Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis †
Abstract
:1. Adipose Derived (AD) Tissue Stromal Vascular Fraction (tSVF): Perspective of Plastic Reconstructive and Aesthetic Surgery
2. Structure and Function of Articular Joint Cartilage
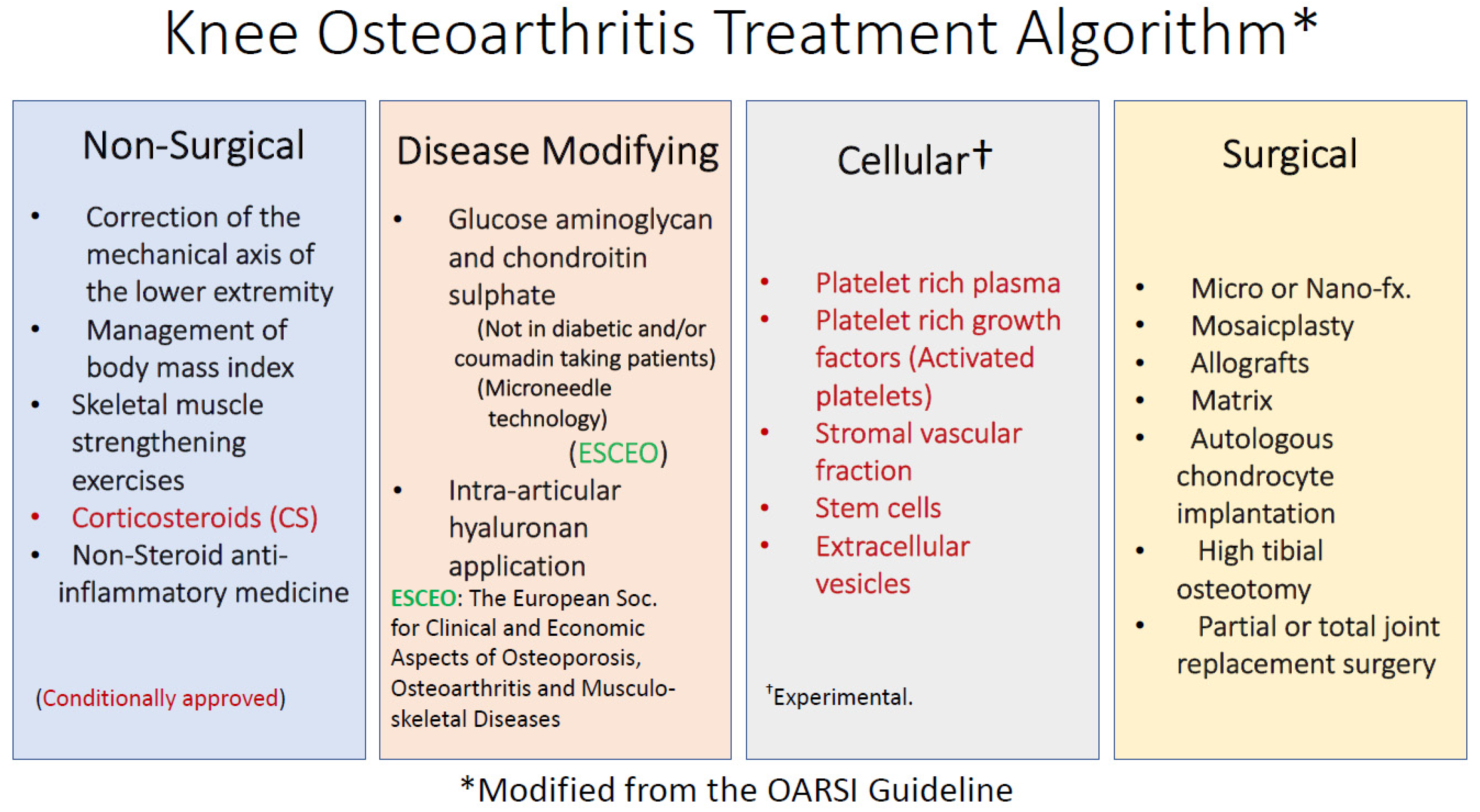
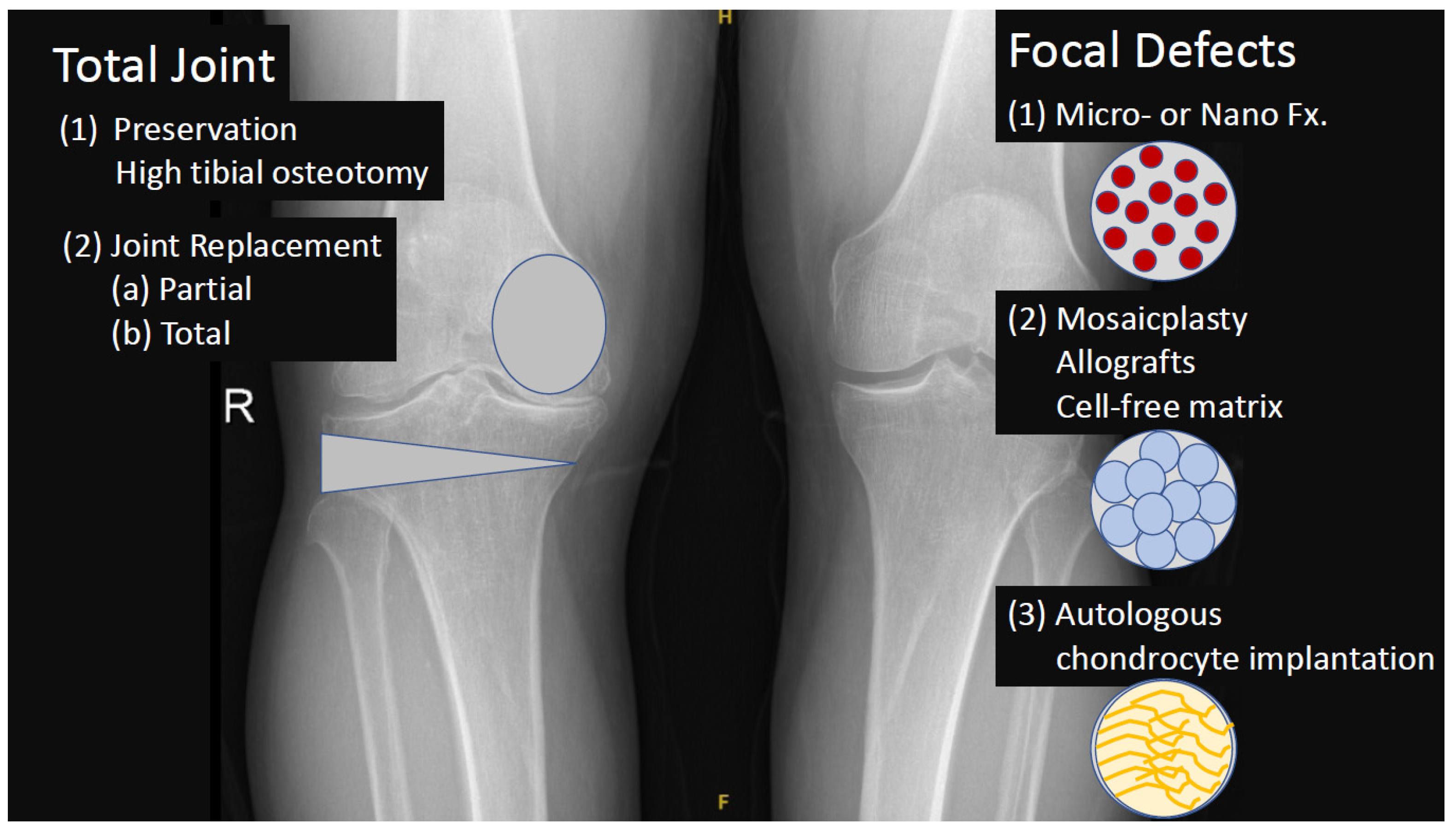
3. Definition and Treatment of Osteoarthritis (OA)
4. Adipose Derived Stromal Vascular Fraction (AD-SVF)
5. Non-Enzymatic tSVF Techniques and Technologies
5.1. Focus on Membrane Properties
5.2. Flow-Cytometry Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Adipose Derived |
| MSCs | Mesenchymal Stem Cells |
| SVF | Stromal Vascular Fraction |
| AD-tSVF | Adipose Derived tissue Stromal Vascular Fraction |
| AD-cSVF | Adipose Derived cellular Stromal Vascular Fraction |
| AD-hSVF | Adipose Derived human Stromal Vascular Fraction |
| PRP | Platelet Rich Plasma |
| ECM | Extracellular Matrix |
| HA | Hyaluronan |
| GAG | Glycosaminoglycan |
| CS | Chondroitin Sulfate |
| KS | Keratan Sulfate |
| OA | Osteoarthritis |
| BMI | Body Mass Index |
| YLD | Global Years Lived with Disability |
| DALYs | Disability Adjusted Life Years |
| USD | United States Dollar |
| NK Cells | Natural Killer Cells |
| IFN | Interferon |
| TNFα | Tumor Necrosis Factor alpha |
| TGFb | Transforming Growth Factor beta |
| IGF-1 | Insulin-like Growth Factor 1 |
| HIF | Hypoxia-Inducible Factor |
| MMPs | Matrix metalloproteinases |
| ADMSCs | Adipose Derived Mesenchymal Stem Cells |
| IL | Interleukin |
| MSCs | Mesenchymal Stem Cells |
| WHO | World Health Organization |
| OP | Osteoporosis |
| FDA | Food and Drug Administration |
| IFATS | The International Federation for Adipose Therapeutics and Science |
| ISCT | The International Society for Cell & Gene Therapy |
| ATMP | Advanced Therapy Medicinal Products |
| ITR2 | Injectable Tissue Replacement and Regeneration |
| KFDA | Korean Food and Drug Administration |
| PDGF | Platelet-Derived Growth Factor |
| VEGF | Vascular Endothelial Growth Factor |
| FGF | Fibroblast Growth Factor |
| FGFR | Fibroblast Growth Factor Receptor |
| TIMPs | Tissue Inhibitors of Metalloproteinases |
| ADAMTS | A Disintegrin and Metalloproteinase with Thrombospondin Motifs |
| MR | Magnetic Resonance |
| CaCl2 | Calcium Chloride 2 |
| VAS | Visual Analogue Scale |
| WOMAC | Western Ontario and McMaster Universities Arthritis Index |
| KOSS | The Knee Osteoarthritis Scoring System |
| PTPRC | Protein Tyrosine Phosphatase Receptor type C |
| LCA | Leukocyte Common Antigen |
| EPISER | Prevalence of Rheumatic Diseases in Adult Population in Spain Study |
| Arg1 | Arginase 1 |
| PBS | Phosphate-Buffered Saline |
| HLA | Human Leucocyte Antigen |
| DMEM | Dulbecco’s Modified Eagle Medium |
| PECAM-1 | Platelet Endothelial Cell Adhesion Molecule |
| FFLL | Female-to-Female Luer-Lock |
| MMPs | Matrix Metalloproteinase |
| COMP | Cartilage oligometric matrix protein |
| GDF-11 | Growth differentiation factor-11 |
References
- Berry, D.C.; Stenesen, D.; Zeve, D.; Graff, J.M. The developmental origins of adipose tissue. Development 2013, 140, 3939–3949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, G.I. Adipose stem cells and skeletal repair. Histol. Histopathol. 2013, 28, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Polly, S.S.; Nichols, A.E.C.; Donnini, E.; Inman, D.J.; Scott, T.J.; Apple, S.M.; Werre, S.R.; Dahlgren, L.A. Adipose-Derived Stromal Vascular Fraction and Cultured Stromal Cells as Trophic Mediators for Tendon Healing. J. Orthop. Res. 2019, 37, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xue, J.; Lu, S.; Yuan, Y.; Liao, Y.; Qiu, J.; Liu, C.; Liao, Q. Anti-inflammatory effect of stromal vascular fraction cells in fat transplantation. Exp. Ther. Med. 2019, 17, 1435–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzouvelekis, A.; Paspaliaris, V.; Koliakos, G.; Ntolios, P.; Bouros, E.; Oikonomou, A.; Zissimopoulos, A.; Boussios, N.; Dardzinski, B.; Gritzalis, D.; et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J. Transl. Med. 2013, 11, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Encinas-Ullán, C.A. Stromal Vascular Fraction for Musculoskeletal Lesions. Int. J. Orthop. 2022, 9, 1658–1668. [Google Scholar] [CrossRef]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ji, Y.H.; Kim, D.W.; Dhong, E.S.; Yoon, E.S. Effects of platelet-rich plasma, adipose-derived stem cells, and stromal vascular fraction on the survival of human transplanted adipose tissue. J. Korean Med. Sci. 2014, 29 (Suppl. S3), S193–S200. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.Z.; Fang, X.H.; Williams, S.J.; Stephenson, L.L.; Baynosa, R.C.; Wong, N.; Khiabani, K.T.; Zamboni, W.A. Analysis for apoptosis and necrosis on adipocytes, stromal vascular fraction, and adipose-derived stem cells in human lipoaspirates after liposuction. Plast. Reconstr. Surg. 2013, 131, 77e–85e. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Rod, E.; Jelec, Z.; Cukelj, F.; Matisic, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Boric, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Lee, D.H.; Kong, C.G.; Shin, Y.W.; Ahmed, S.; Shetty, A.A.; Moon, M.S.; Kim, S.J. Which is better for articular cartilage regeneration, cultured stem cells or concentrated stromal cells? Ann. Transl. Med. 2020, 8, 836. [Google Scholar] [CrossRef] [PubMed]
- James, C.B.; Uhl, T.L. A review of articular cartilage pathology and the use of glucosamine sulfate. J. Athl. Train. 2001, 36, 413–419. [Google Scholar] [PubMed]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Korkusuz, F. Musculoskeletal Research and Basic Science, 1st ed.; Korkusuz, F., Ed.; Springer: Cham, Switzerland, 2016; pp. XIV, 788. [Google Scholar] [CrossRef]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Aubourg, G.; Rice, S.J.; Bruce-Wootton, P.; Loughlin, J. Genetics of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 636–649. [Google Scholar] [CrossRef]
- Hutchison, L.; Grayson, J.; Hiller, C.; D’Souza, N.; Kobayashi, S.; Simic, M. The relationship between knee biomechanics and pain in people with knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res. Hoboken, 2022, ahead of Print. [CrossRef]
- King, L.K.; March, L.; Anandacoomarasamy, A. Obesity & osteoarthritis. Indian J. Med. Res. 2013, 138, 185–193. [Google Scholar]
- Roman-Blas, J.A.; Castañeda, S.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis associated with estrogen deficiency. Arthritis Res. Ther. 2009, 11, 241. [Google Scholar] [CrossRef]
- Valdes, A.M.; Van Oene, M.; Hart, D.J.; Surdulescu, G.L.; Loughlin, J.; Doherty, M.; Spector, T.D. Reproducible genetic associations between candidate genes and clinical knee osteoarthritis in men and women. Arthritis Rheum. 2006, 54, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.L.; Jeyaraman, M. Stromal Vascular Fraction (SVF)—A Revolutionizer in Osteoarthritis Knees. Orthop. Surg. Traumatol. 2020, 4, 11–12. [Google Scholar]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Mato, D.; Sanchez-Piedra, C.; Silva-Fernandez, L.; Sivera, F.; Blanco, F.J.; Perez Ruiz, F.; Juan-Mas, A.; Pego-Reigosa, J.M.; Narvaez, J.; Quilis Marti, N.; et al. Prevalence of rheumatic diseases in adult population in Spain (EPISER 2016 study): Aims and methodology. Reum. Clin. Engl. Ed. 2019, 15, 90–96. [Google Scholar] [CrossRef]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez Robledo, L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- Turkiewicz, A.; Kiadaliri, A.A.; Englund, M. Cause-specific mortality in osteoarthritis of peripheral joints. Osteoarthr. Cartil. 2019, 27, 848–854. [Google Scholar] [CrossRef]
- Total knee replacement: An evidence-based analysis. Ont. Health Technol. Assess Ser. 2005, 5, 1–51.
- Murphy, L.; Helmick, C.G. The impact of osteoarthritis in the United States: A population-health perspective: A population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop. Nurs. 2012, 31, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: A population-based cohort study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.Y.; Zhang, Z.R.; Tang, Z.M.; Hua, F.Z. Benefits and Mechanisms of Exercise Training for Knee Osteoarthritis. Front. Physiol. 2021, 12, 794062. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.C.; Miller, L.E.; Block, J.E. Conservative management of symptomatic knee osteoarthritis: A flawed strategy? Orthop. Rev. Pavia 2013, 5, e2. [Google Scholar] [CrossRef] [PubMed]
- Stellavato, A.; Restaino, O.F.; Vassallo, V.; Cassese, E.; Finamore, R.; Ruosi, C.; Schiraldi, C. Chondroitin Sulfate in USA Dietary Supplements in Comparison to Pharma Grade Products: Analytical Fingerprint and Potential Anti-Inflammatory Effect on Human Osteoartritic Chondrocytes and Synoviocytes. Pharmaceutics 2021, 13, 737. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.D.; Poddar, S.; Tweed, E.M. Clinical inquiries: Do glucosamine and chondroitin worsen blood sugar control in diabetes? J. Fam. Pract. 2006, 55, 1091–1093. [Google Scholar] [PubMed]
- Reginster, J.Y.; Neuprez, A.; Lecart, M.P.; Sarlet, N.; Bruyere, O. Role of glucosamine in the treatment for osteoarthritis. Rheumatol. Int. 2012, 32, 2959–2967. [Google Scholar] [CrossRef] [Green Version]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, D.R.; Ademola, A.; Abbott, J.H.; Sajobi, T.; Hildebrand, K.; Marshall, D.A. Are education, exercise and diet interventions a cost-effective treatment to manage hip and knee osteoarthritis? A systematic review. Osteoarthr. Cartil. 2021, 29, 456–470. [Google Scholar] [CrossRef]
- Detterline, A.J.; Goldstein, J.L.; Rue, J.P.; Bach, B.R., Jr. Evaluation and treatment of osteochondritis dissecans lesions of the knee. J. Knee Surg. 2008, 21, 106–115. [Google Scholar] [CrossRef]
- Paessler, H. Microfracture for treatment of cartilage detects. Zent. Chir. 2000, 125, 500–504. [Google Scholar]
- Erickson, B.J.; Chalmers, P.N.; Yanke, A.B.; Cole, B.J. Surgical management of osteochondritis dissecans of the knee. Curr. Rev. Musculoskelet. Med. 2013, 6, 102–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orth, P.; Gao, L.; Madry, H. Microfracture for cartilage repair in the knee: A systematic review of the contemporary literature. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 670–706. [Google Scholar] [CrossRef] [PubMed]
- Penalver, J.M.; Villalba, J.; Yela-Verdu, C.P.; Sanchez, J.; Balaguer-Castro, M. All-Arthroscopic Nanofractured Autologous Matrix-Induced Chondrogenesis (A-NAMIC) Technique for the Treatment of Focal Chondral Lesions of the Knee. Arthrosc. Tech. 2020, 9, e755–e759. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, K.; El-Khechen, H.A.; Yamashita, F.; Duong, A.; Simunovic, N.; Musahl, V.; Ayeni, O.R. Arthroscopic versus Open Osteochondral Autograft Transplantation (Mosaicplasty) for Cartilage Damage of the Knee: A Systematic Review. J. Knee Surg. 2021, 34, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Greif, D.N.; Kouroupis, D.; Murdock, C.J.; Griswold, A.J.; Kaplan, L.D.; Best, T.M.; Correa, D. Infrapatellar Fat Pad/Synovium Complex in Early-Stage Knee Osteoarthritis: Potential New Target and Source of Therapeutic Mesenchymal Stem/Stromal Cells. Front. Bioeng. Biotechnol. 2020, 8, 860. [Google Scholar] [CrossRef]
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Marcacci, M. Early osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2011, 20, 399–400. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.E.; Bolia, I.K.; Trasolini, N.A. Biological strategies for osteoarthritis: From early diagnosis to treatment. Int. Orthop. 2020, 45, 335–344. [Google Scholar] [CrossRef]
- Christian Lattermann, H.M. Norimasa Nakamura, Elizaveta Kon: Early Osteoarthritis State-of-the-Art Approaches to Diagnosis, Treatment and Controversies; Springer: Heidelberg, Germany, 2022. [Google Scholar]
- Berry, D.C.; Jiang, Y.; Graff, J.M. Emerging Roles of Adipose Progenitor Cells in Tissue Development, Homeostasis, Expansion and Thermogenesis. Trends Endocrinol. Metab. 2016, 27, 574–585. [Google Scholar] [CrossRef]
- Succar, P.; Breen, E.J.; Kuah, D.; Herbert, B.R. Alterations in the Secretome of Clinically Relevant Preparations of Adipose-Derived Mesenchymal Stem Cells Cocultured with Hyaluronan. Stem Cells Int. 2015, 2015, 421253. [Google Scholar] [CrossRef] [Green Version]
- Pak, J.; Lee, J.H.; Pak, N.; Pak, Y.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Cartilage Regeneration in Humans with Adipose Tissue-Derived Stem Cells and Adipose Stromal Vascular Fraction Cells: Updated Status. Int. J. Mol. Sci. 2018, 19, 2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Of, A.; Alexander, R. Understanding Adipose-derived Stromal Vascular Fraction (AD-SVF) Cell Biology and Use on the Basis of Cellular, Chemical, Structural and Paracrine Components: A Concise Review. Prolotherapy 2012, 4., e855–e869. [Google Scholar]
- Manferdini, C.; Maumus, M.; Gabusi, E.; Piacentini, A.; Filardo, G.; Peyrafitte, J.A.; Jorgensen, C.; Bourin, P.; Fleury-Cappellesso, S.; Facchini, A.; et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013, 65, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.K.; Katz, A.J. Review of the adipose derived stem cell secretome. Biochimie 2013, 95, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Keane, T.J.; Roques, A.C.; Patrick, P.S.; Mooney, C.M.; Kuan, W.L.; Pisupati, V.; Oreffo, R.O.C.; Stuckey, D.J.; Watt, F.M.; et al. A blueprint for translational regenerative medicine. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Mukhopadhyay, A. Regenerative Medicine: Laboratory to Clinic; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Dykstra, J.A.; Facile, T.; Patrick, R.J.; Francis, K.R.; Milanovich, S.; Weimer, J.M.; Kota, D.J. Concise Review: Fat and Furious: Harnessing the Full Potential of Adipose-Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2017, 6, 1096–1108. [Google Scholar] [CrossRef]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast Reconstr. Aesthet. Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef]
- Siennicka, K.; Zolocinska, A.; Stepien, K.; Lubina-Dabrowska, N.; Maciagowska, M.; Zolocinska, E.; Slysz, A.; Piusinska-Macoch, R.; Mazur, S.; Zdanowicz, U.; et al. Adipose-Derived Cells (Stromal Vascular Fraction) Transplanted for Orthopedical or Neurological Purposes: Are They Safe Enough? Stem Cells Int. 2016, 2016, 5762916. [Google Scholar] [CrossRef] [Green Version]
- Lana, J.F.S.D.; Lana, A.V.S.D.; da Fonseca, L.F.; Coelho, M.A.; Marques, G.G.; Mosaner, T.; Ribeiro, L.L.; Azzini, G.O.M.; Santos, G.S.; Fonseca, E. Stromal Vascular Fraction for Knee Osteoarthritis–An Update. J. Stem Cells Regen. Med. 2022, 18, 11. [Google Scholar]
- Philipsen, A.; Hansen, A.L.; Jorgensen, M.E.; Brage, S.; Carstensen, B.; Sandbaek, A.; Almdal, T.P.; Gram, J.; Pedersen, E.B.; Lauritzen, T.; et al. Associations of objectively measured physical activity and abdominal fat distribution. Med. Sci. Sport. Exerc. 2015, 47, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Boada-Pladellorens, A.; Avellanet, M.; Pages-Bolibar, E.; Veiga, A. Stromal vascular fraction therapy for knee osteoarthritis: A systematic review. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221117879. [Google Scholar] [CrossRef] [PubMed]
- Comella, K.; Parlo, M.; Daly, R.; Depasquale, V.; Edgerton, E.; Mallory, P.; Schmidt, R.; Drake, W.P. Safety Analysis of Autologous Stem Cell Therapy in a Variety of Degenerative Diseases and Injuries Using the Stromal Vascular Fraction. J. Clin. Med. Res. 2017, 9, 935–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitag, J.; Bates, D.; Boyd, R.; Shah, K.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy—A review. BMC Musculoskelet. Disord. 2016, 17, 230. [Google Scholar] [CrossRef]
- Bianchi, F.; Olivi, E.; Baldassarre, M.; Giannone, F.A.; Laggetta, M.; Valente, S.; Cavallini, C.; Tassinari, R.; Canaider, S.; Pasquinelli, G.; et al. Lipogems, a New Modality of Fat Tissue Handling to Enhance Tissue Repair in Chronic Hind Limb Ischemia. CellR4 2014, 2, e1289. [Google Scholar]
- Murrell, W.D.; Anz, A.W.; Badsha, H.; Bennett, W.F.; Boykin, R.E.; Caplan, A.I. Regenerative treatments to enhance orthopedic surgical outcome. PM R 2015, 7, S41–S52. [Google Scholar] [CrossRef] [Green Version]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Jayaram, P.; Ikpeama, U.; Rothenberg, J.B.; Malanga, G.A. Bone Marrow-Derived and Adipose-Derived Mesenchymal Stem Cell Therapy in Primary Knee Osteoarthritis: A Narrative Review. PM R 2019, 11, 177–191. [Google Scholar] [CrossRef]
- Panchal, J.; Malanga, G.; Sheinkop, M. Safety and Efficacy of Percutaneous Injection of Lipogems Micro-Fractured Adipose Tissue for Osteoarthritic Knees. Am. J. Orthop. Belle Mead NJ 2018, 47. [Google Scholar] [CrossRef]
- Veronese, N.; Cereda, E.; Maggi, S.; Luchini, C.; Solmi, M.; Smith, T.; Denkinger, M.; Hurley, M.; Thompson, T.; Manzato, E.; et al. Osteoarthritis and mortality: A prospective cohort study and systematic review with meta-analysis. Semin. Arthritis Rheum. 2016, 46, 160–167. [Google Scholar] [CrossRef]
- Yokota, N.; Hattori, M.; Ohtsuru, T.; Otsuji, M.; Lyman, S.; Shimomura, K.; Nakamura, N. Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am. J. Sport. Med. 2019, 47, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Sterodimas, A.; Pizzicannella, J.; Dionisi, L.; De Fazio, D.; Calabrese, C.; Garcovich, S. Systematic Review: Allogenic Use of Stromal Vascular Fraction (SVF) and Decellularized Extracellular Matrices (ECM) as Advanced Therapy Medicinal Products (ATMP) in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 4982. [Google Scholar] [CrossRef]
- Torres-Torrillas, M.; Rubio, M.; Damia, E.; Cuervo, B.; Del Romero, A.; Pelaez, P.; Chicharro, D.; Miguel, L.; Sopena, J.J. Adipose-Derived Mesenchymal Stem Cells: A Promising Tool in the Treatment of Musculoskeletal Diseases. Int. J. Mol. Sci. 2019, 20, 3105. [Google Scholar] [CrossRef] [Green Version]
- Behfar, M.; Javanmardi, S.; Sarrafzadeh-Rezaei, F. Comparative study on functional effects of allotransplantation of bone marrow stromal cells and adipose derived stromal vascular fraction on tendon repair: A biomechanical study in rabbits. Cell J. 2014, 16, 263–270. [Google Scholar] [PubMed]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Huang, S.J.; Fu, R.H.; Shyu, W.C.; Liu, S.P.; Jong, G.P.; Chiu, Y.W.; Wu, H.S.; Tsou, Y.A.; Cheng, C.W.; Lin, S.Z. Adipose-derived stem cells: Isolation, characterization, and differentiation potential. Cell Transpl. 2013, 22, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Ceserani, V.; Ferri, A.; Berenzi, A.; Benetti, A.; Ciusani, E.; Pascucci, L.; Bazzucchi, C.; Cocce, V.; Bonomi, A.; Pessina, A.; et al. Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc. Cell 2016, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Pallua, N.; Serin, M.; Wolter, T.P. Characterisation of angiogenetic growth factor production in adipose tissue-derived mesenchymal cells. J. Plast Surg. Hand Surg. 2014, 48, 412–416. [Google Scholar] [CrossRef]
- Strotman, P.K.; Novicoff, W.M.; Nelson, S.J.; Browne, J.A. Increasing Public Interest in Stem Cell Injections for Osteoarthritis of the Hip and Knee: A Google Trends Analysis. J. Arthroplast. 2019, 34, 1053–1057. [Google Scholar] [CrossRef]
- Hurley, E.T.; Yasui, Y.; Gianakos, A.L.; Seow, D.; Shimozono, Y.; Kerkhoffs, G.; Kennedy, J.G. Limited evidence for adipose-derived stem cell therapy on the treatment of osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 3499–3507. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunze, K.N.; Burnett, R.A.; Wright-Chisem, J.; Frank, R.M.; Chahla, J. Adipose-Derived Mesenchymal Stem Cell Treatments and Available Formulations. Curr. Rev. Musculoskelet. Med. 2020, 13, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Lin, G.; Garcia, M.; Ning, H.; Banie, L.; Guo, Y.L.; Lue, T.F.; Lin, C.S. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008, 17, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Black, L.L.; Gaynor, J.; Adams, C.; Dhupa, S.; Sams, A.E.; Taylor, R.; Harman, S.; Gingerich, D.A.; Harman, R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet. Ther. 2008, 9, 192–200. [Google Scholar] [PubMed]
- Pers, Y.M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Pak, J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: A case series. J. Med. Case Rep. 2011, 5, 296. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Gao, Q.; Zhang, Y.; He, Y. Autologous plateletrich plasma promotes proliferation and chondrogenic differentiation of adiposederived stem cells. Mol. Med. Rep. 2015, 11, 1298–1303. [Google Scholar] [CrossRef] [Green Version]
- Van Pham, P.; Bui, K.H.; Ngo, D.Q.; Vu, N.B.; Truong, N.H.; Phan, N.L.; Le, D.M.; Duong, T.D.; Nguyen, T.D.; Le, V.T.; et al. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res. Ther. 2013, 4, 91. [Google Scholar] [CrossRef] [Green Version]
- Pak, J.; Chang, J.J.; Lee, J.H.; Lee, S.H. Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet. Disord. 2013, 14, 337. [Google Scholar] [CrossRef] [Green Version]
- Pak, J.; Lee, J.H.; Lee, S.H. A novel biological approach to treat chondromalacia patellae. PLoS ONE 2013, 8, e64569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Pham, P.; Hong-Thien Bui, K.; Quoc Ngo, D.; Tan Khuat, L.; Kim Phan, N. Transplantation of Nonexpanded Adipose Stromal Vascular Fraction and Platelet-Rich Plasma for Articular Cartilage Injury Treatment in Mice Model. J. Med. Eng. 2013, 2013, 832396. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Akieda, S.; Misumi, K.; Nakayama, K. Osteochondral Regeneration with a Scaffold-Free Three-Dimensional Construct of Adipose Tissue-Derived Mesenchymal Stromal Cells in Pigs. Tissue Eng. Regen. Med. 2018, 15, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biazzo, A.; D’Ambrosi, R.; Masia, F.; Izzo, V.; Verde, F. Autologous adipose stem cell therapy for knee osteoarthritis: Where are we now? Phys. Sport. 2020, 48, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Lee, J.H.; Pak, N.J.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Clinical Protocol of Producing Adipose Tissue-Derived Stromal Vascular Fraction for Potential Cartilage Regeneration. J. Vis. Exp. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Donnenberg, V.S.; Rubin, J.P.; Donnenberg, A.D. Mesenchymal markers on human adipose stem/progenitor cells. Cytom. A 2013, 83, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Köprü, Ç.Z. Kas-iskelet sistemi hastalıklarında stromal vasküler fraksiyon (SVF). TOTBİD 2017, 16, 276–281. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Jacobs, J.J.; Chu, C.R.; Einhorn, T.A. Orthopaedic Basic Science: Foundations of Clinical Practice, 4th ed.; AAOS-American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 2018; pp. 1–371. [Google Scholar]
- Desando, G.; Bartolotti, I.; Martini, L.; Giavaresi, G.; Nicoli Aldini, N.; Fini, M.; Roffi, A.; Perdisa, F.; Filardo, G.; Kon, E.; et al. Regenerative Features of Adipose Tissue for Osteoarthritis Treatment in a Rabbit Model: Enzymatic Digestion Versus Mechanical Disruption. Int. J. Mol. Sci. 2019, 20, 2636. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, P.; Bekerecioglu, M. The Effect of Stromal Vascular Fraction for Patients with Androgenetic Alopecia. J. Turk. Acad. Dermatol. 2020, 14, 107–111. [Google Scholar] [CrossRef]
- Klar, A.S.; Zimoch, J.; Biedermann, T. Skin Tissue Engineering: Application of Adipose-Derived Stem Cells. Biomed. Res. Int. 2017, 2017, 9747010. [Google Scholar] [CrossRef] [Green Version]
- Svalgaard, J.D.; Juul, S.; Vester-Glovinski, P.V.; Haastrup, E.K.; Ballesteros, O.R.; Lynggaard, C.D.; Jensen, A.K.; Fischer-Nielsen, A.; Herly, M.; Munthe-Fog, L. Lipoaspirate Storage Ti.ime and Temperature: Effects on Stromal Vascular Fraction Quality and Cell Composition. Cells Tissues Organs 2020, 209, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Muthu, S.; Jeyaraman, M.; Ranjan, R.; Jha, S.K. Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications. World J. Stem Cells 2021, 13, 1360–1381. [Google Scholar] [CrossRef] [PubMed]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark, B.; Elliot, L.; Thomas, G.; Walter, O.; Jonathan, B.; Shawntae, D.; Sean, B. Prospective Study of Autologous Adipose Derived Stromal Vascular Fraction Containing Stem Cells for the Treatment of Knee Osteoarthritis. Int. J. Stem. Cell Res. Ther. 2019, 6, 064. [Google Scholar] [CrossRef] [Green Version]
- Pers, Y.M.; Quentin, J.; Feirreira, R.; Espinoza, F.; Abdellaoui, N.; Erkilic, N.; Cren, M.; Dufourcq-Lopez, E.; Pullig, O.; Noth, U.; et al. Injection of Adipose-Derived Stromal Cells in the Knee of Patients with Severe Osteoarthritis has a Systemic Effect and Promotes an Anti-Inflammatory Phenotype of Circulating Immune Cells. Theranostics 2018, 8, 5519–5528. [Google Scholar] [CrossRef]
- Cho, H.; Kim, H.; Kim, Y.g.; Kim, K. Recent Clinical Trials in Adipose-derived Stem Cell Mediated Osteoarthritis Treatment. Biotechnol. Bioprocess Eng. 2019, 24, 839–853. [Google Scholar] [CrossRef]
- Jones, I.A.; Wilson, M.; Togashi, R.; Han, B.; Mircheff, A.K.; Thomas Vangsness, C., Jr. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: A study protocol. BMC Musculoskelet. Disord. 2018, 19, 383. [Google Scholar] [CrossRef] [Green Version]
- Mehling, B.; Hric, M.; Salatkova, A.; Vetrak, R.; Santora, D.; Ovariova, M.; Mihalyova, R.; Manvelyan, M. A Retrospective Study of Stromal Vascular Fraction Cell Therapy for Osteoarthritis. J. Clin. Med. Res. 2020, 12, 747–751. [Google Scholar] [CrossRef]
- Tiryaki, T.; Conde-Green, A.; Cohen, S.R.; Canikyan, S.S.; Kocak, P. A 3-step Mechanical Digestion Method to Harvest Adipose-derived Stromal Vascular Fraction. Plast Reconstr. Surg. Glob. Open 2020, 8, e2652. [Google Scholar] [CrossRef]
- Winnier, G.E.; Valenzuela, N.; Peters-Hall, J.; Kellner, J.; Alt, C.; Alt, E.U. Isolation of adipose tissue derived regenerative cells from human subcutaneous tissue with or without the use of an enzymatic reagent. PLoS ONE 2019, 14, e0221457. [Google Scholar] [CrossRef] [Green Version]
- Desando, G.; Cavallo, C.; Sartoni, F.; Martini, L.; Parrilli, A.; Veronesi, F.; Fini, M.; Giardino, R.; Facchini, A.; Grigolo, B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res. Ther. 2013, 15, R22. [Google Scholar] [CrossRef] [Green Version]
- Çerçi, E. Non enzymatic isolation of adipose tissue and stromal vascular fraction derived cells. Ank. Üniversitesi Vet. Fakültesi Derg. 2020, 67, 295–301. [Google Scholar] [CrossRef]
- Riester, S.M.; Denbeigh, J.M.; Lin, Y.; Jones, D.L.; de Mooij, T.; Lewallen, E.A.; Nie, H.; Paradise, C.R.; Radel, D.J.; Dudakovic, A.; et al. Safety Studies for Use of Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells in a Rabbit Model for Osteoarthritis to Support a Phase I Clinical Trial. Stem Cells Transl. Med. 2017, 6, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Şahin, A.A.; Değirmenci, E.; Özturan, K.E.; Fırat, T.; Kükner, A. Effects of adipose tissue-derived stromal vascular fraction on osteochondral defects treated by hyaluronic acid-based scaffold: An experimental study. Jt. Dis. Relat. Surg. 2021, 32, 347. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Ellenhorn, J.D.I. Adipose stromal vascular fraction isolation: A head-to-head comparison of four commercial cell separation systems. Plast Reconstr. Surg. 2013, 132, 932e–939e. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transpl. 2013, 22, 2063–2077. [Google Scholar] [CrossRef]
- van Dongen, J.A.; Harmsen, M.C.; Stevens, H.P. Isolation of Stromal Vascular Fraction by Fractionation of Adipose Tissue. Methods Mol. Biol. 2019, 1993, 91–103. [Google Scholar] [CrossRef]
- Trivisonno, A.; Alexander, R.W.; Baldari, S.; Cohen, S.R.; Di Rocco, G.; Gentile, P.; Magalon, G.; Magalon, J.; Miller, R.B.; Womack, H.; et al. Intraoperative Strategies for Minimal Manipulation of Autologous Adipose Tissue for Cell- and Tissue-Based Therapies: Concise Review. Stem Cells Transl. Med. 2019, 8, 1265–1271. [Google Scholar] [CrossRef] [Green Version]
- Raposio, E.; Simonacci, F.; Perrotta, R.E. Adipose-derived stem cells: Comparison between two methods of isolation for clinical applications. Ann. Med. Surg. 2017, 20, 87–91. [Google Scholar] [CrossRef]
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen. 2015, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Koh, Y.G.; Choi, Y.J.; Kim, S.H.; Yoon, D.S.; Lee, M.; Lee, J.W. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. Vitr. Cell Dev. Biol. Anim. 2015, 51, 142–150. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Messaggio, F.; O, J.; Mendez, A. Differences in exosome content of human adipose tissue processed by non-enzymatic and enzymatic methods. CellR4 2015, 3, e1423. [Google Scholar]
- Detiger, S.E.; Helder, M.N.; Smit, T.H.; Hoogendoorn, R.J. Adverse effects of stromal vascular fraction during regenerative treatment of the intervertebral disc: Observations in a goat model. Eur. Spine J. 2015, 24, 1992–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamenaga, T.; Kuroda, Y.; Nagai, K.; Tsubosaka, M.; Takashima, Y.; Kikuchi, K.; Fujita, M.; Ikuta, K.; Anjiki, K.; Maeda, T. Cryopreserved human adipose-derived stromal vascular fraction maintains fracture healing potential via angiogenesis and osteogenesis in an immunodeficient rat model. Stem Cell Res. Ther. 2021, 12, 110. [Google Scholar]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, R.A.; Aronowitz, J.A.; Dos-Anjos Vilaboa, S. Use of Freshly Isolated Human Adipose Stromal Cells for Clinical Applications. Aesthet. Surg. J. 2017, 37, S4–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisicchia, S.; Bernardi, G.; Pagnotta, S.M.; Tudisco, C. Micro-fragmented stromal-vascular fraction plus microfractures provides better clinical results than microfractures alone in symptomatic focal chondral lesions of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 1876–1884. [Google Scholar] [CrossRef]
- van Dongen, J.A.; Tuin, A.J.; Spiekman, M.; Jansma, J.; van der Lei, B.; Harmsen, M.C. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: A systematic review. J. Tissue Eng. Regen. Med. 2018, 12, e261–e274. [Google Scholar] [CrossRef] [Green Version]
- Ghiasloo, M.; Lobato, R.C.; Diaz, J.M.; Singh, K.; Verpaele, A.; Tonnard, P. Expanding Clinical Indications of Mechanically Isolated Stromal Vascular Fraction: A Systematic Review. Aesthet. Surg. J. 2020, 40, NP546–NP560. [Google Scholar] [CrossRef]
- Hudetz, D.; Boric, I.; Rod, E.; Jelec, Z.; Radic, A.; Vrdoljak, T.; Skelin, A.; Lauc, G.; Trbojevic-Akmacic, I.; Plecko, M.; et al. The Effect of Intra-articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2017, 8, 270. [Google Scholar] [CrossRef] [Green Version]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sport. Med. 2020, 48, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Lee, J.H.; Lee, S.H. Regenerative repair of damaged meniscus with autologous adipose tissue-derived stem cells. Biomed. Res. Int. 2014, 2014, 436029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.D.X.; Wu, C.M.; Dubey, N.K.; Deng, Y.H.; Su, C.W.; Pham, T.T.; Thi Le, P.B.; Sestili, P.; Deng, W.P. Time- and Kellgren(-)Lawrence Grade-Dependent Changes in Intra-Articularly Transplanted Stromal Vascular Fraction in Osteoarthritic Patients. Cells 2019, 8, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onoi, Y.; Hiranaka, T.; Nishida, R.; Takase, K.; Fujita, M.; Hida, Y.; Fujishiro, T.; Okamoto, K. Second-look arthroscopic findings of cartilage and meniscus repair after injection of adipose-derived regenerative cells in knee osteoarthrits: Report of two cases. Regen. Ther. 2019, 11, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Carelli, S.; Messaggio, F.; Canazza, A.; Hebda, D.M.; Caremoli, F.; Latorre, E.; Grimoldi, M.G.; Colli, M.; Bulfamante, G.; Tremolada, C.; et al. Characteristics and Properties of Mesenchymal Stem Cells Derived From Microfragmented Adipose Tissue. Cell Transpl. 2015, 24, 1233–1252. [Google Scholar] [CrossRef] [Green Version]
- Barfod, K.W.; Blond, L. Treatment of osteoarthritis with autologous and microfragmented adipose tissue. Dan Med. J. 2019, 66, A5565. [Google Scholar] [PubMed]
- Cattaneo, G.; De Caro, A.; Napoli, F.; Chiapale, D.; Trada, P.; Camera, A. Micro-fragmented adipose tissue injection associated with arthroscopic procedures in patients with symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 176. [Google Scholar] [CrossRef] [PubMed]
- Yokota, N.; Yamakawa, M.; Shirata, T.; Kimura, T.; Kaneshima, H. Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen. Ther. 2017, 6, 108–112. [Google Scholar] [CrossRef]
- Smyshlyaev, I.A.; Gilfanov, S.I.; Kopylov, V.A.; Gilmutdinov, R.G.; Pulin, I.I.; Korsakov, I.N.; Gilmutdinova, I.R.; Petrikina, A.P.; Eremin, P.S.; Kruchkova, O.V.; et al. Safety and effectıveness of ıntraartıcular admınıstratıon of adıpose-derıved stromal vascular fractıon for treatment of knee artıcular cartılage degeneratıve damage: Prelımınary results of a clınıcal trıal. Travmatol. Ortop. Ross. 2017, 23, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Shevela, E.; Nitsa, N.; Starostina, N.; Baranov, S.; Kozhevnikov, Y.; Popova, N.; Batorov, E.; Ostanin, A.; Chernykh, E. Preliminary clinical results with lipoaspirate stromal vascular cell fraction in treatment of patients with knee osteoarthritis. Med. Immunol. 2017, 19, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Condello, V.; Madonna, V.; Guerriero, M.; Zorzi, C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. J. Exp. Orthop. 2017, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.D.; Tran, T.D.; Nguyen, H.T.; Vu, H.T.; Le, P.T.; Phan, N.L.; Vu, N.B.; Phan, N.K.; Van Pham, P. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Transl. Med. 2017, 6, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bansal, H.; Comella, K.; Leon, J.; Verma, P.; Agrawal, D.; Koka, P.; Ichim, T. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J. Transl. Med. 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, N.; Diamond, R.; Sekyere, E.O.; Thomas, W.D. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: A case series. J. Pain Res. 2015, 8, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Esposito, V.; Passaretti, F.; Perruolo, G.; Ambrosio, M.R.; Valentino, R.; Oriente, F.; Raciti, G.A.; Nigro, C.; Miele, C.; Sammartino, G.; et al. Platelet-Rich Plasma Increases Growth and Motility of Adipose Tissue-Derived Mesenchymal Stem Cells and Controls Adipocyte Secretory Function. J. Cell Biochem. 2015, 116, 2408–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Lee, M.; Koh, Y.G. Additional mesenchymal stem cell injection improves the outcomes of marrow stimulation combined with supramalleolar osteotomy in varus ankle osteoarthritis: Short-term clinical results with second-look arthroscopic evaluation. J. Exp. Orthop. 2016, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Park, E.H.; Kim, Y.C.; Koh, Y.G. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am. J. Sport. Med. 2013, 41, 1090–1099. [Google Scholar] [CrossRef]
- Grossi, P.; Giarratana, S.; Cernei, S.; Grossi, S.; Doniselli, F. Low back pain treated with disc decompression and autologous micro-fragmented adipose tissue: A case report. CellR4 2016, 1772, 4. [Google Scholar]
- Franceschini, M.; Castellaneta, C.; Mineo, G.V. Injection of autologous micro-fragmented adipose tissue for the treatment of post-traumatic degenerative lesion of knee cartilage: A case report. CellR4 2016, 4, e1765. [Google Scholar]
- Bosetti, M.; Borrone, A.; Follenzi, A.; Messaggio, F.; Tremolada, C.; Cannas, M. Human Lipoaspirate as Autologous Injectable Active Scaffold for One-Step Repair of Cartilage Defects. Cell Transpl. 2016, 25, 1043–1056. [Google Scholar] [CrossRef] [Green Version]
- Striano, R.D.; Chen, H.; Bilbool, N.; Azatullah, K.; Hilado, J.; Horan, K. Non-Responsive Knee Pain with Osteoarthritis and Concurrent Meniscal Disease Treated With Autologous Micro-Fragmented Adipose Tissue Under Continuous Ultrasound Guidance. CellR4 2015, 3, e1690. [Google Scholar]
- Lee, S.Y.; Kim, W.; Lim, C.; Chung, S.G. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells 2015, 33, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Choi, Y.J.; Kwon, O.R.; Kim, Y.S. Second-Look Arthroscopic Evaluation of Cartilage Lesions After Mesenchymal Stem Cell Implantation in Osteoarthritic Knees. Am. J. Sport. Med. 2014, 42, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Choi, Y.J.; Kwon, S.K.; Kim, Y.S.; Yeo, J.E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2015, 23, 1308–1316. [Google Scholar] [CrossRef]
- Benzi, R.; Marfia, G.; Bosetti, M.; Beltrami, G.; Magri, A.S.; Versari, S.; Tremolada, C. Microfractured lipoaspirate may help oral bone and soft tissue regeneration: A case report. CellR4 2015, 3, e1583. [Google Scholar]
- Raffaini, M.; Tremolada, C. Micro Fractured and Purified Adipose Tissue Graft (Lipogems®) Can Improve the Orthognathic Surgery Outcomes Both Aesthetically and in Postoperative Healing. CellR4 2014, 2, e1118. [Google Scholar]
- Pham, P.V.; Bui, K.H.-T.; Duong, T.D.; Nguyen, N.T.; Nguyen, T.D.; Le, V.T.; Mai, V.N.T.; Phan, N.L.-C.; Le, D.M.; Ngoc, N.K. Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: A clinical study. Biomed. Res. Ther. 2014, 1, 1–7. [Google Scholar]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Koh, Y.G.; Jo, S.B.; Kwon, O.R.; Suh, D.S.; Lee, S.W.; Park, S.H.; Choi, Y.J. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 2013, 29, 748–755. [Google Scholar] [CrossRef]
- Koh, Y.G.; Choi, Y.J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef]
- Pak, J. Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads. Pain Physician 2012, 15, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Tsubosaka, M.; Matsumoto, T.; Sobajima, S.; Matsushita, T.; Iwaguro, H.; Kuroda, R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet. Disord. 2020, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Santoprete, S.; Marchetti, F.; Rubino, C.; Bedini, M.G.; Nasto, L.A.; Cipolloni, V.; Pola, E. Fresh autologous stromal tissue fraction for the treatment of knee osteoarthritis related pain and disability. Orthop. Rev. 2021, 13, 9161. [Google Scholar] [CrossRef] [PubMed]
- Tsubosaka, M.; Matsumoto, T.; Sobajima, S.; Matsushita, T.; Iwaguro, H.; Kuroda, R. Comparison of Clinical and Imaging Outcomes of Different Doses of Adipose-Derived Stromal Vascular Fraction Cell Treatment for Knee Osteoarthritis. Cell Transplant. 2021, 30, 09636897211067454. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2019, 43, 1123–1134. [Google Scholar] [CrossRef]
- Aletto, C.; Giordano, L.; Quaranta, M.; Zara, A.; Notarfrancesco, D.; Maffulli, N. Short-term results of intra-articular injections of stromal vascular fraction for early knee osteoarthritis. J. Orthop. Surg. Res. 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Labarre, K.W.; Zimmermann, G. Infiltration of the Hoffa’s fat pad with stromal vascular fraction in patients with osteoarthritis of the knee-Results after one year of follow-up. Bone Rep. 2022, 16, 101168. [Google Scholar] [CrossRef]
- Yokota, N.; Lyman, S.; Hanai, H.; Shimomura, K.; Ando, W.; Nakamura, N. Clinical Safety and Effectiveness of Adipose-Derived Stromal Cell vs Stromal Vascular Fraction Injection for Treatment of Knee Osteoarthritis: 2-Year Results of Parallel Single-Arm Trials. Am. J. Sport. Med. 2022, 50, 2659–2668. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; He, B.; Fan, M.; Xiao, M.; Zhang, J.; Chen, D.; Tong, P.; Mao, Q. Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: A minimum 5-year follow-up study. Stem Cell Res. Ther. 2022, 13, 105. [Google Scholar] [CrossRef]
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Delitala, A.; Bianchi, F.; Tremolada, C.; Fontani, V.; Ventura, C. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: A novel approach to multipotency. Cell Transpl. 2014, 23, 1489–1500. [Google Scholar] [CrossRef]
- Fodor, P.B.; Paulseth, S.G. Adipose Derived Stromal Cell (ADSC) Injections for Pain Management of Osteoarthritis in the Human Knee Joint. Aesthet. Surg. J. 2016, 36, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdisa, F.; Gostynska, N.; Roffi, A.; Filardo, G.; Marcacci, M.; Kon, E. Adipose-Derived Mesenchymal Stem Cells for the Treatment of Articular Cartilage: A Systematic Review on Preclinical and Clinical Evidence. Stem Cells Int. 2015, 2015, 597652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, J.D.; Chang, W.-T.; Dragoo, J.L. The use of vibrational energy to isolate adipose-derived stem cells. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1620. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Chang, W. Arthroscopic Harvest of Adipose-Derived Mesenchymal Stem Cells from the Infrapatellar Fat Pad. Am. J. Sport. Med. 2017, 45, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S.; Birnbaum, Z.E. Adipose stromal vascular fraction isolation: A head-to-head comparison of 4 cell separation systems# 2. Ann. Plast. Surg. 2016, 77, 354–362. [Google Scholar]
- Domenis, R.; Lazzaro, L.; Calabrese, S.; Mangoni, D.; Gallelli, A.; Bourkoula, E.; Manini, I.; Bergamin, N.; Toffoletto, B.; Beltrami, C.A. Adipose tissue derived stem cells: In vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res. Ther. 2015, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Patel, P.; Li, H.; Huang, L.T.; Wan, H.; Collins, S.; Connell, T.L.; Xu, H. Physical, biochemical, and biologic properties of fat graft processed via different methods. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3010. [Google Scholar] [CrossRef]
- Hanson, S.E.; Garvey, P.B.; Chang, E.I.; Reece, G.P.; Liu, J.; Baumann, D.P.; Butler, C.E. A randomized prospective time and motion comparison of techniques to process autologous fat grafts. Plast. Reconstr. Surg. 2021, 147, 1035–1044. [Google Scholar] [CrossRef]
- Schafer, M.E.; Hicok, K.C.; Mills, D.C.; Cohen, S.R.; Chao, J.J. Acute adipocyte viability after third-generation ultrasound-assisted liposuction. Aesthetic Surg. J. 2013, 33, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Hivernaud, V.; Lefourn, B.; Robard, M.; Guicheux, J.; Weiss, P. Autologous fat grafting: A comparative study of four current commercial protocols. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 248–256. [Google Scholar] [CrossRef]
- Rodriguez, J.; Pratta, A.-S.; Abbassi, N.; Fabre, H.; Rodriguez, F.; Debard, C.; Adobati, J.; Boucher, F.; Mallein-Gerin, F.; Auxenfans, C. Evaluation of three devices for the isolation of the stromal vascular fraction from adipose tissue and for ASC culture: A comparative study. Stem Cells Int. 2017, 2017, 9289213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Cohen, S.R.; Hicok, K.C.; Shanahan, R.K.; Strem, B.M.; Johnson, C.Y.; Arm, D.M.; Fraser, J.K. Comparison of three different fat graft preparation methods: Gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast. Reconstr. Surg. 2013, 131, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Mestak, O.; Sukop, A.; Hsueh, Y.-S.; Molitor, M.; Mestak, J.; Matejovska, J.; Zarubova, L. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J. Surg. Oncol. 2014, 12, 178. [Google Scholar] [CrossRef] [Green Version]
- Assad, M.; Howell, S.M.; Liu, J.; Reece, G.P.; Chang, E.I.; Garvey, P.B.; Butler, C.E.; Hanson, S.E. The Effect of Lipoaspirate Processing Technique on Complications in Autologous Fat Grafting for Breast Reconstruction: A Propensity Score Analysis Study. Aesthetic Surg. J. 2021, 41, NP1303–NP1309. [Google Scholar] [CrossRef] [PubMed]
- De Fazio, D.; Cingozoglu, C.A.C. Combined mastopexy and augmentation with autologous fat grafting: First results with lipopexy. Plast. Reconstr. Surg. Glob. Open 2020, 8, e1957. [Google Scholar] [CrossRef]
- Sforza, M.; Andjelkov, K.; Zaccheddu, R.; Husein, R.; Atkinson, C. A preliminary assessment of the predictability of fat grafting to correct silicone breast implant-related complications. Aesthetic Surg. J. 2016, 36, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Kuka, G.; Epstein, J.; Aronowitz, J.; Glasgold, M.J.; Rogal, J.G.; Brown, W.; Geronemus, R.G.; Daniels, E.J.; Washenik, K. Cell enriched autologous fat grafts to follicular niche improves hair regrowth in early androgenetic alopecia. Aesthetic Surg. J. 2020, 40, NP328–NP339. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose tissue and mesenchymal stem cells: State of the art and Lipogems® technology development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Magnanelli, S.; Screpis, D.; Di Benedetto, P.; Natali, S.; Causero, A.; Zorzi, C. Open-wedge high tibial osteotomy associated with lipogems® intra-articular injection for the treatment of varus knee osteoarthritis–retrospective study. Acta Bio Med. Atenei Parm. 2020, 91, e2020022. [Google Scholar]
- Randelli, P.; Menon, A.; Ragone, V.; Creo, P.; Bergante, S.; Randelli, F.; De Girolamo, L.; Alfieri Montrasio, U.; Banfi, G.; Cabitza, P. Lipogems product treatment increases the proliferation rate of human tendon stem cells without affecting their stemness and differentiation capability. Stem Cells Int. 2016, 2016, 4373410. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Contreras, M.; Messaggio, F.; Mendez, A.; Ricordi, C. Metabolomic changes in human adipose tissue derived products following non-enzymatic microfacturing. Eur. Rev. Med. Pharm. Sci. 2018, 22, 3249–3260. [Google Scholar]
- Tremolada, C.; Ricordi, C.; Caplan, A.I.; Ventura, C. Mesenchymal stem cells in Lipogems, a reverse story: From clinical practice to basic science. In Mesenchymal Stem Cells; Springer: New York, NY, USA, 2016; pp. 109–122. [Google Scholar]
- Guo, B.; Sawkulycz, X.; Heidari, N.; Rogers, R.; Liu, D.; Slevin, M. Characterisation of novel angiogenic and potent anti-inflammatory effects of micro-fragmented adipose tissue. Int. J. Mol. Sci. 2021, 22, 3271. [Google Scholar] [CrossRef]
- Vinet-Jones, H.; F Darr, K. Clinical use of autologous micro-fragmented fat progressively restores pain and function in shoulder osteoarthritis. Regen. Med. 2020, 15, 2153–2161. [Google Scholar] [CrossRef]
- Sembronio, S.; Tel, A.; Tremolada, C.; Lazzarotto, A.; Isola, M.; Robiony, M. Temporomandibular joint arthrocentesis and microfragmented adipose tissue injection for the treatment of internal derangement and osteoarthritis: A randomized clinical trial. J. Oral Maxillofac. Surg. 2021, 79, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Kokai, L.; Chen, J.; Wang, D.; Wang, S.; Egro, F.M.; Schilling, B.; Sun, H.; Ejaz, A.; Rubin, J.P.; Gusenoff, J.A. Comparison of clinically relevant adipose preparations on articular chondrocyte phenotype in a novel in vitro co-culture model. Stem Cells Dev. 2022, 31, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Casari, G.; Resca, E.; Giorgini, A.; Candini, O.; Petrachi, T.; Piccinno, M.S.; Foppiani, E.M.; Pacchioni, L.; Starnoni, M.; Pinelli, M. Microfragmented adipose tissue is associated with improved ex vivo performance linked to HOXB7 and b-FGF expression. Stem Cell Res. Ther. 2021, 12, 481. [Google Scholar] [CrossRef] [PubMed]
- Coccè, V.; Brini, A.; Giannì, A.B.; Sordi, V.; Berenzi, A.; Alessandri, G.; Tremolada, C.; Versari, S.; Bosetto, A.; Pessina, A. A nonenzymatic and automated closed-cycle process for the isolation of mesenchymal stromal cells in drug delivery applications. Stem Cells Int. 2018, 2018, 4098140. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, R.K.; Blønd, L.; Hölmich, L.R.; Mølgaard, C.; Troelsen, A.; Hölmich, P.; Barfod, K.W. Treatment of osteoarthritis with autologous, micro-fragmented adipose tissue: A study protocol for a randomized controlled trial. Trials 2021, 22, 748. [Google Scholar] [CrossRef]
- Van Genechten, W.; Vuylsteke, K.; Martinez, P.R.; Swinnen, L.; Sas, K.; Verdonk, P. Autologous micro-fragmented adipose tissue (MFAT) to treat symptomatic knee osteoarthritis: Early outcomes of a consecutive case series. J. Clin. Med. 2021, 10, 2231. [Google Scholar] [CrossRef]
- Stefano, B.; Nicholas, E.; Roberto, V.; Elena, M.S.; Bruno, M. Mesenchymal stem cells injection in hip osteoarthritis: Preliminary results. Acta Bio Med. Atenei Parm. 2019, 90, 75. [Google Scholar]
- Shi, Z.; He, J.; He, J.; Xu, Y. Micro-fragmented adipose tissue regulated the biological functions of osteoarthritis synoviocytes by upregulating MiR-92a-3p expression. Tissue Cell 2022, 74, 101716. [Google Scholar] [CrossRef] [PubMed]
- Kaszyński, J.; Bąkowski, P.; Kiedrowski, B.; Stołowski, Ł.; Wasilewska-Burczyk, A.; Grzywacz, K.; Piontek, T. Intra-Articular Injections of Autologous Adipose Tissue or Platelet-Rich Plasma Comparably Improve Clinical and Functional Outcomes in Patients with Knee Osteoarthritis. Biomedicines 2022, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Krześniak, A.M.; Radzimowski, K.; Stolarczyk, A. Comparison of the treatment results of knee osteoarthritis using adipose tissue mesenchymal stromal cells derived through enzymatic digestion and mechanically fragmented adipose tissue. Medicine 2021, 100, e24777. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Péault, B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef] [Green Version]
- Schiavone Panni, A.; Vasso, M.; Braile, A.; Toro, G.; De Cicco, A.; Viggiano, D.; Lepore, F. Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: Identification of a subpopulation with greater response. Int. Orthop. 2019, 43, 7–13. [Google Scholar] [CrossRef]
- Cherian, C.; Malanga, G.A.; Hogaboom, N.; Pollack, M.A.; Dyson-Hudson, T.A. Autologous, micro-fragmented adipose tissue as a treatment for chronic shoulder pain in a wheelchair using individual with spinal cord injury: A case report. Spinal Cord. Ser. Cases 2019, 5, 46. [Google Scholar] [CrossRef]
- Spinelli, M.G.; Lorusso, V.; Palmisano, F.; Morelli, M.; Dell’Orto, P.G.; Tremolada, C.; Montanari, E. Endoscopic repair of a vesicouterine fistula with the injection of microfragmented autologous adipose tissue (Lipogems(®)). Turk. J. Urol. 2020, 46, 398–402. [Google Scholar] [CrossRef]
- Laureti, S.; Gionchetti, P.; Cappelli, A.; Vittori, L.; Contedini, F.; Rizzello, F.; Golfieri, R.; Campieri, M.; Poggioli, G. Refractory Complex Crohn’s Perianal Fistulas: A Role for Autologous Microfragmented Adipose Tissue Injection. Inflamm. Bowel. Dis. 2020, 26, 321–330. [Google Scholar] [CrossRef]
- Copeland, R.; Martin, J. Chronic prosthesis-related residual limb ulcer treated with autologous micro-fragmented adipose tissue. Regen. Ther. 2021, 18, 21–23. [Google Scholar] [CrossRef]
- Topal, U.; Eray, I.C.; Rencüzoğulları, A.; Yalav, O.; Alabaz, Ö. Short-term results of adipose-derived stem cell therapy for the treatment of complex perianal fistula A single center experience. Ann. Ital. Chir. 2019, 90, 583–589. [Google Scholar]
- Lonardi, R.; Leone, N.; Gennai, S.; Trevisi Borsari, G.; Covic, T.; Silingardi, R. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: A randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res. Ther. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casarotti, G.A.; Chiodera, P.; Tremolada, C. Menopause: New frontiers in the treatment of urogenital atrophy. Eur. Rev. Med. Pharm. Sci. 2018, 22, 567–574. [Google Scholar] [CrossRef]
- Ceresa, C.; Borrone, A.; Fracchia, L.; Rinaldi, M.; Marchetti, A.; Tremolada, C.; Bosetti, M. Lipoaspirate Shows In Vitro Potential for Wound Healing. Pharmaceutics 2022, 14, 447. [Google Scholar] [CrossRef]
- Zuin, M.; Ruperto, M.; Balduino, M.; Amodeo, A.; De Zorzi, L.; Roche, B.; Pavanello, M.; Sernagiotto, C. Rectal advancement flap plus adipose lipofilling (RAFAL) for the treatment of rectourethral fistulas after radical prostatectomy. Tech. Coloproctol. 2019, 23, 1003–1007. [Google Scholar] [CrossRef]
- Parente, G.; Pinto, V.; Di Salvo, N.; D’Antonio, S.; Libri, M.; Gargano, T.; Catania, V.D.; Ruggeri, G.; Lima, M. Preliminary Study on the Echo-Assisted Intersphincteric Autologous Microfragmented Adipose Tissue Injection to Control Fecal Incontinence in Children Operated for Anorectal Malformations. Children 2020, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Naldini, G.; Sturiale, A.; Fabiani, B.; Giani, I.; Menconi, C. Micro-fragmented adipose tissue injection for the treatment of complex anal fistula: A pilot study accessing safety and feasibility. Tech. Coloproctol. 2018, 22, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Cicione, C.; Di Taranto, G.; Barba, M.; Isgrò, M.A.; D’Alessio, A.; Cervelli, D.; Sciarretta, F.V.; Pelo, S.; Michetti, F.; Lattanzi, W. In Vitro Validation of a Closed Device Enabling the Purification of the Fluid Portion of Liposuction Aspirates. Plast Reconstr. Surg. 2016, 137, 1157–1167. [Google Scholar] [CrossRef]
- Kavala, A.A.; Turkyilmaz, S. Autogenously derived re.egenerative cell therapy for venous leg ulcers. Arch. Med. Sci. Atheroscler. Dis. 2018, 3, e156–e163. [Google Scholar] [CrossRef]
- Lobascio, P.; Balducci, G.; Minafra, M.; Laforgia, R.; Fedele, S.; Conticchio, M.; Palasciano, N. Adipose-derived stem cells (MYSTEM® EVO Technology) as a treatment for complex transsphincteric anal fistula. Tech. Coloproctol. 2018, 22, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Giudice, G.; Filoni, A.; Maggio, G.; Bonamonte, D.; Maruccia, M.; Nacchiero, E.; Ribatti, D.; Annese, T.; Vestita, M. Use of the Stromal Vascular Fraction in Intermediate-Deep Acute Burns: A Case With Its Own Control. J. Burn Care Res. 2018, 39, 846–849. [Google Scholar] [CrossRef]
- Stevens, H.P.; van Boxtel, J.; van Dijck, R.; van Dongen, J.A. Platelet Rich STROMA, the Combination of PRP and tSVF and Its Potential Effect on Osteoarthritis of the Knee. Appl. Sci. 2020, 10, 4691. [Google Scholar] [CrossRef]
- Stevens, H. ACA-Technik: “stromal vascular fraction”, “platelet-rich plasma” und Mikrofett zur körpereigenen Regeneration und Hautverjüngung. J. Ästhetische Chir. 2019, 12, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Stevens, H.P.; Donners, S.; de Bruijn, J. Introducing Platelet-Rich Stroma: Platelet-Rich Plasma (PRP) and Stromal Vascular Fraction (SVF) Combined for the Treatment of Androgenetic Alopecia. Aesthet Surg. J. 2018, 38, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Copcu, H.E. Supercharged Mechanical Stromal-cell Transfer (MEST). Plast Reconstr. Surg. Glob. Open 2021, 9, e3552. [Google Scholar] [CrossRef]
- Copcu, H.E. Indication-based protocols with different solutions for mechanical stromal-cell transfer. Scars Burn Heal. 2022, 8. [Google Scholar] [CrossRef]
- Zocchi, M.L.; Facchin, F.; Pagani, A.; Bonino, C.; Sbarbati, A.; Conti, G.; Vindigni, V.; Bassetto, F. New perspectives in regenerative medicine and surgery: The bioactive composite therapies (BACTs). Eur. J. Plast. Surg. 2022, 45, 1–25. [Google Scholar] [CrossRef]
- Rossi, M.; Roda, B.; Zia, S.; Vigliotta, I.; Zannini, C.; Alviano, F.; Bonsi, L.; Zattoni, A.; Reschiglian, P.; Gennai, A. Characterization of the Tissue and Stromal Cell Components of Micro-Superficial Enhanced Fluid Fat Injection (Micro-SEFFI) for Facial Aging Treatment. Aesthet Surg. J. 2020, 40, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.R.; Tiryaki, T.; Womack, H.A.; Canikyan, S.; Schlaudraff, K.U.; Scheflan, M. Cellular Optimization of Nanofat: Comparison of Two Nanofat Processing Devices in Terms of Cell Count and Viability. Aesthet Surg. J. Open Forum 2019, 1, ojz028. [Google Scholar] [CrossRef]
- Tiryaki, K.T.; Cohen, S.; Kocak, P.; Canikyan Turkay, S.; Hewett, S. In-Vitro Comparative Examination of the Effect of Stromal Vascular Fraction Isolated by Mechanical and Enzymatic Methods on Wound Healing. Aesthet Surg. J. 2020, 40, 1232–1240. [Google Scholar] [CrossRef]
- Usuelli, F.G.; Grassi, M.; Montrasio, U.A.; De Girolamo, L.; Boga, M. Freshly Isolated Adipose-Derived Stem Cells for the Treatment of Achilles Tendinopathy: A Randomized Prospective Clinical Trial. Foot Ankle Orthop. 2016, 1, 2473011416S2473000006. [Google Scholar] [CrossRef] [Green Version]
- Simunec, D.; Salari, H.; Meyer, J. Treatment of Grade 3 and 4 Osteoarthritis with Intraoperatively Separated Adipose Tissue-Derived Stromal Vascular Fraction: A Comparative Case Series. Cells 2020, 9, 2096. [Google Scholar] [CrossRef] [PubMed]
- Sesé, B.; Sanmartín, J.M.; Ortega, B.; Matas-Palau, A.; Llull, R. Nanofat Cell Aggregates: A Nearly Constitutive Stromal Cell Inoculum for Regenerative Site-Specific Therapies. Plast Reconstr. Surg. 2019, 144, 1079–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caforio, M.; Nobile, C. Intra-Articular Administration of Autologous Purified Adipose Tissue Associated with Arthroscopy Ameliorates Knee Osteoarthritis Symptoms. J. Clin. Med. 2021, 10, 2053. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.E.; Cui, X.; Fink, B.F.; Vasconez, H.C.; Pu, L.L. The viability of autologous fat grafts harvested with the LipiVage system: A comparative study. Ann. Plast Surg. 2008, 60, 594–597. [Google Scholar] [CrossRef]
- Papadopulos, N.A.; Wigand, S.; Kuntz, N.; Piringer, M.; Machens, H.G.; Klüter, H.; Bieback, K.; Karagianni, M. The Impact of Harvesting Systems and Donor Characteristics on Viability of Nucleated Cells in Adipose Tissue: A First Step Towards a Manufacturing Process. J. Craniofac. Surg. 2019, 30, 716–720. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Rubin, J.P.; Pfeifer, M.E.; Moore, L.R.; Donnenberg, V.S.; Donnenberg, A.D. Human adipose stromal vascular cell delivery in a fibrin spray. Cytotherapy 2013, 15, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.I.; Kim, M.S.; Kim, J.H. Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials. Am. J. Sport. Med. 2022. [Google Scholar] [CrossRef]
- Cao, L.; Xiaoming, F.; Zhang, Q.; Fang, J.; Chu, C.; Lv, J.; Ma, Y.; Lu, G.; Yang, K.; Pan, R. An Optimized Method for Adipose Stromal Vascular Fraction Isolation and its Application in Fat Grafting. Aesthetic Plast Surg. 2022, 46, 2500–2508. [Google Scholar] [CrossRef]
- Oato, I.; Mussano, F.; Reano, S.; Boriani, F.; Margara, A.; Ferracini, R.; Adriani, E.; Sabry, O.; Fiorini, M.; Fattori, P. A Novel Method to Optimize Autologous Adipose Tissue Recovery with Extracellular Matrix Preservation. Processes 2020, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- SundarRaj, S.; Deshmukh, A.; Priya, N.; Krishnan, V.S.; Cherat, M.; Majumdar, A.S. Development of a System and Method for Automated Isolation of Stromal Vascular Fraction from Adipose Tissue Lipoaspirate. Stem Cells Int. 2015, 2015, 109353. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Scioli, M.G.; Orlandi, A.; Cervelli, V. Breast Reconstruction with Enhanced Stromal Vascular Fraction Fat Grafting: What Is the Best Method? Plast Reconstr. Surg. Glob. Open 2015, 3, e406. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Grahovac, T.L.; Schafer, M.E.; Shippert, R.D.; Marra, K.G.; Rubin, J.P. Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast Reconstr. Surg. 2013, 132, 351–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papalia, R.; Zampogna, B.; Russo, F.; Vasta, S.; Campi, S.; Saccone, L.; Di Giacomo, G.; Vadala, G.; Denaro, V. Adipose-derived stromal vascular fraction processed with different systems for the treatment of knee osteoarthritis: A pilot study on cell proliferation and clinical results. J. Biol. Regul. Homeost. Agents 2020, 34, 113–119. [Google Scholar]
- Cleveland, E.C.; Albano, N.J.; Hazen, A. Roll, Spin, Wash, or Filter? Processing of Lipoaspirate for Autologous Fat Grafting: An Updated, Evidence-Based Review of the Literature. Plast Reconstr. Surg. 2015, 136, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Shang, H.; Li, Y.; Yang, N.; Patel, N.; Katz, A.J. Isolation of Adipose-Derived Stromal Vascular Fraction Cells Using a Novel Point-of-Care Device: Cell Characterization and Review of the Literature. Tissue Eng. Part. C Methods 2017, 23, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.M.; Mehrkens, A.; Schafer, D.J.; Jaquiery, C.; Guven, S.; Lehmicke, M.; Martinetti, R.; Farhadi, I.; Jakob, M.; Scherberich, A.; et al. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur. Cell Mater. 2010, 19, 127–135. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Ghayor, C.; Weber, F.E. The Use of Adipose Tissue-Derived Progenitors in Bone Tissue Engineering—A Review. Transfus Med. Hemother. 2016, 43, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Popa, E.G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Chondrogenic potential of injectable kappa-carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J. Tissue Eng. Regen. Med. 2015, 9, 550–563. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: A prospective study. Arthroscopy 2014, 30, 1453–1460. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult Mesenchymal Stem Cells: When, Where, and How. Stem Cells Int. 2015, 2015, 628767. [Google Scholar] [CrossRef] [Green Version]
- Berry, R.; Rodeheffer, M.S.; Rosen, C.J.; Horowitz, M.C. Adipose Tissue Residing Progenitors (Adipocyte Lineage Progenitors and Adipose Derived Stem Cells (ADSC). Curr. Mol. Biol. Rep. 2015, 1, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, H.Y.-H.; Lu, V.; Khan, W. Adipose Tissue-Derived Mesenchymal Stem Cells as a Potential Restorative Treatment for Cartilage Defects: A PRISMA Review and Meta-Analysis. Pharmaceuticals 2021, 14, 1280. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Mak, C.; Bojanic, C.; To, K.; Khan, W. Meta-analysis of adipose tissue derived cell-based therapy for the treatment of knee osteoarthritis. Cells 2021, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Mehranfar, S.; Abdi Rad, I.; Mostafavi, E.; Akbarzadeh, A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: An overview of clinical trials. Artif. Cells Nanomed. Biotechnol. 2019, 47, 882–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Pizzicannella, J.; Kothari, A.; Garcovich, S. Impact of the different preparation methods to obtain human adipose-derived stromal vascular fraction cells (AD-SVFs) and human adipose-derived mesenchymal stem cells (AD-MSCs): Enzymatic digestion versus mechanical centrifugation. Int. J. Mol. Sci. 2019, 20, 5471. [Google Scholar] [CrossRef] [Green Version]
- Roffi, A.; Nakamura, N.; Sanchez, M.; Cucchiarini, M.; Filardo, G. Injectable systems for intra-articular delivery of mesenchymal stromal cells for cartilage treatment: A systematic review of preclinical and clinical evidence. Int. J. Mol. Sci. 2018, 19, 3322. [Google Scholar] [CrossRef] [Green Version]
- Pak, J.; Lee, J.H.; Park, K.S.; Park, M.; Kang, L.-W.; Lee, S.H. Current use of autologous adipose tissue-derived stromal vascular fraction cells for orthopedic applications. J. Biomed. Sci. 2017, 24, 9. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Koh, Y.G. Injection of Mesenchymal Stem Cells as a Supplementary Strategy of Marrow Stimulation Improves Cartilage Regeneration After Lateral Sliding Calcaneal Osteotomy for Varus Ankle Osteoarthritis: Clinical and Second-Look Arthroscopic Results. Arthroscopy 2016, 32, 878–889. [Google Scholar] [CrossRef]
- Ude, C.C.; Shah, S.; Ogueri, K.S.; Nair, L.S.; Laurencin, C.T. Stromal Vascular Fraction for Osteoarthritis of the Knee Regenerative Engineering. Regen. Eng. Transl Med. 2022, 8, 210–224. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Mesenchymal stromal cell products for intra-articular knee injections for conservative management of osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X21996953. [Google Scholar] [CrossRef]
- Lavagnolo, U.; Veronese, S.; Negri, S.; Magnan, B.; Sbarbati, A. Lipoaspirate processing for the treatment of knee osteoarthritis: A review of clinical evidences. Biomed. Pharmacother. 2021, 142, 111997. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Otsuka, T.; Bhattacharjee, M.; Laurencin, C.T. Minimally invasive cellular therapies for osteoarthritis treatment. Regen. Eng. Transl. Med. 2021, 7, 76–90. [Google Scholar] [CrossRef]
- Bolia, I.K.; Bougioukli, S.; Hill, W.J.; Trasolini, N.A.; Petrigliano, F.A.; Lieberman, J.R.; Weber, A.E. Clinical efficacy of bone marrow aspirate concentrate versus stromal vascular fraction injection in patients with knee osteoarthritis: A systematic review and meta-analysis. Am. J. Sport. Med. 2022, 50, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Zeng, Y.; Li, M.; Xie, H.; Shen, B. Intra-articular injection of stromal vascular fraction for knee degenerative joint disease: A concise review of preclinical and clinical evidence. Sci. China Life Sci. 2022, 65, 1959–1970. [Google Scholar] [CrossRef]


| Coronary heart disease |
| Peripheral vascular diseases (Burger’s Disease) |
| Dermatopathy |
| Chronic wounds (Pressure ulcers) |
| Ischemia |
| Fistula |
| Liver fibrosis |
| Lipodystrophy |
| Radiation-induced ulcers |
| Alopecia |
| Osteoarthritis (OA) |
| Rheumatoid Arthritis (RA) |
| Systemic lupus erythematosus (SLE) |
| Diabetic foot ulcers |
| Breast cancer-related lymphedema |
| Crohn’s disease |
| Erectile dysfunction |
| Type 2 diabetes mellitus |
| Multiple sclerosis (neurodegenerative diseases) |
| Chronic obstructive pulmonary disease (COPD) |
| Nonunion fractures (High tibial osteotomy etc.) |
| Micromastia |
| Fat and skin grafts |
| Systemic sclerosis |
| Rotator cuff injury |
| Tendinopathy (Achilles tendinitis) |
| Osteochondral defects |
| Anti-inflammatory | Reduces tissue swelling (edema). |
| Anti-apoptotic | Reduces and stops programmed cell death. |
| Anti-fibrotic | Prevents tissue adhesions. |
| Increasing of TIMPs-1, -3, and -4 metalloproteinases | Provides tissue balance (Homeostasis). |
| Inhibition of MMP-1, MMP-3, MMP-13, and MMP-28 metalloproteinases | Provides tissue balance (Homeostasis). |
| Increasing of ADAMTS-4 and -5 | Provides tissue balance (Homeostasis). |
| Regulation of pro-inflammatory molecules | Decreases IL-1b and IL-6 levels. |
| Triggering of IL-1Ra | Reduces the catabolic effect of IL-1. |
| Hyaline cartilage ECM | Increases GAG level. |
| Enzyme Decomposition | Mechanical Separation | |
|---|---|---|
| Initial amount of adipose tissue | 300 mL ↑ | 60 mL ↓ |
| Incubation | (+) | (−) |
| Washing | (+) | (−) |
| Centrifuge | (+) | (−) |
| Device | (+) | (−) |
| Disposable consumable | (+) | (+) |
| Reliability | (+/−) | (+) |
| Bacterial contamination | (+/−) | (−) |
| Enzyme-related side effects in tissue | (+) | (−) |
| Implementation cost | ↑ | ↓ |
| Duration of implementation | 2 h ↑ | 1 h ↓ |
| Number of cells | ↑ | ↓ |
| Cell surface marker | ↓ | ↑ |
| Conventional | Modified Approach | |
|---|---|---|
| Obtaining adipose tissue | - Abdominal fat - Reusable Sorenson type lipoaspiration cannula - Klein’s Translumination solution: Modified Klein solution (500 mL isotonic, 20 mL lidocaine, 2% epinephrine, 2 mL bicarbonate) - 50 mL Luer-Lock syringe | - Abdominal fat - Disposable/Re-usable Coleman style cannula - Klein’s Translumination solution: Modified Klein solution (500 mL isotonic, 20 mL lidocaine, 2% epinephrine, 2 mL bicarbonate) - 50 mL Luer-Lock syringe |
| Mechanical separation/shredding | - Shredding of tissue by shaking with glass ball (shaking time and strength depend on the user) | - Separation by the effect of gravity in a screw form mechanical separator at standard power and time. |
| Pre-filtration | - Polyethylene filtration in a 100 micrometer porous polyethylene bag | -Filtration with the effect of gravity in the 100 micrometer porous device whose base will be supported by a metallic or polymeric cage. |
| Washing | (−) | - Washing in the device |
| Final filtration | -Filtration on 10 micrometer porous polyethylene filters in 10 mL syringes (50 repetitions?) | - Final filtration with the rise of adipose tissue and SVF to the solution surface in serum within the device. |
| Collection of SVF/adipose tissue | - Available in an equivalent system | -Proximal adipose tissue and SVF separation reservoir. |
| Cell counting and characterization | - Cell counting, determination of viability, determination of cell characteristics, and histochemical identification | - Cell counting, determination of viability, determination of cell characteristics, and histochemical identification |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargel, İ.; Tuncel, A.; Baysal, N.; Hartuç-Çevik, İ.; Korkusuz, F. Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 13517. https://doi.org/10.3390/ijms232113517
Vargel İ, Tuncel A, Baysal N, Hartuç-Çevik İ, Korkusuz F. Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis. International Journal of Molecular Sciences. 2022; 23(21):13517. https://doi.org/10.3390/ijms232113517
Chicago/Turabian StyleVargel, İbrahim, Ali Tuncel, Nilsu Baysal, İrem Hartuç-Çevik, and Feza Korkusuz. 2022. "Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis" International Journal of Molecular Sciences 23, no. 21: 13517. https://doi.org/10.3390/ijms232113517
APA StyleVargel, İ., Tuncel, A., Baysal, N., Hartuç-Çevik, İ., & Korkusuz, F. (2022). Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis. International Journal of Molecular Sciences, 23(21), 13517. https://doi.org/10.3390/ijms232113517







