Pharmacogenomics in Psychiatry Practice: The Value and the Challenges
Abstract
:1. Introduction
1.1. CYP450 Genetic and Phenotypic Variations
| Star Allele Functionality | Diplotype Functionality | ||
|---|---|---|---|
| CYP2D6 | |||
| Type of Allele | Examples of Alleles | Phenotype | Diplotype |
| Normal function | *1, *2 | Normal metabolizer | *1/*1, *1/*2 |
| Decreased function | *9, *10, *17, *41 | Intermediate metabolizer | *1/*3, *9/*17 |
| No function | *3, *4, *5, *6 | Ultrarapid metabolizer | *1/*1 × 2 |
| CYP2C9 | |||
| Type of Allele | Examples of Alleles | Phenotype | Diplotype |
| Normal function | *1 | Normal metabolizer | *1/*1 |
| Decreased function | *2, *5, *8, *11 | Intermediate metabolizer | *1/*2, *1/*11 |
| No function | *3, *6, *13 | Poor metabolizer | *2/*3, *3/*5 |
| CYP2C19 | |||
| Type of Allele | Examples of Alleles | Phenotype | Diplotype |
| Normal function | *1 | Normal metabolizer | *1/*1 |
| Increased function | *17 | Intermediate metabolizer | *1/*2, *1/*3 |
| No function | *2, *3 | Rapid metabolizer | *17/*17 |
| CYP2B6 | |||
| Type of Allele | Examples of Alleles | Phenotype | Diplotype |
| Normal function | *1, *2, *5 | Normal metabolizer | *1/*1, *1/*2 |
| Increased function | *4 | Intermediate metabolizer | *4/*8 |
| No function | *8 | Ultrarapid metabolizer | *4/*4 |

1.2. CYP450 Genetic Variations and Antidepressants
| Main Enzyme | Medications and Transmitters Affected (Paired to the Gene) | ||||
|---|---|---|---|---|---|
| Anti-Depressant | Anti-Psychotics | Mood-Stabilizer | Neurotransmitter | References | |
| CYP2C19 | Imipramine, Amitriptyline, Trimipramine, Clomipramine, Citalopram, Escitalopram | - | - | Dopamine and Serotonin | [28,29] |
| CYP2D6 | Amitriptyline, Fluvoxamine, Paroxetine, Venlafaxine, Fluoxetine, Mirtazapine, Vortioxetine. | Aripiprazole, Risperidone, Brexpiprazole | - | Dopamine | [15,35,38] |
| CYP1A2 | Amitriptyline, Clomipramine, Trimipramine, Imipramine, Doxepin, Mirtazapine. | Olanzapine, Asenapine, Clozapine | - | - | [39,40] |
| CYP3A4 | Mirtazapine | Haloperidol, Clozapine, Aripiprazole, Quetiapine, Levomepromazine, Brexpiprazole | Carbamazepine | - | [39,41,42] |
| CYP2C9 | - | - | Valproic acid | - | [43] |
| CYP2C8 | - | - | Carbamazepine | - | [44] |
| CYP2B6 | Sertraline | - | - | - | [45] |
1.3. CYP450 Genetic Variations and Antipsychotics
1.4. CYP450 Genetic Variations and Mood Stabilizers
2. The Influence of CYP2D6 and CYP2C19 Variants on Neurotransmitters
2.1. Dopamine Synthesis via CYP2D6
2.2. Serotonin Metabolism via CYP2D6 and CYP2C19
3. Human Leukocyte Antigen (HLA) Gene in Psychiatry
4. Gene Variants Predicting Predisposition to Obesity and Metabolic Syndrome
4.1. Genes Associated with Obesity
4.2. Genes Associated with Metabolic Syndrome
4.2.1. ADRA1A
4.2.2. eNOS
5. Impact of Variability in SNP Frequency among Ethnicities
5.1. CYP2D6 Isoenzyme
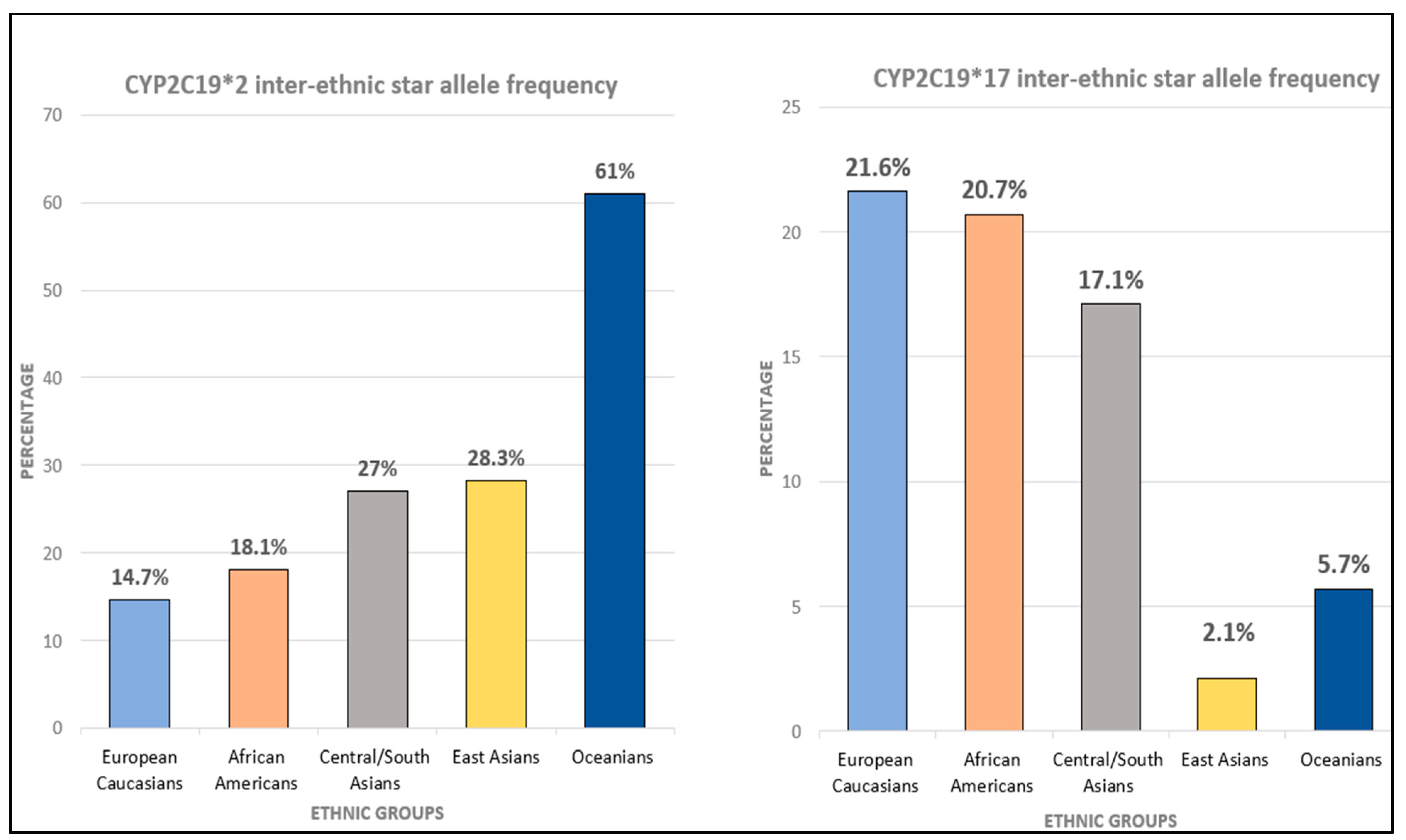
5.2. Ethnic Variation of CYP2C19
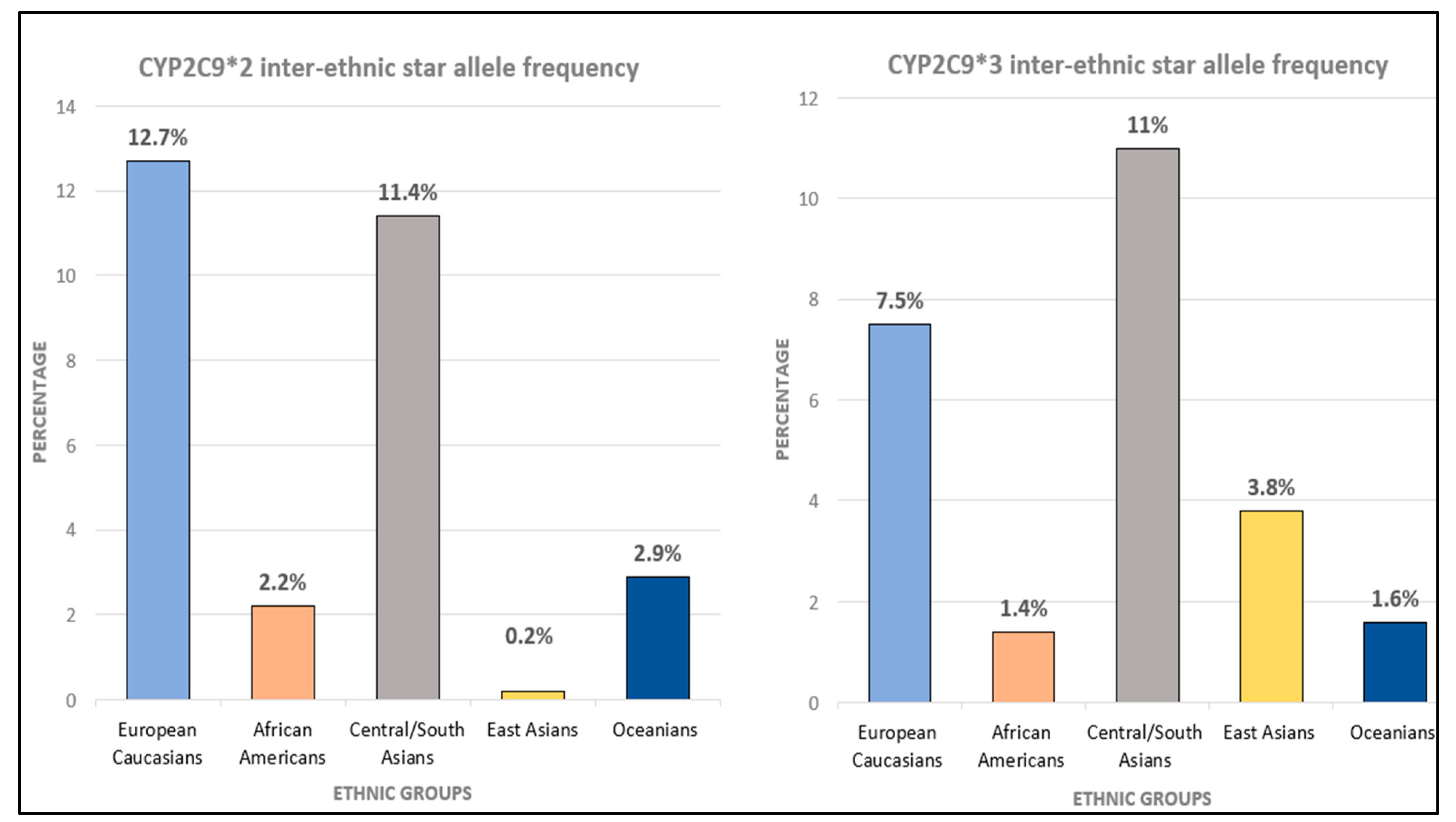
5.3. Ethnic Variation of CYP2C9
5.4. Ethnic Variation of HLA (Human Leukocyte Antigen)
6. Non-Genetic Factors Influencing Cytochrome P450s Activity
7. Pharmacogenomics and the MENA Region
8. Pharmacogenomics in Real World of Psychiatry Practice
9. Challenges
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, K.; De Torres, I. A world of depression. Nature 2014, 515, 180–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrman, H.; Kieling, C.; McGorry, P.; Horton, R.; Sargent, J.; Patel, V. Reducing the global burden of depression: A Lancet–World Psychiatric Association Commission. Lancet 2019, 393, e42–e43. [Google Scholar] [CrossRef]
- Shalimova, A.; Babasieva, V.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B.; Mwinyi, J. Therapy response prediction in major depressive disorder: Current and novel genomic markers influencing pharmacokinetics and pharmacodynamics. Pharmacogenomics 2021, 22, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.M.D. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar]
- Yang, Y.; Botton, M.R.; Scott, E.R.; Scott, S.A. Sequencing the CYP2D6 gene: From variant allele discovery to clinical pharmacogenetic testing. Pharmacogenomics 2017, 18, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [CrossRef]
- Sims, R.J., 3rd; Chen, C.F.; Santos-Rosa, H.; Kouzarides, T.; Patel, S.S.; Reinberg, D. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J. Biol. Chem. 2005, 280, 41789–41792. [Google Scholar] [CrossRef] [Green Version]
- Sim, S.C.; Ingelman-Sundberg, M. The Human Cytochrome P450 (CYP) Allele Nomenclature website: A peer-reviewed database of CYP variants and their associated effects. Hum. Genom. 2010, 4, 278. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.-F. Polymorphism of human cytochrome P450 2D6 and its clinical significance. Clin. Pharmacokinet. 2009, 48, 761–804. [Google Scholar] [CrossRef]
- Lin, E.; Lin, C.-H.; Lane, H.-Y. Precision psychiatry applications with pharmacogenomics: Artificial intelligence and machine learning approaches. Int. J. Mol. Sci. 2020, 21, 969. [Google Scholar] [CrossRef] [Green Version]
- Darney, K.; Lautz, L.; Béchaux, C.; Wiecek, W.; Testai, E.; Amzal, B.; Dorne, J. Human variability in polymorphic CYP2D6 metabolism: Implications for the risk assessment of chemicals in food and emerging designer drugs. Environ. Int. 2021, 156, 106760. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, A.M.; Dang, C.H. Basic review of the cytochrome p450 system. J. Adv. Pract. Oncol. 2013, 4, 263. [Google Scholar] [PubMed]
- Stavropoulou, E.; Pircalabioru, G.G.; Bezirtzoglou, E. The role of cytochromes P450 in infection. Front. Immunol. 2018, 9, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guengerich, F.P.; Waterman, M.R.; Egli, M. Recent structural insights into cytochrome P450 function. Trends Pharmacol. Sci. 2016, 37, 625–640. [Google Scholar] [CrossRef] [Green Version]
- Hicks, J.K.; Swen, J.J.; Thorn, C.F.; Sangkuhl, K.; Kharasch, E.D.; Ellingrod, V.L.; Skaar, T.C.; Müller, D.J.; Gaedigk, A.; Stingl, J.C. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Tricyclic Antidepressants. Clin. Pharmacol. Ther. 2013, 93, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Gopisankar, M.G. CYP2D6 pharmacogenomics. Egypt. J. Med. Hum. Genet. 2017, 18, 309–313. [Google Scholar] [CrossRef]
- Wang, H.; An, N.; Wang, H.; Gao, Y.; Liu, D.; Bian, T.; Zhu, J.; Chen, C. Evaluation of the effects of 20 nonsynonymous single nucleotide polymorphisms of CYP2C19 on S-mephenytoin 4′-hydroxylation and omeprazole 5′-hydroxylation. Drug Metab. Dispos. 2011, 39, 830–837. [Google Scholar] [CrossRef]
- Trojer, P.; Li, G.; Sims, R.J., 3rd; Vaquero, A.; Kalakonda, N.; Boccuni, P.; Lee, D.; Erdjument-Bromage, H.; Tempst, P.; Nimer, S.D.; et al. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell 2007, 129, 915–928. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, J.P.; Peter, A.P.; Shaman, J.A. Consequences of CYP2D6 copy-number variation for pharmacogenomics in psychiatry. Front. Psychiatry 2019, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Häkkinen, K.; Kiiski, J.I.; Lähteenvuo, M.; Jukuri, T.; Suokas, K.; Niemi-Pynttäri, J.; Kieseppä, T.; Männynsalo, T.; Wegelius, A.; Haaki, W. Implementation of CYP2D6 copy-number imputation panel and frequency of key pharmacogenetic variants in Finnish individuals with a psychotic disorder. Pharm. J. 2022, 22, 166–172. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An evidence-based framework for evaluating pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrell, P.B.; McLeod, H.L. Carbamazepine, HLA-B* 1502 and risk of Stevens–Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics 2008, 9, 1543–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huddart, R.; Fohner, A.E.; Whirl-Carrillo, M.; Wojcik, G.L.; Gignoux, C.R.; Popejoy, A.B.; Bustamante, C.D.; Altman, R.B.; Klein, T.E. Standardized biogeographic grouping system for annotating populations in pharmacogenetic research. Clin. Pharmacol. Ther. 2019, 105, 1256–1262. [Google Scholar] [CrossRef]
- PharmGKB. PharmGKB Biogeographical Groups. Available online: https://www.pharmgkb.org/page/biogeographicalGroups (accessed on 7 February 2022).
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
- Yasui-Furukori, N.; Nakagami, T.; Kaneda, A.; Inoue, Y.; Suzuki, A.; Otani, K.; Kaneko, S. Inverse correlation between clinical response to paroxetine and plasma drug concentration in patients with major depressive disorders. Hum. Psychopharmacol. Clin. Exp. 2011, 26, 602–608. [Google Scholar] [CrossRef]
- Vo, N.; Goodman, R.H. CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 2001, 276, 13505–13508. [Google Scholar] [CrossRef] [Green Version]
- Fricke-Galindo, I.; Céspedes-Garro, C.; Rodrigues-Soares, F.; Naranjo, M.; Delgado, A.; De Andrés, F.; López-López, M.; Peñas-Lledó, E.; LLerena, A. Interethnic variation of CYP2C19 alleles,‘predicted’phenotypes and ‘measured’metabolic phenotypes across world populations. Pharm. J. 2016, 16, 113–123. [Google Scholar]
- Schenk, P.; van Vliet, M.; Mathot, R.; van Gelder, T.; Vulto, A.; Van Fessem, M.; Rij, V.-V.; Lindemans, J.; Bruijn, J.; Van Schaik, R. The CYP2C19* 17 genotype is associated with lower imipramine plasma concentrations in a large group of depressed patients. Pharm. J. 2010, 10, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Kirchheiner, J.; Nickchen, K.; Bauer, M.; Wong, M.; Licinio, J.; Roots, I.; Brockmöller, J. Pharmacogenetics of antidepressants and antipsychotics: The contribution of allelic variations to the phenotype of drug response. Mol. Psychiatry 2004, 9, 442–473. [Google Scholar] [PubMed]
- Rudberg, I.; Mohebi, B.; Hermann, M.; Refsum, H.; Molden, E. Impact of the ultrarapid CYP2C19* 17 allele on serum concentration of escitalopram in psychiatric patients. Clin. Pharmacol. Ther. 2008, 83, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Adams-Cioaba, M.A.; Min, J. Structure and function of histone methylation binding proteins. Biochem. Cell Biol. Biochim. Biol. Cell. 2009, 87, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Imipramine Therapy and CYP2D6 and CYP2C19 Genotype. In Medical Genetics Summaries; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2017. [Google Scholar]
- Khalid, M.M.; Waseem, M. Tricyclic Antidepressant Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Anttila, S.A.; Leinonen, E.V. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001, 7, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Citrome, L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: A systematic review of the efficacy and safety profile for this newly approved antipsychotic–what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int. J. Clin. Pract. 2015, 69, 978–997. [Google Scholar] [PubMed]
- Brittain, H.G. Profiles of Drug Substances, Excipients, and Related Methodology; Academic press: Cambridge, MA, USA, 2020; Volume 45. [Google Scholar]
- Ayano, G. Psychotropic medications metabolized by cytochromes P450 (CYP1A2) enzyme and relevant drug interactions: Review of articles. Austin J. Pharmacol. Ther. 2016, 4, 2–5. [Google Scholar]
- DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00502 (accessed on 10 July 2022).
- DrugBank Online. Available online: https://go.drugbank.com/drugs/DB09128 (accessed on 10 July 2022).
- Monostory, K.; Nagy, A.; Tóth, K.; Bűdi, T.; Kiss, Á.; Déri, M.; Csukly, G. Relevance of CYP2C9 function in valproate therapy. Curr. Neuropharmacol. 2019, 17, 99–106. [Google Scholar] [CrossRef]
- Fuhr, L.M.; Marok, F.Z.; Hanke, N.; Selzer, D.; Lehr, T. Pharmacokinetics of the CYP3A4 and CYP2B6 Inducer Carbamazepine and Its Drug–Drug Interaction Potential: A Physiologically Based Pharmacokinetic Modeling Approach. Pharmaceutics 2021, 13, 270. [Google Scholar] [CrossRef]
- Obach, R.S.; Cox, L.M.; Tremaine, L.M. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: An in vitro study. Drug Metab. Dispos. 2005, 33, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Altar, C.A.; Hornberger, J.; Shewade, A.; Cruz, V.; Garrison, J.; Mrazek, D. Clinical validity of cytochrome P450 metabolism and serotonin gene variants in psychiatric pharmacotherapy. Int. Rev. Psychiatry 2013, 25, 509–533. [Google Scholar] [CrossRef] [PubMed]
- Walden, L.M.; Brandl, E.J.; Tiwari, A.K.; Cheema, S.; Freeman, N.; Braganza, N.; Kennedy, J.L.; Müller, D.J. Genetic testing for CYP2D6 and CYP2C19 suggests improved outcome for antidepressant and antipsychotic medication. Psychiatry Res. 2019, 279, 111–115. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/2015 (accessed on 8 June 2022).
- Ravyn, D.; Ravyn, V.; Lowney, R.; Nasrallah, H.A. CYP450 pharmacogenetic treatment strategies for antipsychotics: A review of the evidence. Schizophr. Res. 2013, 149, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Spina, E.; de Leon, J. Clinical applications of CYP genotyping in psychiatry. J. Neural Transm. 2015, 122, 5–28. [Google Scholar] [CrossRef] [Green Version]
- Senner, F.; Kohshour, M.O.; Abdalla, S.; Papiol, S.; Schulze, T.G. The genetics of response to and side effects of Lithium treatment in bipolar disorder: Future research perspectives. Front. Pharmacol. 2021, 12, 638882. [Google Scholar] [CrossRef]
- Carrascal-Laso, L.; Isidoro-García, M.; Ramos-Gallego, I.; Franco-Martín, M.A. Influence of the CYP450 Genetic Variation on the Treatment of Psychotic Disorders. J. Clin. Med. 2021, 10, 4275. [Google Scholar] [CrossRef]
- Brandl, E.J.; Chowdhury, N.I.; Tiwari, A.K.; Lett, T.A.; Meltzer, H.Y.; Kennedy, J.L.; Müller, D.J. Genetic variation in CYP3A43 is associated with response to antipsychotic medication. J. Neural Transm. 2015, 122, 29–34. [Google Scholar] [CrossRef]
- Hodgson, K.; Tansey, K.; Dernovšek, M.Z.; Hauser, J.; Henigsberg, N.; Maier, W.; Mors, O.; Placentino, A.; Rietschel, M.; Souery, D. Genetic differences in cytochrome P450 enzymes and antidepressant treatment response. J. Psychopharmacol. 2014, 28, 133–141. [Google Scholar] [CrossRef]
- Calafato, M.S.; Austin-Zimmerman, I.; Thygesen, J.H.; Sairam, M.; Metastasio, A.; Marston, L.; Abad-Santos, F.; Bhat, A.; Harju-Seppänen, J.; Irizar, H. The effect of CYP2D6 variation on antipsychotic-induced hyperprolactinaemia: A systematic review and meta-analysis. Pharm. J. 2020, 20, 629–637. [Google Scholar] [CrossRef]
- Moons, T.; De Roo, M.; Claes, S.; Dom, G. Relationship between P-glycoprotein and second-generation antipsychotics. Pharmacogenomics 2011, 12, 1193–1211. [Google Scholar] [CrossRef]
- Young, R.M.; Lawford, B.R.; Barnes, M.; Burton, S.C.; Ritchie, T.; Ward, W.K.; Noble, E.P. Prolactin levels in antipsychotic treatment of patients with schizophrenia carrying the DRD2* A1 allele. Br. J. Psychiatry 2004, 185, 147–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shad, M.U. Genetic Testing for Antipsychotic Pharmacotherapy: Bench to Bedside. Behav. Sci. 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, T.; Sellitto, C.; Manzo, V.; Colucci, F.; Giudice, V.; Stefanelli, B.; Iuliano, A.; Corrivetti, G.; Filippelli, A. Pharmacogenetics of carbamazepine and valproate: Focus on polymorphisms of drug metabolizing enzymes and transporters. Pharmaceuticals 2021, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Salloum, N.C.; McCarthy, M.J.; Leckband, S.G.; Kelsoe, J.R. Towards the clinical implementation of pharmacogenetics in bipolar disorder. BMC Med. 2014, 12, 90. [Google Scholar] [CrossRef] [Green Version]
- Franco, V.; Perucca, E. The pharmacogenomics of epilepsy. Expert Rev. Neurother. 2015, 15, 1161–1170. [Google Scholar] [CrossRef]
- Saiz-Rodríguez, M.; Almenara, S.; Navares-Gómez, M.; Ochoa, D.; Román, M.; Zubiaur, P.; Koller, D.; Santos, M.; Mejía, G.; Borobia, A.M. Effect of the most relevant CYP3A4 and CYP3A5 polymorphisms on the pharmacokinetic parameters of 10 CYP3A substrates. Biomedicines 2020, 8, 94. [Google Scholar] [CrossRef]
- Panomvana, D.; Traiyawong, T.; Towanabut, S. Effect of CYP3A5 genotypes on the pharmacokinetics of carbamazepine when used as monotherapy or co-administered with phenytoin, phenobarbital or valproic acid in Thai patients. J. Pharm. Pharm. Sci. 2013, 16, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Yin, T.; Ma, H.-Y.; Liu, D.-Q.; Sheng, Y.-H.; Wang, C.; Zhou, B.-T. Effects of CYP3A4/5 and ABCB1 genetic polymorphisms on carbamazepine metabolism and transport in Chinese patients with epilepsy treated with carbamazepine in monotherapy and bitherapy. Epilepsy Res. 2015, 117, 52–57. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Bipolar Disorder: Assessment and Management; NICE Clinical Guidelines; NICE, National Institute for Health and Care Excellence: London, UK, 2019; No. 185. [Google Scholar]
- Niwa, T.; Yasuda, S.; Yamamoto, Y.; Murakami, M.; Ishii, R. Contribution of the human cytochrome P450 2C subfamily to the metabolism of and the interactions with endogenous compounds including steroid hormones. Die Pharm. Int. J. Pharm. Sci. 2021, 76, 611–613. [Google Scholar]
- Haduch, A.; Bromek, E.; Daniel, W.A. Role of brain cytochrome P450 (CYP2D) in the metabolism of monoaminergic neurotransmitters. Pharmacol. Rep. 2013, 65, 1519–1528. [Google Scholar] [CrossRef]
- Kirchheiner, J.; Seeringer, A.; Godoy, A.L.; Ohmle, B.; Maier, C.; Beschoner, P.; Sim, E.-J.; Viviani, R. CYP2D6 in the brain: Genotype effects on resting brain perfusion. Mol. Psychiatry 2011, 16, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jukić, M.M.; Opel, N.; Ström, J.; Carrillo-Roa, T.; Miksys, S.; Novalen, M.; Renblom, A.; Sim, S.C.; Peñas-Lledó, E.M.; Courtet, P. Elevated CYP2C19 expression is associated with depressive symptoms and hippocampal homeostasis impairment. Mol. Psychiatry 2017, 22, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Haduch, A.; Daniel, W.A. The engagement of brain cytochrome P450 in the metabolism of endogenous neuroactive substrates: A possible role in mental disorders. Drug Metab. Rev. 2018, 50, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Stingl, J.C.; Scholl, C.; Bosch, J.E.; Viviani, R. Genetic polymorphism of CYP2C19 and subcortical variability in the human adult brain. Transl. Psychiatry 2021, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Bang, M.; Kim, A.; Cho, D.-Y.; Lee, S.-H. Influence of cytochrome P450 2D6 polymorphism on hippocampal white matter and treatment response in schizophrenia. NPJ Schizophr. 2021, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Peñas-LLedó, E.M.; LLerena, A. CYP2D6 variation, behaviour and psychopathology: Implications for pharmacogenomics-guided clinical trials. Br. J. Clin. Pharmacol. 2014, 77, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Niwa, T.; Yanai, M.; Matsumoto, M.; Shizuku, M. Effect of cytochrome P450 (CYP) 2D6 genetic polymorphism on the inhibitory action of antidepressants on CYP2D6-mediated dopamine formation from p-tyramine. J. Pharm. Pharm. Sci. 2018, 21, 135–142. [Google Scholar] [CrossRef]
- Cheng, J.; Zhen, Y.; Miksys, S.; Beyoğlu, D.; Krausz, K.W.; Tyndale, R.F.; Yu, A.; Idle, J.R.; Gonzalez, F.J. Potential role of CYP2D6 in the central nervous system. Xenobiotica 2013, 43, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Deneris, E.; Gaspar, P. Serotonin neuron development: Shaping molecular and structural identities. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e301. [Google Scholar] [CrossRef]
- Huisinga, K.L.; Brower-Toland, B.; Elgin, S.C. The contradictory definitions of heterochromatin: Transcription and silencing. Chromosoma 2006, 115, 110–122. [Google Scholar] [CrossRef]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC®) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 2017, 102, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 ubiquitin ligases: Styles, structures and functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Karnes, J.H.; Rettie, A.E.; Somogyi, A.A.; Huddart, R.; Fohner, A.E.; Formea, C.M.; Ta Michael Lee, M.; Llerena, A.; Whirl-Carrillo, M.; Klein, T.E. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA-B genotypes and phenytoin dosing: 2020 update. Clin. Pharmacol. Ther. 2021, 109, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.; Keogh, M.C.; Ishii, H.; Peterson, C.L.; Buratowski, S.; Lieberman, J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell 2005, 20, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Wójcikowski, J.; Danek, P.J.; Basińska-Ziobroń, A.; Pukło, R.; Daniel, W.A. In vitro inhibition of human cytochrome P450 enzymes by the novel atypical antipsychotic drug asenapine: A prediction of possible drug–drug interactions. Pharmacol. Rep. 2020, 72, 612–621. [Google Scholar] [CrossRef] [Green Version]
- Zubiaur, P.; Soria-Chacartegui, P.; Koller, D.; Navares-Gómez, M.; Ochoa, D.; Almenara, S.; Saiz-Rodríguez, M.; Mejía-Abril, G.; Villapalos-García, G.; Román, M. Impact of polymorphisms in transporter and metabolizing enzyme genes on olanzapine pharmacokinetics and safety in healthy volunteers. Biomed. Pharmacother. 2021, 133, 111087. [Google Scholar] [CrossRef]
- Prior, T.I.; Baker, G.B. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J. Psychiatry Neurosci. 2003, 28, 99–112. [Google Scholar]
- Dean, L.; Kane, M. Phenytoin Therapy and HLA-B* 15: 02 and CYP2C9 Genotype; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2021. [Google Scholar]
- Nordquist, H.; Jamil, R.T. Biochemistry, HLA Antigens; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Tamouza, R.; Krishnamoorthy, R.; Leboyer, M. Understanding the genetic contribution of the human leukocyte antigen system to common major psychiatric disorders in a world pandemic context. Brain Behav. Immun. 2021, 91, 731–739. [Google Scholar] [CrossRef]
- Sterner, K.N.; Weckle, A.; Chugani, H.T.; Tarca, A.L.; Sherwood, C.C.; Hof, P.R.; Kuzawa, C.W.; Boddy, A.M.; Abbas, A.; Raaum, R.L. Dynamic gene expression in the human cerebral cortex distinguishes children from adults. PLoS ONE 2012, 7, e37714. [Google Scholar] [CrossRef]
- Boulanger, L.M. Immune proteins in brain development and synaptic plasticity. Neuron 2009, 64, 93–109. [Google Scholar] [CrossRef] [Green Version]
- Numata, S.; Umehara, H.; Ohmori, T.; Hashimoto, R. Clozapine pharmacogenetic studies in schizophrenia: Efficacy and agranulocytosis. Front. Pharmacol. 2018, 9, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Clerc, S.; Taing, L.; Fond, G.; Meary, A.; Llorca, P.; Blanc, O.; Beaune, P.; Rajagopal, K.; Jamain, S.; Tamouza, R. A double amino-acid change in the HLA-A peptide-binding groove is associated with response to psychotropic treatment in patients with schizophrenia. Transl. Psychiatry 2015, 5, e608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloypan, C.; Koomdee, N.; Satapornpong, P.; Tempark, T.; Biswas, M.; Sukasem, C. A comprehensive review of HLA and severe cutaneous adverse drug reactions: Implication for clinical pharmacogenomics and precision medicine. Pharmaceuticals 2021, 14, 1077. [Google Scholar] [CrossRef]
- Jeiziner, C.; Wernli, U.; Suter, K.; Hersberger, K.E.; Meyer zu Schwabedissen, H.E. HLA-associated adverse drug reactions-scoping review. Clin. Transl. Sci. 2021, 14, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.-L.; Shiao, M.-S.; Hui, R.C.-Y.; Su, S.-C.; Wang, C.-W.; Chang, Y.-C.; Chung, W.-H. HLA association with drug-induced adverse reactions. J. Immunol. Res. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.S.; Zhang, M.W.; Mak, A.; Ho, R.C. Metabolic syndrome in psychiatry: Advances in understanding and management. Adv. Psychiatr. Treat. 2014, 20, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Chen, J.; Yin, Z.; Wang, L.; Peng, L. The association between depression and metabolic syndrome and its components: A bidirectional two-sample Mendelian randomization study. Transl. Psychiatry 2021, 11, 633. [Google Scholar] [CrossRef]
- Malan-Mueller, S.; Kilian, S.; van den Heuvel, L.L.; Bardien, S.; Asmal, L.; Warnich, L.; Emsley, R.A.; Hemmings, S.M.; Seedat, S. A systematic review of genetic variants associated with metabolic syndrome in patients with schizophrenia. Schizophr. Res. 2016, 170, 1–17. [Google Scholar] [CrossRef]
- OMIM. Online Mendelian Inheritance in Man. Available online: https://www.omim.org/ (accessed on 6 September 2022).
- GWAS Catalog. The NHGRI-EBI Catalog of Published Genome-Wide Association Studies. Available online: https://www.ebi.ac.uk/gwas/ (accessed on 6 September 2022).
- Claussnitzer, M.; Dankel, S.N.; Kim, K.-H.; Quon, G.; Meuleman, W.; Haugen, C.; Glunk, V.; Sousa, I.S.; Beaudry, J.L.; Puviindran, V. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 2015, 373, 895–907. [Google Scholar] [CrossRef] [Green Version]
- Ensembl. Ensembl Genome Browser. Available online: http://asia.ensembl.org/index.html (accessed on 6 September 2022).
- Mao, L.; Fang, Y.; Campbell, M.; Southerland, W.M. Population differentiation in allele frequencies of obesity-associated SNPs. BMC Genom. 2017, 18, 861. [Google Scholar] [CrossRef]
- Nagy, K.; Fiatal, S.; Sándor, J.; Ádány, R. Distinct penetrance of obesity-associated susceptibility alleles in the hungarian general and Roma populations. Obes. Facts 2017, 10, 444–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boiko, A.S.; Pozhidaev, I.V.; Paderina, D.Z.; Bocharova, A.V.; Mednova, I.A.; Fedorenko, O.Y.; Kornetova, E.G.; Loonen, A.J.; Semke, A.V.; Bokhan, N.A. Search for Possible Associations of FTO Gene Polymorphic Variants with Metabolic Syndrome, Obesity and Body Mass Index in Schizophrenia Patients. Pharm. Pers. Med. 2021, 14, 1123. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chiu, H.-J.; Loh, E.-W.; Chan, C.-H.; Hwu, T.-M.; Liu, Y.-R.; Lan, T.-H. Association of the ADRA1A gene and the severity of metabolic abnormalities in patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Buzas, B.; Belfer, I.; Hipp, H.; Lorincz, I.; Evans, C.; Phillips, G.; Taubman, J.; Max, M.; Goldman, D. Haplotype block and superblock structures of the alpha1-adrenergic receptor genes reveal echoes from the chromosomal past. Mol. Genet. Genom. 2004, 272, 519–529. [Google Scholar] [CrossRef]
- Ren, D.; Cai, X.; Lin, Q.; Ye, H.; Teng, J.; Li, J.; Ding, X.; Zhang, Z. Impact of linkage disequilibrium heterogeneity along the genome on genomic prediction and heritability estimation. Genet. Sel. Evol. 2022, 54, 47. [Google Scholar] [CrossRef]
- Johnson, G.C.; Esposito, L.; Barratt, B.J.; Smith, A.N.; Heward, J.; Di Genova, G.; Ueda, H.; Cordell, H.J.; Eaves, I.A.; Dudbridge, F. Haplotype tagging for the identification of common disease genes. Nat. Genet. 2001, 29, 233–237. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Mohammed, A.K.; Vinodson, B.; Clerici, M.; Kazmi, U.; Hussain, T.; Draz, H.M. Variants of endothelial nitric oxide synthase gene are associated with components of metabolic syndrome in an Arab population. Endocr. J. 2012, 59, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.L.; Ruiz, R.; Gonzalez, M.A.; Ramirez-Lorca, R.; Couto, C.; Ramos, A.; Gutierrez-Tous, R.; Rivera, J.M.; Ruiz, A.; Real, L.M. Association of NOS3 gene with metabolic syndrome in hypertensive patients. Thromb. Haemost. 2004, 92, 413–418. [Google Scholar] [CrossRef]
- Miranda, J.A.; Belo, V.A.; Souza-Costa, D.C.; Lanna, C.M.; Tanus-Santos, J.E. eNOS polymorphism associated with metabolic syndrome in children and adolescents. Mol. Cell. Biochem. 2013, 372, 155–160. [Google Scholar] [CrossRef]
- Tanus-Santos, J.E.; Desai, M.; Flockhart, D.A. Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharm. Genom. 2001, 11, 719–725. [Google Scholar] [CrossRef]
- Zhu, F.; Zykova, T.A.; Peng, C.; Zhang, J.; Cho, Y.Y.; Zheng, D.; Yao, K.; Ma, W.Y.; Lau, A.T.; Bode, A.M.; et al. Phosphorylation of H2AX at Ser139 and a new phosphorylation site Ser16 by RSK2 decreases H2AX ubiquitination and inhibits cell transformation. Cancer Res 2011, 71, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saruwatari, J.; Nakashima, H.; Tsuchimine, S.; Nishimura, M.; Ogusu, N.; Yasui-Furukori, N. Possible impact of the CYP2D6* 10 polymorphism on the nonlinear pharmacokinetic parameter estimates of paroxetine in Japanese patients with major depressive disorders. Pharm. Pers. Med. 2014, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Thour, A.; Marwaha, R. Amitriptyline; StatPearls Publishing: Treasure Island, FL, 2019. [Google Scholar]
- Cruz, L.; Soares, P.; Correia, M. Ubiquitin-Specific Proteases: Players in Cancer Cellular Processes. Pharmaceuticals 2021, 14, 848. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, K.W.; Song, W.-J.; Kim, S.-H.; Jee, Y.-K.; Lee, S.-M.; Kang, H.-R.; Park, H.-W.; Cho, S.-H.; Park, S.-H. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011, 97, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y. HLA-B allele associations with certain drugs are not confirmed in Japanese patients with severe cutaneous drug reactions. Acta Derm. Venereol. 2008, 88, 616–618. [Google Scholar]
- Aggarwal, R.; Sharma, M.; Modi, M.; Kumar Garg, V.; Salaria, M. HLA-B∗ 1502 is associated with carbamazepine induced Stevens–Johnson syndrome in North Indian population. Hum. Immunol. 2014, 75, 1120–1122. [Google Scholar] [CrossRef]
- Chang, C.C.; Too, C.L.; Murad, S.; Hussein, S.H. Association of HLA-B* 1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens–Johnson syndrome in the multi-ethnic Malaysian population. Int. J. Dermatol. 2011, 50, 221–224. [Google Scholar] [CrossRef]
- He, X.-J.; Jian, L.-Y.; He, X.-L.; Wu, Y.; Xu, Y.-Y.; Sun, X.-J.; Miao, L.-Y.; Zhao, L.-M. Association between the HLA-B* 15: 02 allele and carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis in Han individuals of northeastern China. Pharmacol. Rep. 2013, 65, 1256–1262. [Google Scholar] [CrossRef]
- Alfirevic, A.; Jorgensen, A.L.; Williamson, P.R.; Chadwick, D.W.; Park, B.K.; Pirmohamed, M. HLA-B locus in Caucasian patients with carbamazepine hypersensitivity. Pharmacogenomics 2006, 7. [Google Scholar]
- Kaniwa, N.; Saito, Y.; Aihara, M.; Matsunaga, K.; Tohkin, M.; Kurose, K.; Furuya, H.; Takahashi, Y.; Muramatsu, M.; Kinoshita, S. HLA-B* 1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia 2010, 51, 2461–2465. [Google Scholar] [CrossRef]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperavičiūtė, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; de Bakker, P.I. HLA-A* 3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genin, E.; Chen, D.; Hung, S.; Sekula, P.; Schumacher, M.; Chang, P.; Tsai, S.; Wu, T.; Bellón, T.; Tamouza, R. HLA-A* 31: 01 and different types of carbamazepine-induced severe cutaneous adverse reactions: An international study and meta-analysis. Pharm. J. 2014, 14, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Mushiroda, T.; Yowang, A.; Takahashi, A.; Kubo, M.; Shirakata, Y.; Ikezawa, Z.; Iijima, M.; Shiohara, T.; Hashimoto, K. Genome-wide association study identifies HLA-A* 3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum. Mol. Genet. 2011, 20, 1034–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yampayon, K.; Sukasem, C.; Limwongse, C.; Chinvarun, Y.; Tempark, T.; Rerkpattanapipat, T.; Kijsanayotin, P. Influence of genetic and non-genetic factors on phenytoin-induced severe cutaneous adverse drug reactions. Eur. J. Clin. Pharmacol. 2017, 73, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Ng, C.; Too, C.; Choon, S.; Lee, C.; Chung, W.; Hussein, S.; Lim, K.; Murad, S. Association of HLA-B* 15: 13 and HLA-B* 15: 02 with phenytoin-induced severe cutaneous adverse reactions in a Malay population. Pharm. J. 2017, 17, 170–173. [Google Scholar] [CrossRef]
- Su, S.C.; Chen, C.B.; Chang, W.C.; Wang, C.W.; Fan, W.L.; Lu, L.Y.; Nakamura, R.; Saito, Y.; Ueta, M.; Kinoshita, S. HLA Alleles and CYP 2C9* 3 as Predictors of Phenytoin Hypersensitivity in East Asians. Clin. Pharmacol. Ther. 2019, 105, 476–485. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Martínez-Juárez, I.E.; Monroy-Jaramillo, N.; Jung-Cook, H.; Falfán-Valencia, R.; Ortega-Vázquez, A.; Alonso-Vilatela, M.E.; López-López, M. HLA-A* 02: 01: 01/-B* 35: 01: 01/-C* 04: 01: 01 haplotype associated with lamotrigine-induced maculopapular exanthema in Mexican Mestizo patients. Pharmacogenomics 2014, 15, 1881–1891. [Google Scholar] [CrossRef]
- Nelson, E.M.; Philbrick, A.M. Avoiding serotonin syndrome: The nature of the interaction between tramadol and selective serotonin reuptake inhibitors. Ann. Pharmacother. 2012, 46, 1712–1716. [Google Scholar] [CrossRef]
- Xie, C.; Pogribna, M.; Word, B.; Lyn-Cook Jr, L.; Lyn-Cook, B.D.; Hammons, G.J. In vitro analysis of factors influencing CYP 1A2 expression as potential determinants of interindividual variation. Pharmacol. Res. Perspect. 2017, 5, e00299. [Google Scholar] [CrossRef] [Green Version]
- Klomp, S.D.; Manson, M.L.; Guchelaar, H.-J.; Swen, J.J. Phenoconversion of cytochrome P450 metabolism: A systematic review. J. Clin. Med. 2020, 9, 2890. [Google Scholar] [CrossRef]
- Lesche, D.; Mostafa, S.; Everall, I.; Pantelis, C.; Bousman, C.A. Impact of CYP1A2, CYP2C19, and CYP2D6 genotype-and phenoconversion-predicted enzyme activity on clozapine exposure and symptom severity. Pharm. J. 2020, 20, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs—Best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-F.; Hu, A.-L.; Xie, L.; Liu, J.-J.; Wu, Q.; Liu, J. Age-associated changes of cytochrome P450 and related phase-2 gene/proteins in livers of rats. PeerJ 2019, 7, e7429. [Google Scholar] [CrossRef] [PubMed]
- Mangó, K.; Kiss, Á.F.; Fekete, F.; Erdős, R.; Monostory, K. CYP2B6 allelic variants and non-genetic factors influence CYP2B6 enzyme function. Sci. Rep. 2022, 12, 2984. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.F. Probing the world of cytochrome P450 enzymes. Mol. Interv. 2004, 4, 157. [Google Scholar]
- Jithesh, P.V.; Abuhaliqa, M.; Syed, N.; Ahmed, I.; El Anbari, M.; Bastaki, K.; Sherif, S.; Umlai, U.-K.; Jan, Z.; Gandhi, G. A population study of clinically actionable genetic variation affecting drug response from the Middle East. NPJ Genom. Med. 2022, 7, 10. [Google Scholar] [CrossRef]
- Meas, R.; Mao, P. Histone ubiquitylation and its roles in transcription and DNA damage response. DNA Repair 2015, 36, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Harrach, S.; Schmidt-Lauber, C.; Pap, T.; Pavenstädt, H.; Schlatter, E.; Schmidt, E.; Berdel, W.; Schulze, U.; Edemir, B.; Jeromin, S. MATE1 regulates cellular uptake and sensitivity to imatinib in CML patients. Blood Cancer J. 2016, 6, e470. [Google Scholar] [CrossRef]
- Sanli, T.; Rashid, A.; Liu, C.; Harding, S.; Bristow, R.G.; Cutz, J.-C.; Singh, G.; Wright, J.; Tsakiridis, T. Ionizing radiation activates AMP-activated kinase (AMPK): A target for radiosensitization of human cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 221–229. [Google Scholar] [CrossRef]
- El Rouby, N.; Shahin, M.H.; Bader, L.; Khalifa, S.I.; Elewa, H. Genomewide association analysis of warfarin dose requirements in Middle Eastern and North African populations. Clin. Transl. Sci. 2022, 15, 558–566. [Google Scholar] [CrossRef]
- National Library of Medicine. VKORC1 Vitamin K Epoxide Reductase Complex Subunit 1 [Homo Sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/79001 (accessed on 10 April 2022).
- Runcharoen, C.; Fukunaga, K.; Sensorn, I.; Iemwimangsa, N.; Klumsathian, S.; Tong, H.; Vo, N.S.; Le, L.; Hlaing, T.M.; Thant, M. Prevalence of pharmacogenomic variants in 100 pharmacogenes among Southeast Asian populations under the collaboration of the Southeast Asian Pharmacogenomics Research Network (SEAPharm). Hum. Genome Var. 2021, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahayri, Z.N.; Patrinos, G.P.; Wattanapokayakit, S.; Iemwimangsa, N.; Fukunaga, K.; Mushiroda, T.; Chantratita, W.; Ali, B.R. Variation in 100 relevant pharmacogenes among emiratis with insights from understudied populations. Sci. Rep. 2020, 10, 21310. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.R.; Wegmann, D.; Ehm, M.G.; Kessner, D.; St. Jean, P.; Verzilli, C.; Shen, J.; Tang, Z.; Bacanu, S.-A.; Fraser, D. An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science 2012, 337, 100–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghamdi, J.; Padmanabhan, S. Fundamentals of complex trait genetics and association studies. Handb. Pharm. Stratif. Med. 2014, 1, 235–257. [Google Scholar]
- Jarrar, Y.; Musleh, R.; Ghanim, M.; AbuKhader, I.; Jarrar, Q. Assessment of the need for pharmacogenomics education among pharmacists in the West Bank of Palestine. Int. J. Clin. Pract. 2021, 75, e14435. [Google Scholar] [CrossRef]
- Van Westrhenen, R.; Aitchison, K.J.; Ingelman-Sundberg, M.; Jukić, M.M. Pharmacogenomics of antidepressant and antipsychotic treatment: How far have we got and where are we going? Front. Psychiatry 2020, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Jukić, M.M.; Haslemo, T.; Molden, E.; Ingelman-Sundberg, M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2,087 patients. Am. J. Psychiatry 2018, 175, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Haslemo, T.; Eliasson, E.; Jukić, M.M.; Ingelman-Sundberg, M.; Molden, E. Significantly lower CYP2D6 metabolism measured as the O/N-desmethylvenlafaxine metabolic ratio in carriers of CYP2D6* 41 versus CYP2D6* 9 or CYP2D6* 10: A study on therapeutic drug monitoring data from 1003 genotyped Scandinavian patients. Br. J. Clin. Pharmacol. 2019, 85, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Cicali, E.J.; Weitzel, K.W.; Elsey, A.R.; Orlando, F.A.; Vinson, M.; Mosley, S.; Smith, D.M.; Davis, R.; Drum, L.; Estores, D. Challenges and lessons learned from clinical pharmacogenetic implementation of multiple gene–drug pairs across ambulatory care settings. Genet. Med. 2019, 21, 2264–2274. [Google Scholar] [CrossRef]
- Luzum, J.A.; Luzum, M.J. Physicians’ attitudes toward pharmacogenetic testing before and after pharmacogenetic education. Pers. Med. 2016, 13, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Mikat-Stevens, N.A.; Larson, I.A.; Tarini, B.A. Primary-care providers’ perceived barriers to integration of genetics services: A systematic review of the literature. Genet. Med. 2015, 17, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirmohamed, M. Acceptance of biomarker-based tests for application in clinical practice: Criteria and obstacles. Clin. Pharmacol. Ther. 2010, 88, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Barboza, A.B.; McElroy, S.L.; Veldic, M.; Singh, B.; Kung, S.; Romo-Nava, F.; Nunez, N.A.; Cabello-Arreola, A.; Coombes, B.J.; Prieto, M. Potential pharmacogenomic targets in bipolar disorder: Considerations for current testing and the development of decision support tools to individualize treatment selection. Int. J. Bipolar Disord. 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Shugg, T.; Pasternak, A.L.; London, B.; Luzum, J.A. Prevalence and types of inconsistencies in clinical pharmacogenetic recommendations among major US sources. NPJ Genom. Med. 2020, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.; Thangaraj, K.; Patterson, N.; Price, A.L.; Singh, L. Reconstructing Indian population history. Nature 2009, 461, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.W.; Brett, A.S. Race and Pharmacogenomics—Personalized Medicine or Misguided Practice? JAMA 2021, 325, 625–626. [Google Scholar] [CrossRef]
- Jithesh, P.V.; Scaria, V. From Genomes to Genomic Medicine: Enabling Personalized and Precision Medicine in the Middle East; Future Medicine: London, UK, 2017; Volume 14, pp. 377–382. [Google Scholar]

| Enzyme | Star Allele (Genotype) | Diplotype Examples | Metabolizer (Phenotype) | CPIC ® Guidelines | Reference |
|---|---|---|---|---|---|
| CYP2D6 | *4, *10 | *4/*41 *4/*10 *10/*10 *10/*41 | Intermediate | Dosing recommendations for TCAs:
| [31,78,79] |
| *4/*4 | Poor | Dosing recommendations for TCAs:
| |||
| CYP2C9 | *2, *3 | *1/*2 *1/*3 *2/*17 *3/*17 | Intermediate | Dosing recommendation for phenytoin/fosphenytoin based on HLA-B*15:02:
| [80,81] |
| *2/*2 *3/*3 *2/*3 | Poor | Dosing recommendation for phenytoin/fosphenytoin based on HLA-B*15:02:
| [80,81] | ||
| CYP2C19 | *2, *17 | *17/*17 | Ultra-rapid | Dosing recommendations for the tertiary amines:
| [31,82] |
| *1/*17 | Rapid | ||||
| *1/*2 *2/*17 *3/*17 | Intermediate | Dosing recommendations for the tertiary amines:
|
| Enzyme | Selected Psychotropic(s) Affected by Enzyme | Alternative Psychotropics | Reference |
|---|---|---|---|
| CYP2D6 | Tricyclic antidepressants | Secondary amines, Desipramine Nortriptyline. | [79] |
| CYP2C9 | Olanzapine | Asenapine, Quetiapine. | [83,84,85,86] |
| Phenytoin Fosphenytoin | Eslicarbazepine, Lamotrigine, Phenobarbital. | ||
| CYP2C19 | Sertraline Escitalopram Citalopram | Fluoxetine, Fluvoxamine, Paroxetine. | [31,83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alchakee, A.; Ahmed, M.; Eldohaji, L.; Alhaj, H.; Saber-Ayad, M. Pharmacogenomics in Psychiatry Practice: The Value and the Challenges. Int. J. Mol. Sci. 2022, 23, 13485. https://doi.org/10.3390/ijms232113485
Alchakee A, Ahmed M, Eldohaji L, Alhaj H, Saber-Ayad M. Pharmacogenomics in Psychiatry Practice: The Value and the Challenges. International Journal of Molecular Sciences. 2022; 23(21):13485. https://doi.org/10.3390/ijms232113485
Chicago/Turabian StyleAlchakee, Aminah, Munazza Ahmed, Leen Eldohaji, Hamid Alhaj, and Maha Saber-Ayad. 2022. "Pharmacogenomics in Psychiatry Practice: The Value and the Challenges" International Journal of Molecular Sciences 23, no. 21: 13485. https://doi.org/10.3390/ijms232113485





