Generation and Characterization of Native and Sialic Acid-Deficient IgE
Abstract
:1. Introduction
2. Results
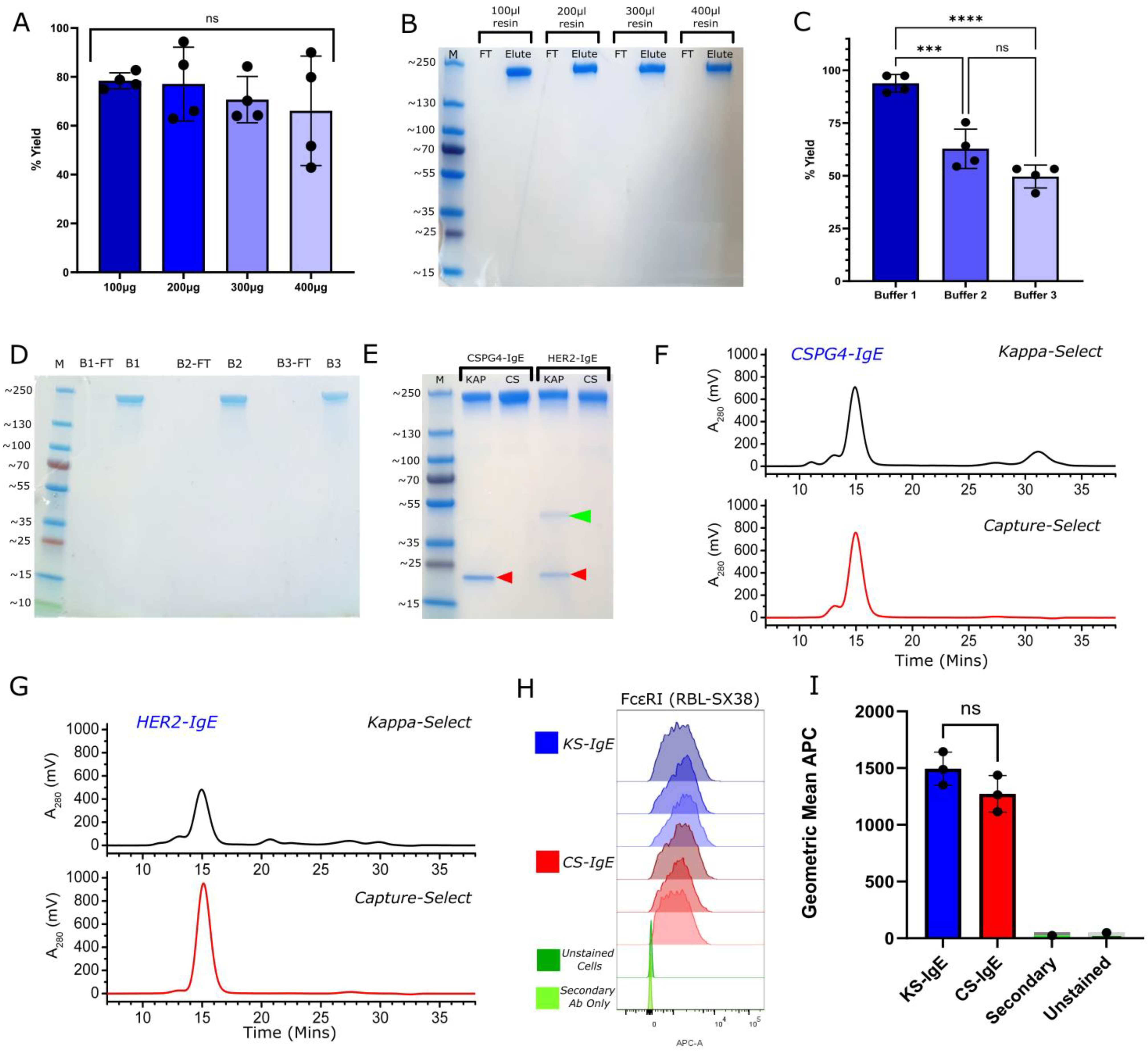
2.1. High Purity of Full-Length IgE Antibody Yields via IgE Class-Specific Affinity Chromatography
2.2. Generation and Purification of Sialic Acid-Deficient IgE
2.3. Glyco-Analysis Retention of Complex Glycan Structures and Loss of Sialic Acid Residus on Glyco-Engineered IgE
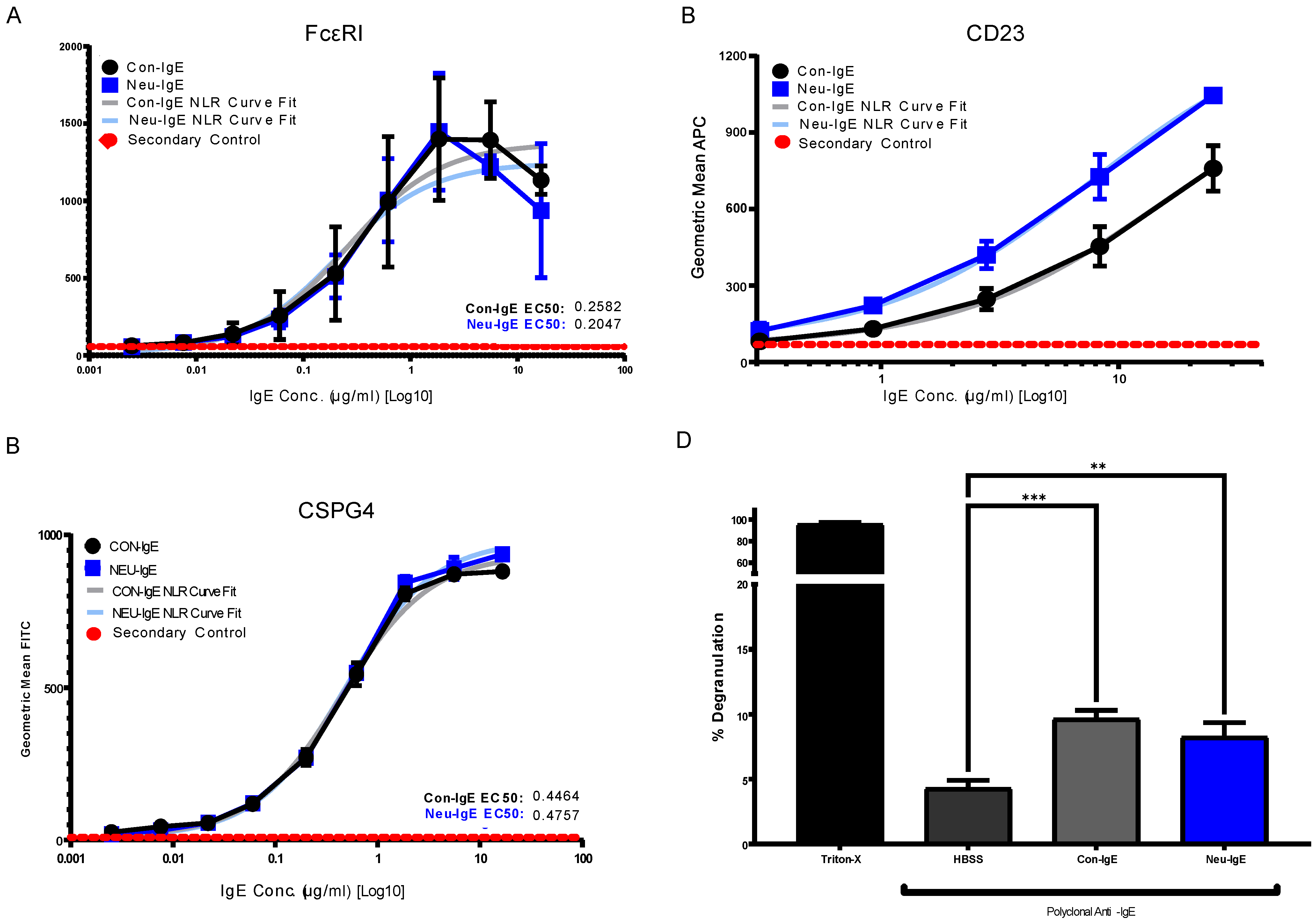
2.4. Glyco-Engineered IgE retains Fc and Fab Region-Mediated Binding to Immune and Target Antigen Expressing Cells and can Trigger Cellular Degranulation
3. Discussion
4. Materials and Methods
4.1. Production of Recombinant IgE in Culture Using Human Embryonic Kidney Expi293F Cells
4.2. Packing of Chromatography Columns and Purification of Recombinant IgE from Culture Supernatants
4.3. Dialysis of Recombinant IgE from Culture
4.4. Production and Purification of Sialic Acid-Deficient IgE using Glycosidase Enzymes
4.5. Confirmation of Antibody Purification via Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.6. Size Exclusion Chromatography (SEC)
4.7. Glycoanalysis of Con-IgE and Neu-IgE by Hydrophobic Interaction Liquid Chromatography (HILIC) HPLC
4.8. Flow Cytometric Evaluations of IgE Binding to Cell Surface Receptors and Antigens
4.9. IgE-Mediated Degranulation of RBL-SX38 Cells
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gould, H.J.; Sutton, B.J.; Beavil, A.J.; Beavil, R.L.; McCloskey, N.; Cooker, H.A.; Fear, D.; Smurthwaite, L. The biology of IGE and the basis of allergic disease. Annu. Rev. Immunol. 2003, 21, 579–628. [Google Scholar] [CrossRef] [PubMed]
- Sutton, B.J.; Davies, A.M.; Baz, H.J.; Karagiannis, S.N. IgE Antibodies: From Structure to Function and Clinical Translation. Antibodies 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukai, K.; Tsai, M.; Starkl, P.; Marichal, T.; Galli, S.J. IgE and mast cells in host defense against parasites and venoms. Semin. Immunopathol. 2016, 38, 581–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelman, F.D.; Urban, F.J., Jr. The other side of the coin: The protective role of the TH2 cytokines. J. Allergy Clin. Immunol. 2001, 107, 772–780. [Google Scholar] [CrossRef] [PubMed]
- McCraw, A.J.; Chauhan, J.; Bax, H.J.; Stavraka, C.; Osborn, G.; Grandits, M.; López-Abente, J.; Josephs, D.H.; Spicer, J.; Wagner, G.K.; et al. Insights from IgE Immune Surveillance in Allergy and Cancer for Anti-Tumour IgE Treatments. Cancers 2021, 13, 4460. [Google Scholar] [CrossRef]
- Karagiannis, S.N.; Chauhan, J.; Bax, H.J.; Stavraka, C.; Osborn, G.; Grandits, M.; López-Abente, J.; Josephs, D.H.; Spicer, J.; Wagner, G.K.; et al. Therapeutic IgE Antibodies: Harnessing a Macrophage-Mediated Immune Surveillance Mechanism against Cancer. Cancer Res. 2017, 77, 2779–2783. [Google Scholar] [CrossRef] [Green Version]
- Karagiannis, S.N.; Josephs, D.H.; Karagiannis, P.; Gilbert, A.E.; Saul, L.; Rudman, S.M.; Dodev, T.; Koers, A.; Blower, P.J.; Corrigan, C.; et al. Recombinant IgE antibodies for passive immunotherapy of solid tumours: From concept towards clinical application. Cancer Immunol. Immunother. 2012, 61, 1547–1564. [Google Scholar] [CrossRef]
- Dhaliwal, B.; Pang, M.O.Y.; Keeble, A.H.; James, L.K.; Gould, H.J.; McDonnell, J.M.; Sutton, B.J.; Beavil, A.J. IgE binds asymmetrically to its B cell receptor CD23. Sci. Rep. 2017, 7, 45533. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, J.; McCraw, A.J.; Nakamura, M.; Osborn, G.; Sow, H.S.; Cox, V.F.; Stavraka, C.; Josephs, D.H.; Spicer, J.F.; Karagiannis, S.N.; et al. IgE Antibodies against Cancer: Efficacy and Safety. Antibodies 2020, 9, 55. [Google Scholar] [CrossRef]
- Spicer, J.; Basu, B.; Montes, A.; Banerji, U.; Kristeleit, R.; Veal, G.J.; Corrigan, C.; Till, S.; Nintos, G.; Brier, T.; et al. Abstract CT141: Phase 1 trial of MOv18, a first-in-class IgE antibody therapy for cancer. Cancer Res. 2020, 80, CT141. [Google Scholar] [CrossRef]
- Plomp, R.; Hensbergen, P.J.; Rombouts, Y.; Zauner, G.; Dragan, I.; Koeleman, C.A.M.; Deelder, A.M.; Wuhrer, M. Site-specific N-glycosylation analysis of human immunoglobulin e. J. Proteome Res. 2014, 13, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.M.; Cosgrave, E.F.J.; Struwe, W.B.; Wormald, M.; Davey, G.P.; Jefferins, R.; Rudd, P.M. Glycosylation and Fc receptors. Curr. Top Microbiol. Immunol. 2014, 382, 165–199. [Google Scholar] [PubMed]
- Plomp, R.; Bondt, A.; de Haan, N.; Rombouts, Y.; Wuhrer, M. Recent Advances in Clinical Glycoproteomics of Immunoglobulins (Igs). Mol. Cell. Proteomics 2016, 15, 2217–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björklund, J.E.; Karlsson, T.; Magnusson, C.G. N-glycosylation influences epitope expression and receptor binding structures in human IgE. Mol. Immunol. 1999, 36, 213–221. [Google Scholar] [CrossRef]
- Garman, S.C.; Wurzburg, B.A.; Tarchevskaya, S.S.; Kinet, J.-P.; Jardetzky, T.S. Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc epsilonRI alpha. Nature 2000, 406, 259–266. [Google Scholar] [CrossRef]
- Henry, A.J.; McDonnell, J.M.; Ghirlando, R.; Sutton, B.J.; Gould, H.J. Conformation of the isolated cepsilon3 domain of IgE and its complex with the high-affinity receptor, FcepsilonRI. Biochemistry 2000, 39, 7406–7413. [Google Scholar] [CrossRef]
- Nettleton, M.Y.; Kochan, J.P. Role of glycosylation sites in the IgE Fc molecule. Int. Arch. Allergy Immunol. 1995, 107, 328–329. [Google Scholar] [CrossRef] [Green Version]
- Shade, K.-T.C.; Platzer, B.; Washburn, N.; Mani, V.; Bartsch, Y.C.; Conroy, M.; Pagan, J.D.; Bosques, C.; Mempel, T.R.; Fiebiger, E.; et al. A single glycan on IgE is indispensable for initiation of anaphylaxis. J. Exp. Med. 2015, 212, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Basu, M.; Hakimi, J.; Dharm, E.; Kondas, J.A.; Tsien, W.H.; Pilson, R.S.; Lin, P.; Gilfillan, A.; Haring, P. Purification and characterization of human recombinant IgE-Fc fragments that bind to the human high affinity IgE receptor. J. Biol. Chem. 1993, 268, 13118–13127. [Google Scholar] [CrossRef]
- Doré, K.A.; Davies, A.M.; Drinkwater, N.; Beavil, A.J.; McDonnell, J.M.; Sutton, B.J. Thermal sensitivity and flexibility of the Cε3 domains in immunoglobulin E. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1336–1347. [Google Scholar] [CrossRef]
- Robertson, M.W.; Liu, F.T. Heterogeneous IgE glycoforms characterized by differential recognition of an endogenous lectin (IgE-binding protein). J. Immunol. 1991, 147, 3024–3030. [Google Scholar] [PubMed]
- Robertson, M.W.; Albrandt, K.; Keller, D.; Liu, F.T. Human IgE-binding protein: A soluble lectin exhibiting a highly conserved interspecies sequence and differential recognition of IgE glycoforms. Biochemistry 1990, 29, 8093–8100. [Google Scholar] [CrossRef] [PubMed]
- Shade, K.-T.C.; Conroy, M.E.; Washburn, N.; Kitaoka, M.; Huynh, D.J.; Laprise, E.; Patil, S.U.; Shreffler, W.G.; Anthony, R.M. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature 2020, 582, 265–270. [Google Scholar] [CrossRef]
- Badloe, F.M.S.; De Vriese, S.; De Bruyn Carlier, T.; Vandersichel, E.; Scheffel, J.; Maurer, M.; Ring, J.; Gutermuth, J.; Krohn, I.K. A Novel Method for Total IgE Purification from Human Serum. J. Immunol. 2022, 208, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Pomés, A.; Mueller, G.A.; Chruszcz, M. Structural Aspects of the Allergen-Antibody Interaction. Front. Immunol. 2020, 11, 2067. [Google Scholar] [CrossRef]
- Dorrington, K.J.; Bennich, H.H. Structure-function relationships in human immunoglobulin E. Immunol. Rev. 1978, 41, 3–25. [Google Scholar] [CrossRef]
- Wu, G.; Hitchen, P.G.; Panico, M.; North, S.J.; Barbouche, M.-R.; Binet, D.; Morris, H.R.; Dell, A.; Haslam, S.M. Glycoproteomic studies of IgE from a novel hyper IgE syndrome linked to PGM3 mutation. Glycoconj. J. 2016, 33, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Montero-Morales, L.; Maresch, D.; Crescioli, S.; Castilho, A.; Ilieva, K.M.; Mele, S.; Karagiannis, S.N.; Altmann, F.; Steinkellner, H. In Planta Glycan Engineering and Functional Activities of IgE Antibodies. Front. Bioeng. Biotechnol. 2019, 7, 242. [Google Scholar] [CrossRef]
- Crescioli, S.; Chiaruttini, G.; Mele, S.; Ilieva, K.M.; Pellizzari, G.; Spencer, D.I.R.; Gardner, R.A.; Lacy, K.E.; Spicer, J.F.; Tutt, A.N.J.; et al. Engineering and stable production of recombinant IgE for cancer immunotherapy and AllergoOncology. J. Allergy Clin. Immunol. 2018, 141, 1519–1523. [Google Scholar] [CrossRef] [Green Version]
- Ilieva, K.M.; Fazekas-Singer, J.; Bax, H.J.; Crescioli, S.; Montero-Morales, L.; Mele, S.; Sow, H.S.; Stavraka, C.; Josephs, D.H.; Spicer, J.F.; et al. AllergoOncology: Expression platform development and functional profiling of an anti-HER2 IgE antibody. Allergy 2019, 74, 1985–1989. [Google Scholar] [CrossRef]
- Deschepper, F.M.; Zoppi, R.; Pirro, M.; Hensbergen, P.J.; Dall’Olio, F.; Kotsisas, M.; Gardner, R.A.; Spencer, D.I.R.; Videira, P.A. L1CAM as an E-selectin Ligand in Colon Cancer. Int. J. Mol. Sci. 2020, 21, 8286. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Schwab, I.; Lux, A.; Nimmerjhn, F. The role of sialic acid as a modulator of the anti-inflammatory activity of IgG. Semin. Immunopathol. 2012, 34, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Majewska, N.I.; Tajeda, M.L.; Betenbaugh, M.J.; Agarwal, N. N-Glycosylation of IgG and IgG-Like Recombinant Therapeutic Proteins: Why Is It Important and How Can We Control It? Annu. Rev. Chem. Biomol. Eng. 2020, 11, 311–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.T. IgG Fc Glycosylation in Human Immunity. Curr. Top Microbiol. Immunol. 2019, 423, 63–75. [Google Scholar] [PubMed]
- Wang, X.; Mathieu, M.; Brezski, R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell 2018, 9, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [Green Version]
- Peipp, M.; van Bueren, J.J.L.; Schneider-Merck, T.; Bleeker, W.W.K.; Dechant, M.; Beyer, T.; Repp, R.; var Berkel, P.H.C.; Vink, T.; van de Winkel, J.G.J.; et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood 2008, 112, 2390–2399. [Google Scholar] [CrossRef]
- Okazaki, A.; Shoji-Hosaka, E.; Nakamura, K.; Wakitani, M.; Uchida, K.; Kakita, S.; Tsumoto, K.; Kumagai, I.; Shitara, K. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J. Mol. Biol. 2004, 336, 1239–1249. [Google Scholar] [CrossRef]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef] [Green Version]
- Parekh, R.B.; Dwek, R.A.; Sutton, B.J.; Fernandes, D.L.; Leung, A.; Stanworth, D.; Rademacher, T.W.; Mizuochi, T.; Taniguchi, T.; Matsuda, K.; et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 1985, 316, 452–457. [Google Scholar] [CrossRef]
- Tomana, M.; Schrohenloher, R.E.; Koopman, W.J.; Alarcän, G.S.; Paul, W.A. Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis Rheum. 1988, 31, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.C.; Ilieva, K.M.; Visconti, A.; Beaumont, M.; Kiddle, S.J.; Debson, R.J.B.; Mangino, M.; Lim, E.M.; Pezer, M.; Stevens, C.J.; et al. Dysregulated Antibody, Natural Killer Cell and Immune Mediator Profiles in Autoimmune Thyroid Diseases. Cells 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Dammen-Brower, K.; Epler, P.; Zhu, S.; Bernstein, Z.J.; Stabach, P.R.; Braddock, D.T.; Spangler, J.B.; Yarema, K.J. Strategies for Glycoengineering Therapeutic Proteins. Front Chem. 2022, 10, 863118. [Google Scholar]


| Peak Number | Observed MS | Calculated MS | Predicted Monosaccharide Composition | Suggested Structure |
|---|---|---|---|---|
| 1 | 740.42 2+ | 740.33 2+ | H3N3F1-PROC | 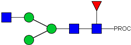 |
| 2, 3 | 841.95 2+ | 841.87 2+ | H3N4F1-PROC | 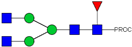 |
| 4, 5 | 727.90 2+ | 727.81 2+ | H5N2-PROC | 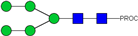 |
| 6 | 943.50 2+ | 943.41 2+ | H3N5F1-PROC | 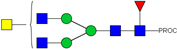 |
| 7 | 943.50 2+ | 943.41 2+ | H3N5F1-PROC | 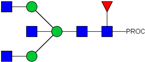 |
| 8 | 922.98 2+ | 922.89 2+ | H4N4F1-PROC | 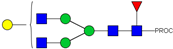 |
| 9 | 914.97 2+ | 914.89 2+ | H3N4F2-PROC | 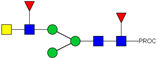 |
| 10 | 922.98 2+ | 922.89 2+ | H4N4F1-PROC | 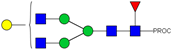 |
| 11 | 943.50 2+ | 943.41 2+ | H3N5F1-PROC | 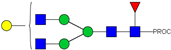 |
| 1045.05 2+ | 1044.94 2+ | H3N6F1-PROC | 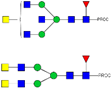 | |
| 12 | 808.92 2+ | 808.84 2+ | H6N2-PROC | 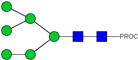 |
| 13 | 683.37 3+ | 683.29 3+ | H4N5F1-PROC | 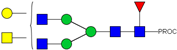 |
| 678.03 3+ | 677.96 3+ | H3N5F2-PROC |  | |
| 14 | 683.36 3+ | 683.29 3+ | H4N5F1-PROC | 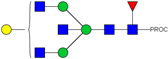 |
| 15 | 683.37 3+ | 683.29 3+ | H4N5F1-PROC | 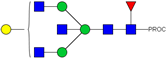 |
| 726.39 3+ | 726.30 3+ | H3N5F1S1-PROC | 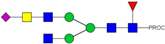 | |
| 16 | 996.02 2+ | 995.92 2+ | H4N4F2-PROC | 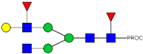 |
| 17 | 669.68 3+ | 669.61 3+ | H5N4F1 |  |
| 18 | 1024.50 2+ | 1024.43 2+ | H4N5F1-PROC | 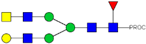 |
| 745.73 3+ | 745.65 3+ | H3N6F2-PROC |  | |
| 751.06 3+ | 750.98 3+ | H4N6F1-PROC | 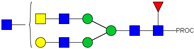 | |
| 19 | 712.72 3+ | 712.63 3+ | H4N4F1S1-PROC | 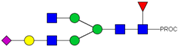 |
| 20 | 794.07 3+ | 794.00 3+ | H3N6F1S1-PROC |  |
| 21 | 889.94 2+ | 889.86 2+ | H7N2-PROC | 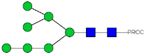 |
| 22 | 737.43 3+ | 737.31 3+ | H5N5F1-PROC | 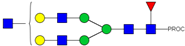 |
| 23 | 737.43 3+ | 737.31 3+ | H5N5F1-PROC | 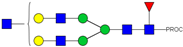 |
| 766.72 3+ | 766.65 3+ | H5N4F1S1-PROC |  | |
| 780.42 3+ | 780.32 3+ | H4N5F1S1-PROC | 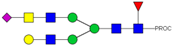 | |
| 24, 25 | 848.10 3+ | 848.02 3+ | H4N6F1S1-PROC | 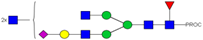 |
| 26 | 780.41 3+ | 780.32 3+ | H4N5F1S1-PROC | 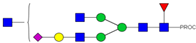 |
| 805.08 3+ | 805.00 3+ | H5N6F1-PROC |  | |
| 27 | 805.09 3+ | 805.00 3+ | H5N6F1-PROC | 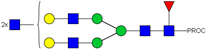 |
| 28 | 766.72 3+ | 766.65 3+ | H5N4F1S1-PROC | 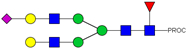 |
| 805.08 3+ | 805.00 3+ | H5N6F1-PROC | 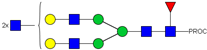 | |
| 29 | 834.43 3+ | 834.34 3+ | H5N5F1S1-PROC | 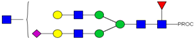 |
| 848.10 3+ | 848.02 3+ | H4N6F1S1-PROC | 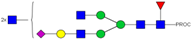 | |
| 30 | 834.43 3+ | 834.34 3+ | H5N5F1S1-PROC |  |
| 863.77 3+ | 863.68 3+ | H5N4F1S2-PROC |  | |
| 877.42 3+ | 877.35 3+ | H4N5F1S2-PROC | 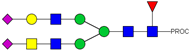 | |
| 31 | 970.95 2+ | 970.89 2+ | H8N2-PROC |  |
| 32, 33 | 902.13 3+ | 902.03 3+ | H5N6F1S1-PROC | 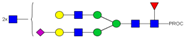 |
| 34 | 902.13 3+ | 902.03 3+ | H5N6F1S1-PROC | 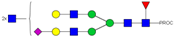 |
| 863.77 3+ | 863.68 3+ | H5N4F1S2-PROC | 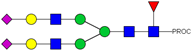 | |
| 35 | - | - | - | - |
| 36 | 859.10 3+ | 859.02 3+ | H6N6F1-PROC | 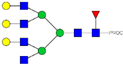 |
| 37 | 859.10 3+ | 859.02 3+ | H6N6F1-PROC | 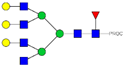 |
| 931.43 3+ | 931.37 3+ | H5N5F1S2-PROC |  | |
| 38 | 888.44 3+ | 888.36 3+ | H6N5F1S1-PROC | 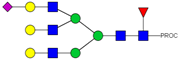 |
| 39 | 1051.97 2+ | 1051.92 2+ | H9N2-PROC | 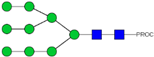 |
| 40 | 956.12 3+ | 956.05 3+ | H6N6F1S1-PROC |  |
| 999.15 3+ | 999.06 3+ | H5N6F1S2-PROC | 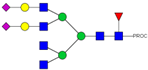 | |
| 42 | 985.47 3+ | 985.39 3+ | H6N5F1S2-PROC | 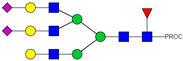 |
| 43 | 985.47 3+ | 985.39 3+ | H6N5F1S2-PROC | 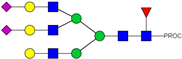 |
| 913.12 3+ | 913.04 3+ | H7N6F1-PROC | 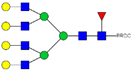 | |
| 44, 45 | 790.16 4+ | 790.06 4+ | H6N6F1S2-PROC |  |
| 46 | 1010.14 3+ | 1010.07 3+ | H7N6F1S1-PROC | 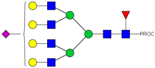 |
| 47 | 1082.48 3+ | 1082.42 3+ | H6N5F1S3-PROC | 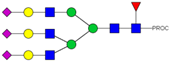 |
| 48 | - | - | - | - |
| 49 | 862.83 4+ | 862.84 4+ | H6N6F1S3-PROC | 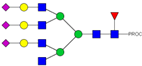 |
| 50, 51 | 830.66 4+ | 830.58 4+ | H7N6F1S2-PROC |  |
| 52, 53 | 903.44 4+ | 903.35 4+ | H7N6F1S3-PROC | 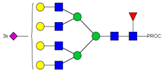 |
| 54, 55 | 976.19 4+ | 976.12 4+ | H7N6F1S4-PROC | 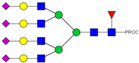 |
| Peak Number | Observed MS | Calculated MS | Predicted Monosaccharide Composition | Suggested Structure |
|---|---|---|---|---|
| 1 | 740.42 2+ | 740.33 2+ | H3N3F1-PROC | 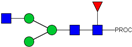 |
| 2, 3 | 841.95 2+ | 841.87 2+ | H3N4F1-PROC | 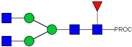 |
| 4, 5 | 727.90 2+ | 727.81 2+ | H5N2-PROC | 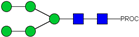 |
| 6 | 943.50 2+ | 943.41 2+ | H3N5F1-PROC |  |
| 7 | 943.50 2+ | 943.41 2+ | H3N5F1-PROC | 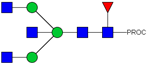 |
| 8 | 922.98 2+ | 922.89 2+ | H4N4F1-PROC |  |
| 9 | 914.97 2+ | 914.89 2+ | H3N4F2-PROC | 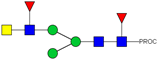 |
| 10 | 922.98 2+ | 922.89 2+ | H4N4F1-PROC | 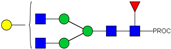 |
| 11 | 943.48 2+ | 943.41 2+ | H3N5F1-PROC | 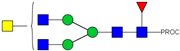 |
| 1045.05 2+ | 1044.94 2+ | H3N6F1-PROC |  | |
| 12 | 808.92 2+ | 808.84 2+ | H6N2-PROC | 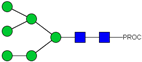 |
| 13 | 683.37 3+ | 683.29 3+ | H4N5F1-PROC | 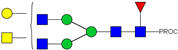 |
| 678.07 3+ | 677.96 3+ | H3N5F2-PROC |  | |
| 14 | 683.36 3+ | 683.29 3+ | H4N5F1-PROC | 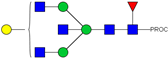 |
| 15 | 683.38 3+ | 683.29 3+ | H4N5F1-PROC | 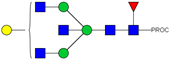 |
| 16 | 996.02 2+ | 995.92 2+ | H4N4F2-PROC | 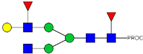 |
| 17 | 669.68 3+ | 669.61 3+ | H5N4F1-PROC | 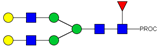 |
| 18 | 1024.48 2+ | 1024.43 2+ | H4N5F1-PROC |  |
| 745.74 3+ | 745.65 3+ | H3N6F2-PROC | 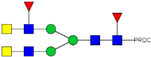 | |
| 751.06 3+ | 750.98 3+ | H4N6F1-PROC | 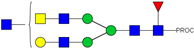 | |
| 20, 21 | 889.94 2+ | 889.86 2+ | H3N6F1S1-PROC |  |
| 22, 23 | 737.40 3+ | 737.31 3+ | H5N5F1-PROC | 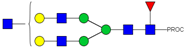 |
| 26, 27, 28 | 805.08 3+ | 805.00 3+ | H5N6F1-PROC | 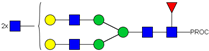 |
| 30 | 791.42 3+ | 791.33 3+ | H5N5F1S1-PROC | 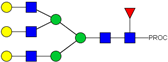 |
| 31 | 970.95 2+ | 970.89 2+ | H8N2-PROC | 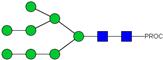 |
| 32 | 791.42 3+ | 791.33 3+ | H5N5F1S1-PROC | 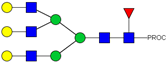 |
| 36, 37, 38 | 859.10 3+ | 859.02 3+ | H6N6F1-PROC | 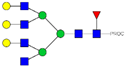 |
| 39 | 1051.97 2+ | 1051.92 2+ | H9N2-PROC | 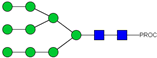 |
| 40, 41 | 926.78 3+ | 926.71 3+ | H6N6F1S1-PROC | 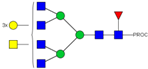 |
| 42, 43, 44 | 913.12 3+ | 913.04 3+ | H7N6F1-PROC | 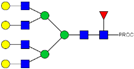 |
| Buffer Name | Preparation |
|---|---|
| Buffer 1 | 50 mM Sodium Citrate + 50 mM Sodium Chloride, pH 3.5 |
| Buffer 2 | 0.1 M Glycine, pH 2.3 |
| Buffer 3 | 20 mM Citric Acid, pH 3.0 |
| Neutralization Buffer | 1 M Tris, pH 8.2 |
| Calcium Buffer | 0.1 mM CaCl2 |
| FACS Buffer | 1× HBSS, 2% FBS |
| Reducing Buffer | 50 mM Dithiothreitol (DTT) in 4× Laemmli Protein Sample Buffer |
| T-PBS | 0.1% Tween + PBS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCraw, A.J.; Gardner, R.A.; Davies, A.M.; Spencer, D.I.R.; Grandits, M.; Wagner, G.K.; McDonnell, J.M.; Karagiannis, S.N.; Chenoweth, A.; Crescioli, S. Generation and Characterization of Native and Sialic Acid-Deficient IgE. Int. J. Mol. Sci. 2022, 23, 13455. https://doi.org/10.3390/ijms232113455
McCraw AJ, Gardner RA, Davies AM, Spencer DIR, Grandits M, Wagner GK, McDonnell JM, Karagiannis SN, Chenoweth A, Crescioli S. Generation and Characterization of Native and Sialic Acid-Deficient IgE. International Journal of Molecular Sciences. 2022; 23(21):13455. https://doi.org/10.3390/ijms232113455
Chicago/Turabian StyleMcCraw, Alex J., Richard A. Gardner, Anna M. Davies, Daniel I. R. Spencer, Melanie Grandits, Gerd K. Wagner, James M. McDonnell, Sophia N. Karagiannis, Alicia Chenoweth, and Silvia Crescioli. 2022. "Generation and Characterization of Native and Sialic Acid-Deficient IgE" International Journal of Molecular Sciences 23, no. 21: 13455. https://doi.org/10.3390/ijms232113455
APA StyleMcCraw, A. J., Gardner, R. A., Davies, A. M., Spencer, D. I. R., Grandits, M., Wagner, G. K., McDonnell, J. M., Karagiannis, S. N., Chenoweth, A., & Crescioli, S. (2022). Generation and Characterization of Native and Sialic Acid-Deficient IgE. International Journal of Molecular Sciences, 23(21), 13455. https://doi.org/10.3390/ijms232113455










