Ontogenetic Changes in the Expression of the Lin28 Protein in the Rat Hypothalamic Tuberal Nuclei
Abstract
1. Introduction
2. Results
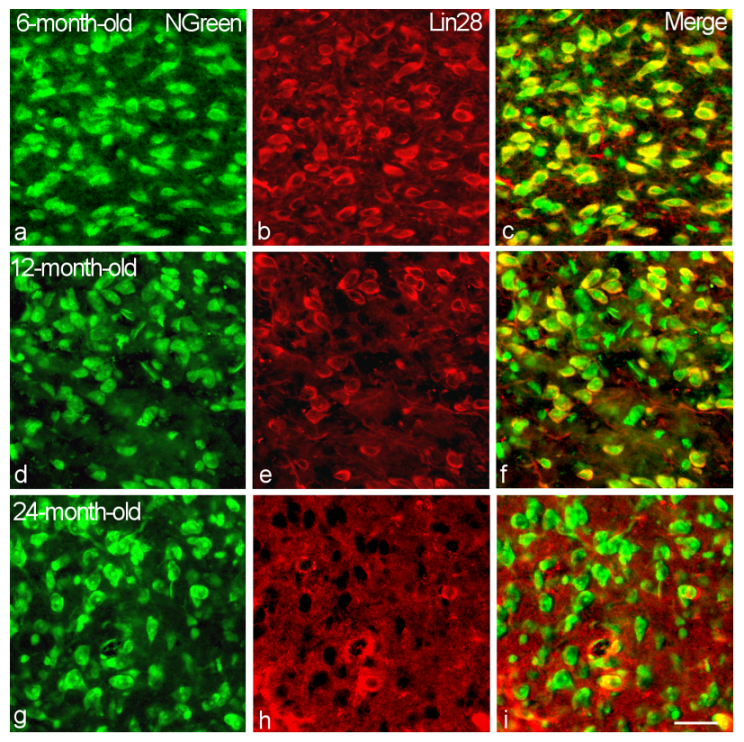
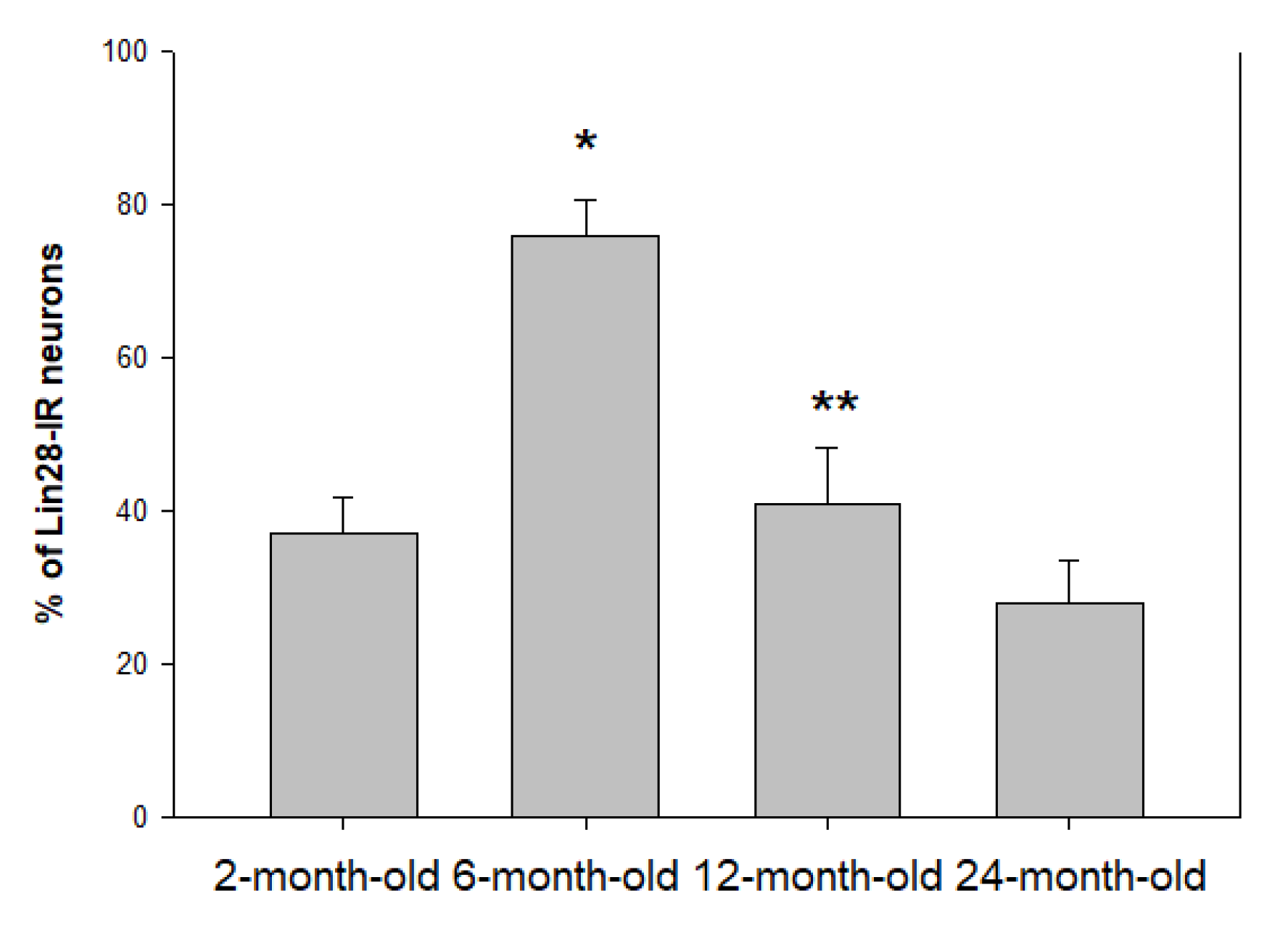
2.1. Location and Percentage of Lin28-IR Neurons
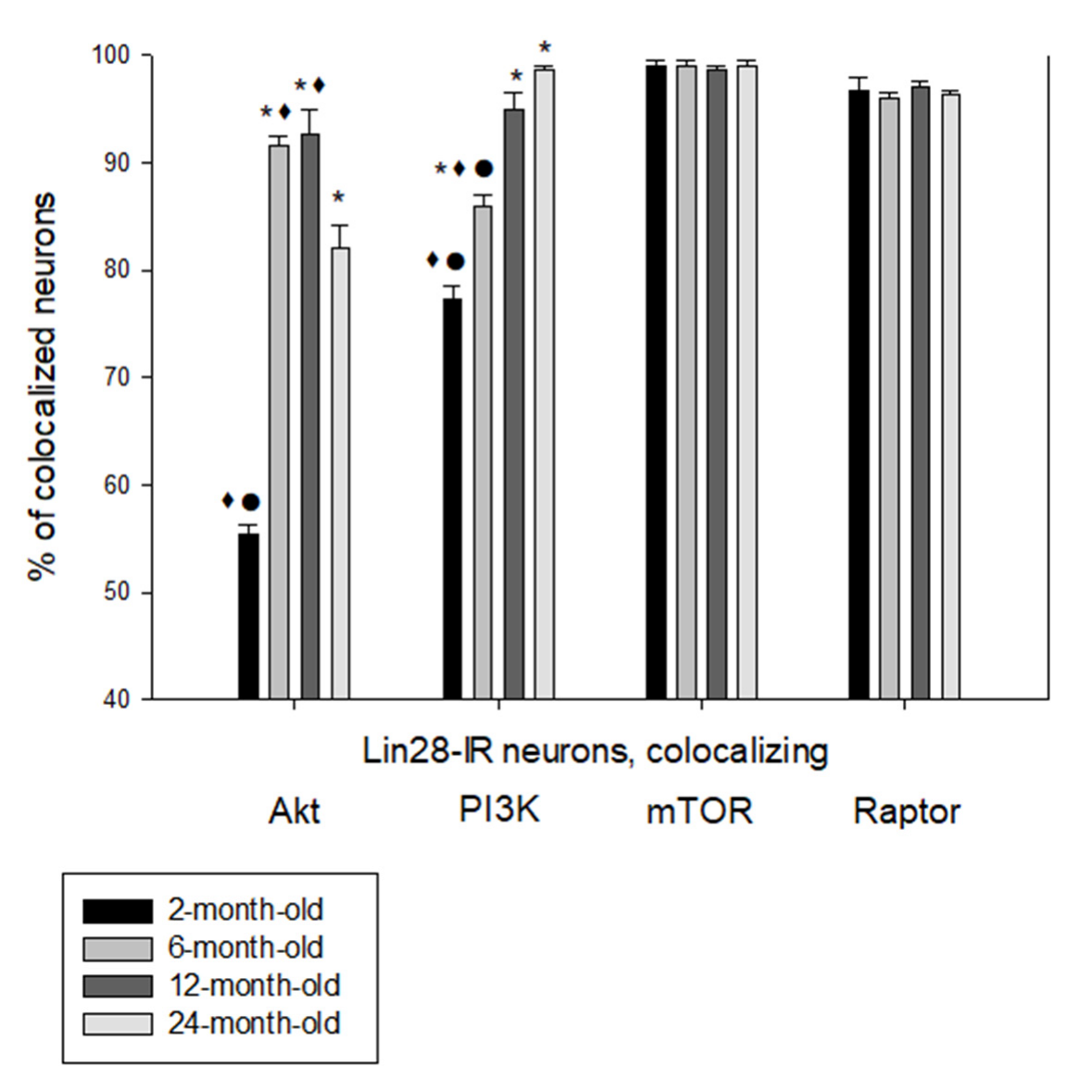
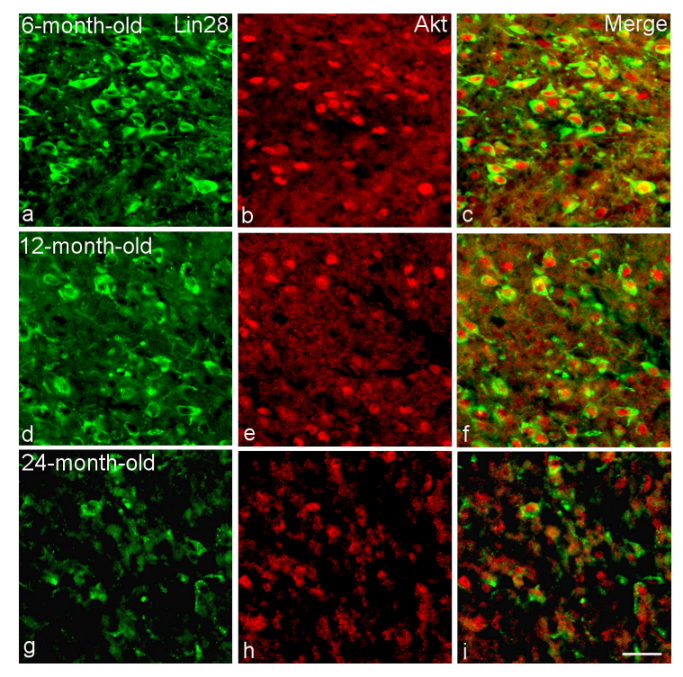
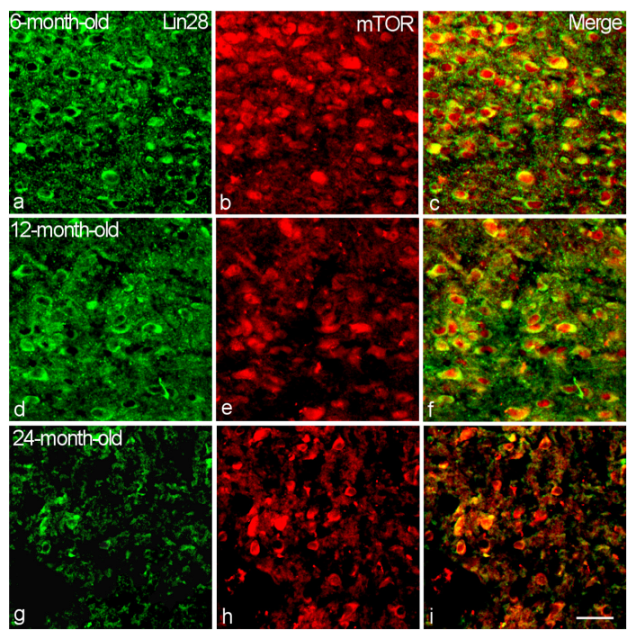
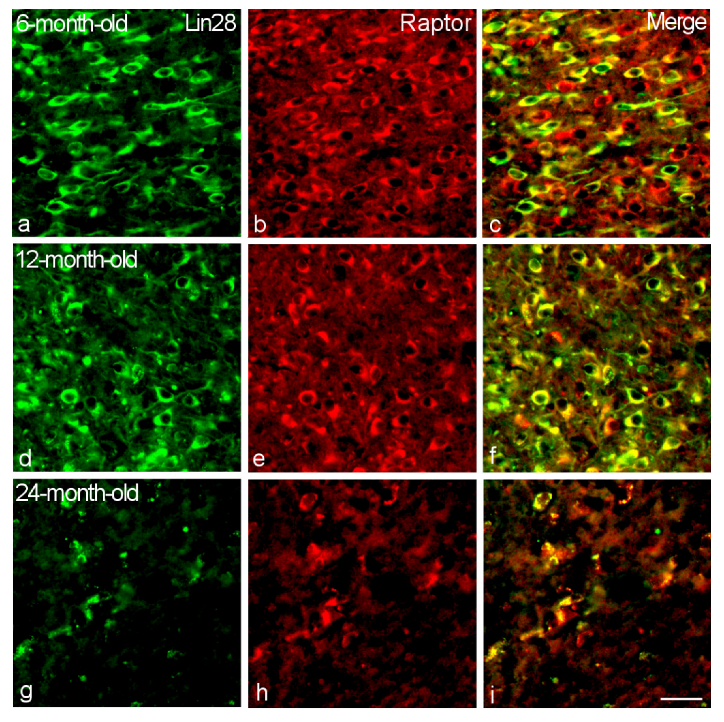
2.2. Colocalization of Lin28-IR Neurons with Components of Insulin Signaling
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Immunohistochemistry
4.3. Image Acquisition
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambros, V.; Horvitz, H.R. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 1984, 226, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.R.; Daley, G.Q.; Gregory, R.I. Selective blockade of microRNA processing by Lin28. Science 2008, 320, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Polytarchou, C.; Thornton, J.E.; LaPierre, R.J.; Pothoulakis, C.; Hagan, J.P.; Iliopoulos, D.; Gregory, R.I. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell 2011, 147, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. A mirror of two faces: Lin28 as a master regulator of both miRNA and mRNA. Wiley Interdiscip. Rev. RNA 2012, 3, 483–494. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Katsura, A.; Miyazono, K. A role of uridylation pathway for blockade of let-7 microRNA biogenesis by Lin28B. Cancer Sci. 2015, 106, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- BÜssing, I.; Slack, F.J.; Grosshans, H. let-7 microRNAs in development, stem cells and cancer. Trends Mol. Med. 2008, 14, 400–409. [Google Scholar] [CrossRef]
- Lin, Z.; Radaeva, M.; Cherkasov, A.; Dong, X. Lin28 Regulates Cancer Cell Stemness for Tumour Progression. Cancers 2022, 14, 4640. [Google Scholar] [CrossRef]
- Zhu, H.; Shyh-Chang, N.; Segrè, A.V.; Shinoda, G.; Shah, S.P.; Einhorn, W.S.; Takeuchi, A.; Engreitz, J.M.; Hagan, J.P.; Kharas, M.G.; et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011, 147, 81–94. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Zhu, H. Lin28 and let-7 in cell metabolism and cancer. Transl. Pediatr. 2015, 4, 4–11. [Google Scholar] [CrossRef]
- Wu, K.; Ahmad, T.; Eri, R. LIN28A: A multifunctional versatile molecule with future therapeutic potential. World J. Biol. Chem. 2022, 13, 35–46. [Google Scholar] [CrossRef]
- Jun-Hao, E.T.; Gupta, R.R.; Shyh-Chang, N. Lin28 and let-7 in the Metabolic Physiology of Aging. Trends Endocrinol. Metab. 2016, 27, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Chen, S.; Li, D.; Yang, J.; Zhao, X.; Qin, M.; Guo, M.; Chen, C.; He, Z.; et al. Let-7 as a Promising Target in Aging and Aging-Related Diseases: A Promise or a Pledge. Biomolecules 2022, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, G.; Shyh-Chang, N.; Soysa, T.Y.; Zhu, H.; Seligson, M.T.; Shah, S.P.; Abo-Sido, N.; Yabuuchi, A.; Hagan, J.P.; Gregory, R.I.; et al. Fetal deficiency of lin28 programs life-long aberrations in growth and glucose metabolism. Stem Cells 2013, 31, 1563–1573. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Lamming, D.W. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016, 23, 990–1003. [Google Scholar] [CrossRef]
- Kim, J.D.; Toda, C.; Ramírez, C.M.; Fernández-Hernando, C.; Diano, S. Hypothalamic Ventromedial Lin28a Enhances Glucose Metabolism in Diet-Induced Obesity. Diabetes 2017, 66, 2102–2111. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, M.S.; Jia, B.; Yan, J.; Zuniga-Hertz, J.P.; Han, C.; Cai, D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 2017, 548, 52–57. [Google Scholar] [CrossRef]
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79. [Google Scholar] [CrossRef]
- Masliukov, P.M.; Nozdrachev, A.D. Hypothalamic Regulatory Mechanisms of Aging. J. Evol. Biochem. Phys. 2021, 57, 473–491. [Google Scholar] [CrossRef]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef]
- Tran, L.T.; Park, S.; Kim, S.K.; Lee, J.S.; Kim, K.W.; Kwon, O. Hypothalamic control of energy expenditure and thermogenesis. Exp. Mol. Med. 2022, 54, 358–369. [Google Scholar] [CrossRef]
- Moiseev, K.Y.; Vishnyakova, P.A.; Porseva, V.V.; Masliukov, A.P.; Spirichev, A.A.; Emanuilov, A.I.; Masliukov, P.M. Changes of nNOS expression in the tuberal hypothalamic nuclei during ageing. Nitric Oxide 2020, 100–101, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakova, P.A.; Moiseev, K.Y.; Spirichev, A.A.; Emanuilov, A.I.; Nozdrachev, A.D.; Masliukov, P.M. Expression of calbindin and calretinin in the dorsomedial and ventromedial hypothalamic nuclei during aging. Anat. Rec. 2021, 304, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Sangiao-Alvarellos, S.; Manfredi-Lozano, M.; Ruiz-Pino, F.; Navarro, V.M.; Sánchez-Garrido, M.A.; Leon, S.; Dieguez, C.; Cordido, F.; Matagne, V.; Dissen, G.A.; et al. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty. Endocrinology 2013, 154, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Varela, L.; Martínez-Sánchez, N.; Gallego, R.; Vázquez, M.J.; Roa, J.; Gándara, M.; Schoenmakers, E.; Nogueiras, R.; Chatterjee, K.; Tena-Sempere, M.; et al. Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J. Pathol. 2012, 227, 209–222. [Google Scholar] [CrossRef]
- Anfimova, P.A.; Moiseev, K.Y.; Porseva, V.V.; Pankrasheva, L.G.; Masliukov, P.M. mTOR Expression in Neurons of the Rat Tuberal Hypothalamus in Aging. J. Evol. Biochem. Physiol. 2022, 58, 1464–1470. [Google Scholar] [CrossRef]
- Vellai, T.; Takacs-Vellai, K.; Zhang, Y.; Kovacs, A.L.; Orosz, L.; Müller, F. Genetics: Influence of TOR kinase on lifespan in C. elegans. Nature 2003, 426, 620. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Robida-Stubbs, S.; Glover-Cutter, K.; Lamming, D.W.; Mizunuma, M.; Narasimhan, S.D.; Neumann-Haefelin, E.; Sabatini, D.M.; Blackwell, T.K. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012, 15, 713–724. [Google Scholar] [CrossRef]
- Jiang, S. A Regulator of Metabolic Reprogramming: MicroRNA Let-7. Transl. Oncol. 2019, 12, 1005–1013. [Google Scholar] [CrossRef]
- Porseva, V.V.; Levshin, N.Y.; Moiseev, K.Y.; Pankrasheva, L.G.; Baranov, A.A.; Pavlov, A.V.; Nozdrachev, A.D.; Masliukov, P.M. Let-7a, mir-9, mir-132, and mir-218 microRNA Expression in the Dorsomedial and Ventromedial Hypothalamic Nuclei during Aging in Rats. Adv. Gerontol. 2021, 11, 346–350. [Google Scholar] [CrossRef]
- Yang, M.; Yang, S.L.; Herrlinger, S.; Liang, C.; Dzieciatkowska, M.; Hansen, K.C.; Desai, R.; Nagy, A.; Niswander, L.; Moss, E.G.; et al. Lin28 promotes the proliferative capacity of neural progenitor cells in brain development. Development 2015, 142, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Khor, S. Hypothalamic microinflammation. Handb Clin. Neurol. 2021, 181, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, Y.; Yi, C.X.; Tong, Q.; Cai, D.; Liu, T.; Xu, Y.; Yi, C.X.; Tong, Q.; Cai, D. The hypothalamus for whole-body physiology: From metabolism to aging. Protein Cell 2022, 13, 394–421. [Google Scholar] [CrossRef] [PubMed]
- Cavadas, C.; Aveleira, C.A.; Souza, G.F.; Velloso, L.A. The pathophysiology of defective proteostasis in the hypothalamus - from obesity to ageing. Nat. Rev. Endocrinol. 2016, 12, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Bozadjieva-Kramer, N.; Ross, R.A.; Johnson, D.Q.; Fenselau, H.; Haggerty, D.L.; Atwood, B.; Lowell, B.; Flak, J.N. The Role of Mediobasal Hypothalamic PACAP in the Control of Body Weight and Metabolism. Endocrinology 2021, 162, bqab012. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Quinn, B.; Toga, A.W.; Motamed, S.; Merlic, C.A. Fluoro nissl green: A novel fluorescent counterstain for neuroanatomy. Neurosci. Lett. 1995, 184, 169–172. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anfimova, P.A.; Pankrasheva, L.G.; Moiseev, K.Y.; Shirina, E.S.; Porseva, V.V.; Masliukov, P.M. Ontogenetic Changes in the Expression of the Lin28 Protein in the Rat Hypothalamic Tuberal Nuclei. Int. J. Mol. Sci. 2022, 23, 13468. https://doi.org/10.3390/ijms232113468
Anfimova PA, Pankrasheva LG, Moiseev KY, Shirina ES, Porseva VV, Masliukov PM. Ontogenetic Changes in the Expression of the Lin28 Protein in the Rat Hypothalamic Tuberal Nuclei. International Journal of Molecular Sciences. 2022; 23(21):13468. https://doi.org/10.3390/ijms232113468
Chicago/Turabian StyleAnfimova, Polina A., Lydia G. Pankrasheva, Konstantin Yu. Moiseev, Elizaveta S. Shirina, Valentina V. Porseva, and Petr M. Masliukov. 2022. "Ontogenetic Changes in the Expression of the Lin28 Protein in the Rat Hypothalamic Tuberal Nuclei" International Journal of Molecular Sciences 23, no. 21: 13468. https://doi.org/10.3390/ijms232113468
APA StyleAnfimova, P. A., Pankrasheva, L. G., Moiseev, K. Y., Shirina, E. S., Porseva, V. V., & Masliukov, P. M. (2022). Ontogenetic Changes in the Expression of the Lin28 Protein in the Rat Hypothalamic Tuberal Nuclei. International Journal of Molecular Sciences, 23(21), 13468. https://doi.org/10.3390/ijms232113468









