y.J.; Kim, K.H.; An, H.S.; Lee, J.Y.; Jeong, E.A.; Lee, J.; Shin, H.J.; et al. Skeletal Lipocalin-2 Is Associated with Iron-Related Oxidative Stress in ob/ob Mice with Sarcopenia. Antioxidants 2021, 10, 758. [Google Scholar] [CrossRef]
- Foreman, N.A.; Hesse, A.S.; Ji, L.L. Redox Signaling and Sarcopenia: Searching for the Primary Suspect. Int. J. Mol. Sci. 2021, 22, 9045. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, L.; López-Hoyos, M.; Muñoz-Cacho, P.; Martínez-Taboada, V.M. Aging is associated with circulating cytokine dysregulation. Cell. Immunol. 2012, 273, 124–132. [Google Scholar] [CrossRef]
- Duggal, N.A.; Pollock, R.D.; Lazarus, N.R.; Harridge, S.; Lord, J.M. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018, 17, e12750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshounpour, M.T.; Habibi, A.; Ranjbar, R. Impact of combined exercise training on plasma concentration of Apelin, resistin and insulin resistance in patients with type 2 diabetics’ male. Hormozgan Med. J. 2016, 20. [Google Scholar]
- Ferrari, U.; Schmidmaier, R.; Jung, T.; Reincke, M.; Martini, S.; Schoser, B.; Bidlingmaier, M.; Drey, M. IGF-I/IGFBP3/ALS Deficiency in Sarcopenia: Low GHBP Suggests GH Resistance in a Subgroup of Geriatric Patients. J. Clin. Endocrinol. Metab. 2021, 106, e1698–e1707. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Rosso, C.; Caviglia, G.P.; Ribaldone, D.G.; Bugianesi, E. The Impact of Dysmetabolic Sarcopenia Among Insulin Sensitive Tissues: A Narrative Review. Front. Endocrinol. 2021, 12, 716533. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Lubrano, C.; Sergi, G.; Coin, A.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Sarcopenic Obesity and Metabolic Syndrome in Adult Caucasian Subjects. J. Nutr. Health Aging 2016, 20, 958–963. [Google Scholar] [CrossRef]
- Oflazoglu, U.; Caglar, S.; Yılmaz, H.E.; Önal, H.T.; Varol, U.; Salman, T.; Yildiz, Y.; Unal, S.; Guc, Z.G.; Kucukzeybek, Y.; et al. The relationship between sarcopenia detected in newly diagnosed colorectal cancer patients and FGF21, irisin and CRP levels. Eur. Geriatr. Med. 2022, 13, 795–803. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Maio, M.C.; Barbalho, S.M.; Guiguer, E.L.; Araújo, A.C.; de Alvares Goulart, R.; Flato, U.A.P.; Júnior, E.B.; Detregiachi, C.R.P.; Dos Santos Haber, J.F.; et al. Organokines in Rheumatoid Arthritis: A Critical Review. Int. J. Mol. Sci. 2022, 23, 6193. [Google Scholar] [CrossRef]
- Choi, K.M. The Impact of Organokines on Insulin Resistance, Inflammation, and Atherosclerosis. Endocrinol. Metab. (Seoul) 2016, 31, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Yang, L.; Yan, S.; Zheng, H.; Liang, L.; Cai, X.; Liao, M. Fetuin A promotes lipotoxicity in β cells through the TLR4 signaling pathway and the role of pioglitazone in anti-lipotoxicity. Mol. Cell. Endocrinol. 2015, 412, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wu, C.H.; Hsu, L.W.; Chen, P.W.; Yu, J.R.; Chang, C.S.; Tsai, W.C.; Liu, P.Y. Serum vitamin D, intact parathyroid hormone, and Fetuin A concentrations were associated with geriatric sarcopenia and cardiac hypertrophy. Sci. Rep. 2017, 7, 40996. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Gray, S.R.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Biomarkers Profile of People With Sarcopenia: A Cross-sectional Analysis From UK Biobank. J. Am. Med. Dir. Assoc. 2020, 21, e2011–e2017. [Google Scholar] [CrossRef]
- Pereira, S.L.; Shoemaker, M.E.; Gawel, S.; Davis, G.J.; Luo, M.; Mustad, V.A.; Cramer, J.T. Biomarker Changes in Response to a 12-Week Supplementation of an Oral Nutritional Supplement Enriched with Protein, Vitamin D and HMB in Malnourished Community Dwelling Older Adults with Sarcopenia. Nutrients 2022, 14, 1196. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Hwang, S.Y.; Choi, J.H.; Lee, H.J.; Chung, H.S.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; et al. Association of leukocyte cell-derived chemotaxin 2 (LECT2) with NAFLD, metabolic syndrome, and atherosclerosis. PLoS ONE 2017, 12, e0174717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Bennett, S.; Li, Y.; Liu, M.; Xu, J. The molecular structure and role of LECT2 or CHM-II in arthritis, cancer, and other diseases. J. Cell. Physiol. 2022, 237, 480–488. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef]
- Xue, Y.; Hu, S.; Chen, C.; He, J.; Sun, J.; Jin, Y.; Zhang, Y.; Zhu, G.; Shi, Q.; Rui, Y. Myokine Irisin promotes osteogenesis by activating BMP/SMAD signaling via αV integrin and regulates bone mass in mice. Int. J. Biol. Sci. 2022, 18, 572–584. [Google Scholar] [CrossRef]
- Santos, J.P.M.d.; Maio, M.C.d.; Lemes, M.A.; Laurindo, L.F.; Haber, J.F.d.S.; Bechara, M.D.; Prado, P.S.d.; Rauen, E.C.; Costa, F.; Pereira, B.C.d.A.; et al. Non-Alcoholic Steatohepatitis (NASH) and Organokines: What Is Now and What Will Be in the Future. Int. J. Mol. Sci. 2022, 23, 498. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, M.; Larijani, B.; Keshtkar, A.A.; Nasli-Esfahani, E.; Alatab, S.; Mohajeri-Tehrani, M.R. Mechanisms involved in altered bone metabolism in diabetes: A narrative review. J. Diabetes Metab. Disord. 2016, 15, 52. [Google Scholar] [CrossRef]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. N. Y. Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Esposito, P.; Picciotto, D.; Battaglia, Y.; Costigliolo, F.; Viazzi, F.; Verzola, D. Myostatin: Basic biology to clinical application. Adv. Clin. Chem. 2022, 106, 181–234. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; McFarlane, C.; Kambadur, R.; Kukreti, H.; Bonala, S.; Srinivasan, S. Myostatin: Expanding horizons. IUBMB Life 2015, 67, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Pacicca, D.M.; Brown, T.; Watkins, D.; Kover, K.; Yan, Y.; Prideaux, M.; Bonewald, L. Elevated glucose acts directly on osteocytes to increase sclerostin expression in diabetes. Sci. Rep. 2019, 9, 17353. [Google Scholar] [CrossRef] [Green Version]
- Dumic-Cule, I.; Ivanac, G.; Lucijanic, T.; Katičić, D.; Jurin, I.; Birkic, D.; Rahelic, D.; Blaslov, K. Type 2 diabetes and osteoporosis: Current knowledge. Endrocrine Oncol. Metab. 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Delezie, J.; Weihrauch, M.; Maier, G.; Tejero, R.; Ham, D.J.; Gill, J.F.; Karrer-Cardel, B.; Rüegg, M.A.; Tabares, L.; Handschin, C. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 16111–16120. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Moon, H.Y.; van Praag, H. On the Run for Hippocampal Plasticity. Cold Spring Harb. Perspect. Med. 2018, 8, a029736. [Google Scholar] [CrossRef]
- Lee, S.Y.; Wang, T.Y.; Chen, S.L.; Chang, Y.H.; Chen, P.S.; Huang, S.Y.; Tzeng, N.S.; Wang, L.J.; Lee, I.H.; Chen, K.C.; et al. The correlation between plasma brain-derived neurotrophic factor and cognitive function in bipolar disorder is modulated by the BDNF Val66Met polymorphism. Sci. Rep. 2016, 6, 37950. [Google Scholar] [CrossRef]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Ait Eldjoudi, D.; Cordero Barreal, A.; Gonzalez-Rodríguez, M.; Ruiz-Fernández, C.; Farrag, Y.; Farrag, M.; Lago, F.; Capuozzo, M.; Gonzalez-Gay, M.A.; Mera Varela, A.; et al. Leptin in Osteoarthritis and Rheumatoid Arthritis: Player or Bystander? Int. J. Mol. Sci. 2022, 23, 2859. [Google Scholar] [CrossRef]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Hacham, M.; White, R.M.; Argov, S.; Segal, S.; Apte, R.N. Interleukin-6 and interleukin-10 are expressed in organs of normal young and old mice. Eur. Cytokine Netw. 2004, 15, 37–46. [Google Scholar] [PubMed]
- Pfeiffer, S.E.M.; Quesada-Masachs, E.; McArdle, S.; Zilberman, S.; Yesildag, B.; Mikulski, Z.; von Herrath, M. Effect of IL4 and IL10 on a human in vitro type 1 diabetes model. Clin. Immunol. 2022, 241, 109076. [Google Scholar] [CrossRef]
- Beppu, L.Y.; Mooli, R.G.R.; Qu, X.; Marrero, G.J.; Finley, C.A.; Fooks, A.N.; Mullen, Z.P.; Frias, A.B., Jr.; Sipula, I.; Xie, B.; et al. Tregs facilitate obesity and insulin resistance via a Blimp-1/IL-10 axis. JCI Insight 2021, 6, e140644. [Google Scholar] [CrossRef]
- Son, J.S.; Kim, H.J.; Son, Y.; Lee, H.; Chae, S.A.; Seong, J.K.; Song, W. Effects of exercise-induced apelin levels on skeletal muscle and their capillarization in type 2 diabetic rats. Muscle Nerve 2017, 56, 1155–1163. [Google Scholar] [CrossRef]
- Naranjo, J.D.; Dziki, J.L.; Badylak, S.F. Regenerative Medicine Approaches for Age-Related Muscle Loss and Sarcopenia: A Mini-Review. Gerontology 2017, 63, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef] [Green Version]
- Gould, P.W.; Zemel, B.S.; Taratuta, E.G.; Baker, J.F. Circulating Fibroblast Growth Factor-21 Levels in Rheumatoid Arthritis: Associations With Disease Characteristics, Body Composition, and Physical Functioning. J. Rheumatol. 2021, 48, 504–512. [Google Scholar] [CrossRef]
- Leuchtmann, A.B.; Adak, V.; Dilbaz, S.; Handschin, C. The Role of the Skeletal Muscle Secretome in Mediating Endurance and Resistance Training Adaptations. Front. Physiol. 2021, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V. The Interplay between Mitochondrial Morphology and Myomitokines in Aging Sarcopenia. Int. J. Mol. Sci. 2020, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Alamdari, K.A.; Symonds, M.E.; Nobari, H.; Carlos-Vivas, J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: Systematic review and meta-analysis. Horm. (Athens) 2021, 20, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Priego, T.; Martín, A.I.; González-Hedström, D.; Granado, M.; López-Calderón, A. Role of hormones in sarcopenia. Vitam Horm 2021, 115, 535–570. [Google Scholar] [CrossRef]
- China, S.P.; Pal, S.; Chattopadhyay, S.; Porwal, K.; Kushwaha, S.; Bhattacharyya, S.; Mittal, M.; Gurjar, A.A.; Barbhuyan, T.; Singh, A.K.; et al. Globular adiponectin reverses osteo-sarcopenia and altered body composition in ovariectomized rats. Bone 2017, 105, 75–86. [Google Scholar] [CrossRef]
- Jung, S.H.; Park, H.S.; Kim, K.S.; Choi, W.H.; Ahn, C.W.; Kim, B.T.; Kim, S.M.; Lee, S.Y.; Ahn, S.M.; Kim, Y.K.; et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J. Nutr. Biochem. 2008, 19, 371–375. [Google Scholar] [CrossRef]
- Abou-Samra, M.; Selvais, C.M.; Dubuisson, N.; Brichard, S.M. Adiponectin and Its Mimics on Skeletal Muscle: Insulin Sensitizers, Fat Burners, Exercise Mimickers, Muscling Pills … or Everything Together? Int. J. Mol. Sci. 2020, 21, 2620. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.J.; Kang, X.R.; Bian, T.; Shen, Z.M.; Jiang, Y.; Sun, B.; Hu, H.B.; Chen, Y.S. lncRNA DLEU2 acts as a miR-181a sponge to regulate SEPP1 and inhibit skeletal muscle differentiation and regeneration. Aging 2020, 12, 24033–24056. [Google Scholar] [CrossRef]
- Verma, M.; Kapoor, N.; Chaudhary, A.; Sharma, P.; Ghosh, N.; Sidana, S.; Kakkar, R.; Kalra, S. Prevalence and Determinants of Sarcopenic Obesity in Older Adults: Secondary Data Analysis of the Longitudinal Ageing Study in India (LASI) Wave 1 Survey (2017–18). Adv. Ther. 2022, 39, 4094–4113. [Google Scholar] [CrossRef]
- Qu, Z.; Huang, J.; Yang, F.; Hong, J.; Wang, W.; Yan, S. Sex hormone-binding globulin and arthritis: A Mendelian randomization study. Arthritis Res. Ther. 2020, 22, 118. [Google Scholar] [CrossRef] [PubMed]
- Kornicka-Garbowska, K.; Bourebaba, L.; Röcken, M.; Marycz, K. Sex Hormone Binding Globulin (SHBG) Mitigates ER Stress in Hepatocytes In Vitro and Ex Vivo. Cells 2021, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Sáez-López, C.; Barbosa-Desongles, A.; Hernández, C.; Selva, D.M. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol. Metab. 2015, 26, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Osiecka-Iwan, A.; Hyc, A.; Radomska-Lesniewska, D.M.; Rymarczyk, A.; Skopinski, P. Antigenic and immunogenic properties of chondrocytes. Implications for chondrocyte therapeutic transplantation and pathogenesis of inflammatory and degenerative joint diseases. Cent. Eur. J. Immunol. 2018, 43, 209–219. [Google Scholar] [CrossRef]
- Huang, H.H.; Yeh, C.; Chen, J.C.; Lee, T.H.; Chen, S.C.; Lee, W.J.; Chen, C.Y. Does bariatric surgery influence plasma levels of fetuin-A and leukocyte cell-derived chemotaxin-2 in patients with type 2 diabetes mellitus? PeerJ 2018, 6, e4884. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Bedogni, G.; Fioravanti, A. Circulating Mir-140 and leptin improve the accuracy of the differential diagnosis between psoriatic arthritis and rheumatoid arthritis: A case-control study. Transl. Res. 2022, 239, 18–34. [Google Scholar] [CrossRef]



| Classification | Organokine | Role in Sarcopenia and Metabolic Repercussions | Expression in Sarcopenia | General Actions | Roles in Sarcopenia | References |
|---|---|---|---|---|---|---|
| MYOKINES | Irisin | Dyslipidemia, sarcopenic obesity, and diabetes. | ↓ | In ↑ concentrations: ↓ body weight and↑ insulin sensitivity. | In ↓ concentrations, it predicts sarcopenic obesity and ↑ insulin resistance. It is inversely correlated with the occurrence of dyslipidemia. | [68,69] |
| Myostatin | Sarcopenic obesity and diabetes. | ↑, it is inversely proportional to skeletal muscle mass. | Generates muscle waste and correlates positively with a pro-inflammatory. | In ↑ concentrations, sarcopenic obesity and its driven diabetes can be predicted by myostatin levels. Additionally, it impairs muscle growth and contributes to muscle insulin resistance. | [70,71,72] | |
| Sclerostin | Sarcopenia and diabetes. | ↓ | Formation and regulation of bone mass and decrease in bone mass in diabetic patients. | In ↑ concentrations, it is significantly associated with a lower risk of sarcopenia. Hyperglycemia directly increases sclerostin production. | [73,74,75,76] | |
| BDNF | Dyslipidemia, sarcopenia, obesity, and diabetes. | ↓ | ↑ Memory, ↑ insulin sensitivity, regulates lipid metabolism, and acts in myogenesis process. | In ↓ concentrations, it predicts sarcopenia, obesity, ↑ insulin resistance, and promotes dyslipidemia. | [77,78,79,80] | |
| IL-6 | Sarcopenia, sarcopenic obesity, metabolic syndrome, and diabetes. | ↑ | ↑ Insulin resistance, ↑ inflammation, ↑ muscle volume, and strength. | In ↑ concentrations, sarcopenic obesity, metabolic syndrome, and diabetes, as long as IL-6 is a pro-inflammatory factor. | [10,25,81,82] | |
| IL-15 | Sarcopenia, sarcopenic obesity, obesity, and oxidative stress. | ↓ | ↓ Inflammation, ↓ oxidative stress, ↓ obesity, and ↓ glucose levels. | Prevents the reduction of muscle mass, increases glucose uptake by the skeletal muscle, promotes muscle hypertrophy, and reduces subcutaneous fat and adipose tissue mass. | [10,80,83,84,85] | |
| ADIPOKINES | Leptin | Sarcopenic obesity. | ↑ | ↑ Lipolysis and ↑ insulin sensitivity by skeletal muscles. | Risk predictor for sarcopenic obesity. In ↓ concentrations, leptin can induce insulin resistance by decreasing glucose consumption by muscles. | [86,87]. |
| LCN2 | Sarcopenic obesity and diabetes. | ↑ | ↑ Insulin resistance, ↑ inflammation, and ↑ oxidative stress. | In ↑ concentrations, sarcopenic obesity involves metabolic disorders such as obesity, insulin resistance, and type 2 diabetes. | [25,88,89,90] | |
| IL-6 | Sarcopenia, sarcopenic obesity, metabolic syndrome, and diabetes. | ↑ | ↑ Inflammation and ↑ insulin resistance by inhibiting the expression of IRS1 and GLUT4 in adipocytes. | In ↑ concentrations, sarcopenic obesity, metabolic syndrome, and diabetes. | [10,79,81,91] | |
| IL-10 | Sarcopenia, sarcopenic obesity, obesity, oxidative stress, and autoimmune diabetes. | ↓ | ↓ Inflammation, oxidative stress, and obesity in skeletal muscle. | In spite of an increase in the levels of IL-10 in older humans, in ↑ concentrations it promotes an anti-inflammatory environment and induces protein synthesis in individuals with sarcopenic obesity. | [10,72,92] | |
| IL-15 | Sarcopenia, sarcopenic obesity, obesity, and oxidative stress. | ↓ | ↓ Inflammation, ↓ oxidative stress, ↓ obesity, and ↓ glucose levels. | Prevents the reduction of muscle mass, promotes muscle hypertrophy, and reduces glucose levels, subcutaneous fat, and adipose tissue mass. | [10,80,85,93] | |
| Apelin | Sarcopenia, obesity, and diabetes. | ↓ | ↓ Inflammation, ↓ glucose levels, and ↓ insulin resistance index. | Improves muscle function in an anti-inflammatory way, increases the regenerative abilities of muscles, and also improves metabolism in obesity and DM2. | [10,80,94] | |
| IGF-1 | Sarcopenia, sarcopenic obesity, and oxidative stress. | ↓ | ↑ Muscle hypertrophy and ↓ atrophy. | In ↓ concentrations, it promotes sarcopenia through ↓ protein synthesis, autophagy, and ↓ muscle regeneration. It also promotes sarcopenic obesity. | [95,96,97] (Naranjo et al., 2017) | |
| FGF-21 | Sarcopenia, sarcopenic obesity, obesity, and oxidative stress. | ↓ | Regulates glucose and lipid metabolism, antioxidant, ↓ obesity, and ↓ sarcopenia. | In ↓ concentrations, it promotes sarcopenia and obesity since it is directly linked with muscle mass along with irisin. | [10,80,83,98] | |
| Adiponectin | Sarcopenia, sarcopenic obesity, obesity, and oxidative stress. | ↑ | ↓ Inflammation, ↓ atherosclerosis, ↓ oxidative stress, and ↑ insulin resistance. | In ↑ concentrations, it promotes muscle hypertrophy and ↓ sarcopenic obesity. At the muscle level, ↑free fatty acid oxidation and glucose uptake. At the liver level, ↓ gluconeogenesis. | [10,99] | |
| HEPATOKINES | Fetuin A | Sarcopenic obesity and diabetes. | ↑ | ↑ Insulin resistance, ↑ inflammation, and ↑ oxidative stress. | In ↑ concentrations, it contributes to a state of obesity, sarcopenia, sarcopenic obesity, and insulin resistance through a pro-inflammatory scenario. | [25,100,101,102] |
| SHBG | Sarcopenia, sarcopenic obesity, diabetes, and CVD. | ↓ | ↑ Inflammation, ↑ oxidative stress, ↑ obesity, ↑ fatty liver disease, and ↑ CVD. | ↑ Levels of SHBG are related to sarcopenia in both sexes of older individuals. | [25,103,104] | |
| LECT-2 | Insulin resistance and obesity. | Probably ↑ | In ↑ concentrations: ↑ insulin resistance, obesity, and NAFLD. | Although LECT-2 has no known implications for the mechanism of sarcopenia, it acts directly on skeletal muscle by positively correlating with insulin resistance and obesity. | [99,105,106] | |
| IGF-1 | Sarcopenia, sarcopenic obesity, and oxidative stress. | ↓ | ↑ Muscle hypertrophy, ↓ atrophy, and acts on oxidative stress. | In ↓ concentrations, ↓ protein synthesis, autophagy, and ↓ muscle regeneration, and it promotes sarcopenia and also sarcopenic obesity. | [10,95,107] | |
| OSTEOKINES | Osteocalcin | Sarcopenia, sarcopenic obesity, and diabetes. | ↓ | In ↑ concentrations: ↑ insulin sensitivity, uptake of glucose, anti-inflammatory, and ↑ muscle mass. | In ↑ concentrations, it contributes to the survival and function of pancreatic β cells, thereby increasing insulin secretion. It also contributes to muscle hypertrophy and strength. Consequently, its reduction would promote the opposite effect. | [108,109] |
| Irisin | Dyslipidemia, sarcopenic obesity, and diabetes. | ↓ | In ↑ concentrations: ↓ body weight and↑insulin sensitivity. | In ↓ concentrations, it predicts sarcopenic obesity and ↑ insulin resistance. | [33,108,110,111]. | |
| Sclerostin | Sarcopenia and diabetes. | ↓ | Formation and regulation of bone mass and decreased bone mass in diabetic patients. | In ↑ concentrations, it is prospectively associated with a lower risk of sarcopenia. | [73,75,76,112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minniti, G.; Pescinini-Salzedas, L.M.; Minniti, G.A.d.S.; Laurindo, L.F.; Barbalho, S.M.; Vargas Sinatora, R.; Sloan, L.A.; Haber, R.S.d.A.; Araújo, A.C.; Quesada, K.; et al. Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. Int. J. Mol. Sci. 2022, 23, 13452. https://doi.org/10.3390/ijms232113452
Minniti G, Pescinini-Salzedas LM, Minniti GAdS, Laurindo LF, Barbalho SM, Vargas Sinatora R, Sloan LA, Haber RSdA, Araújo AC, Quesada K, et al. Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. International Journal of Molecular Sciences. 2022; 23(21):13452. https://doi.org/10.3390/ijms232113452
Chicago/Turabian StyleMinniti, Giulia, Letícia Maria Pescinini-Salzedas, Guilherme Almeida dos Santos Minniti, Lucas Fornari Laurindo, Sandra Maria Barbalho, Renata Vargas Sinatora, Lance Alan Sloan, Rafael Santos de Argollo Haber, Adriano Cressoni Araújo, Karina Quesada, and et al. 2022. "Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise" International Journal of Molecular Sciences 23, no. 21: 13452. https://doi.org/10.3390/ijms232113452
APA StyleMinniti, G., Pescinini-Salzedas, L. M., Minniti, G. A. d. S., Laurindo, L. F., Barbalho, S. M., Vargas Sinatora, R., Sloan, L. A., Haber, R. S. d. A., Araújo, A. C., Quesada, K., Haber, J. F. d. S., Bechara, M. D., & Sloan, K. P. (2022). Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. International Journal of Molecular Sciences, 23(21), 13452. https://doi.org/10.3390/ijms232113452










