MK2 Promotes the Development and Progression of Pancreatic Neuroendocrine Tumors Mediated by Macrophages and Metabolomic Factors
Abstract
1. Introduction
2. Results
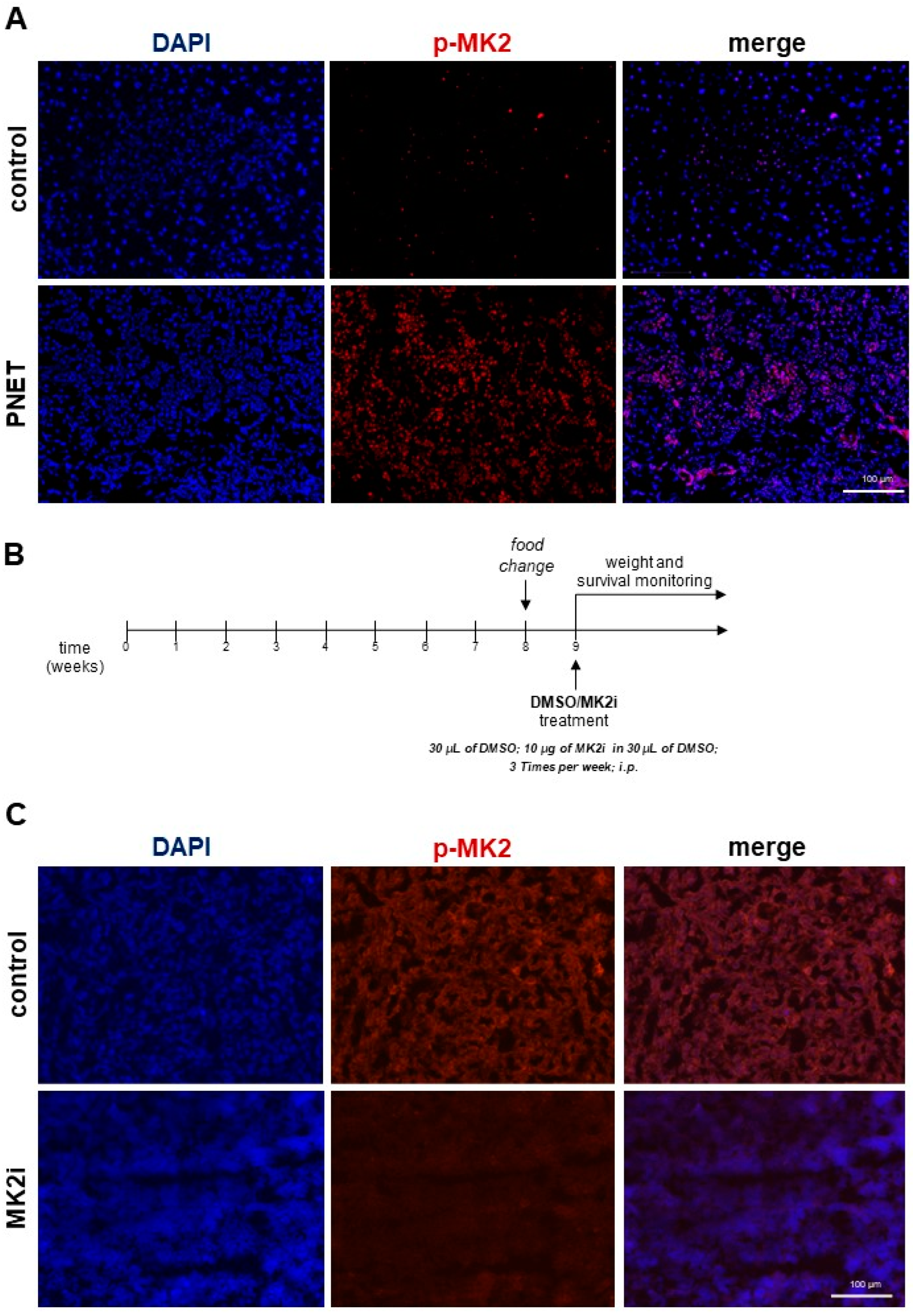
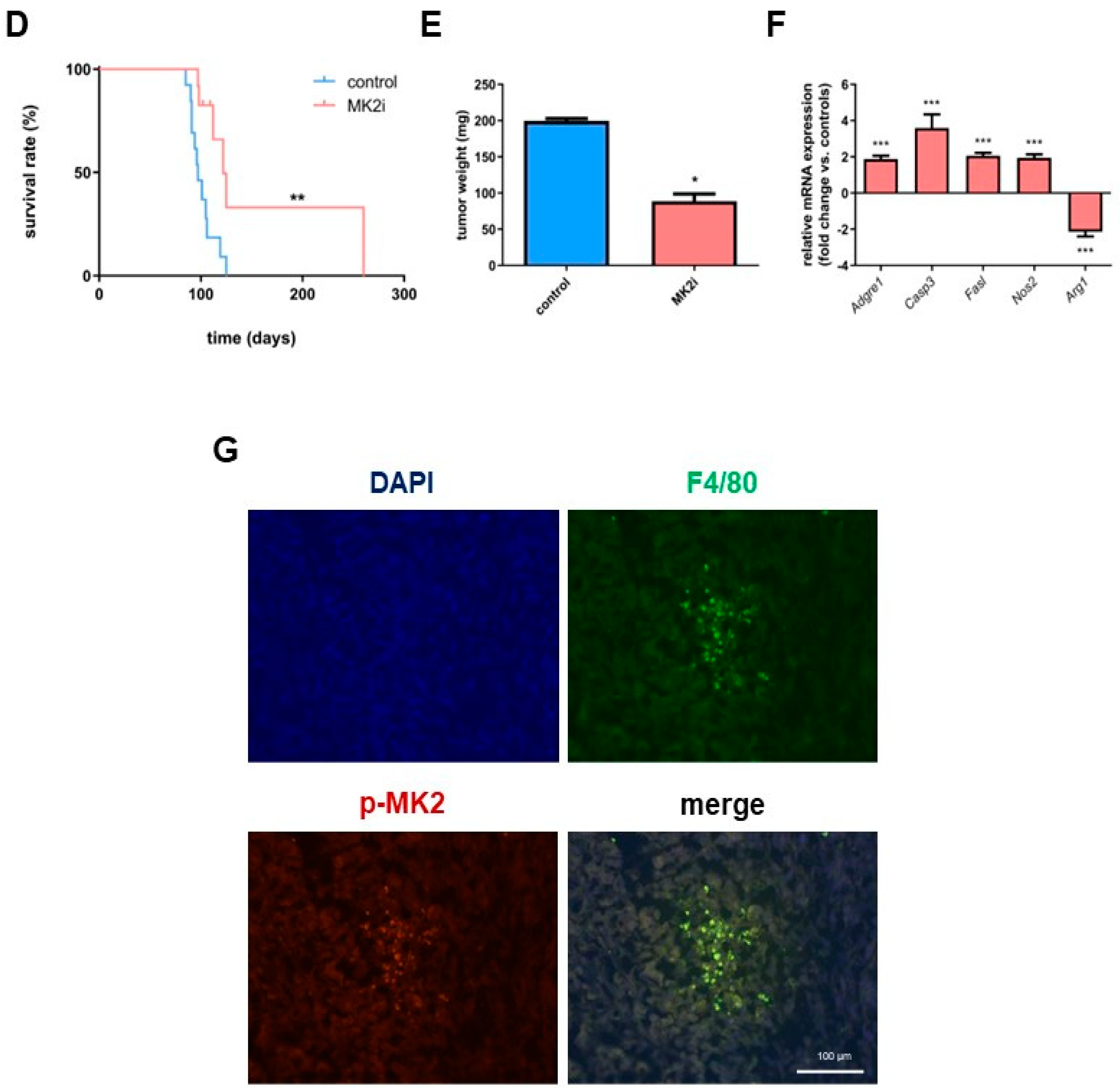
2.1. Inhibition of MK2 Improves Survival and Prevents the Progression of Pancreatic Neuroendocrine Tumors
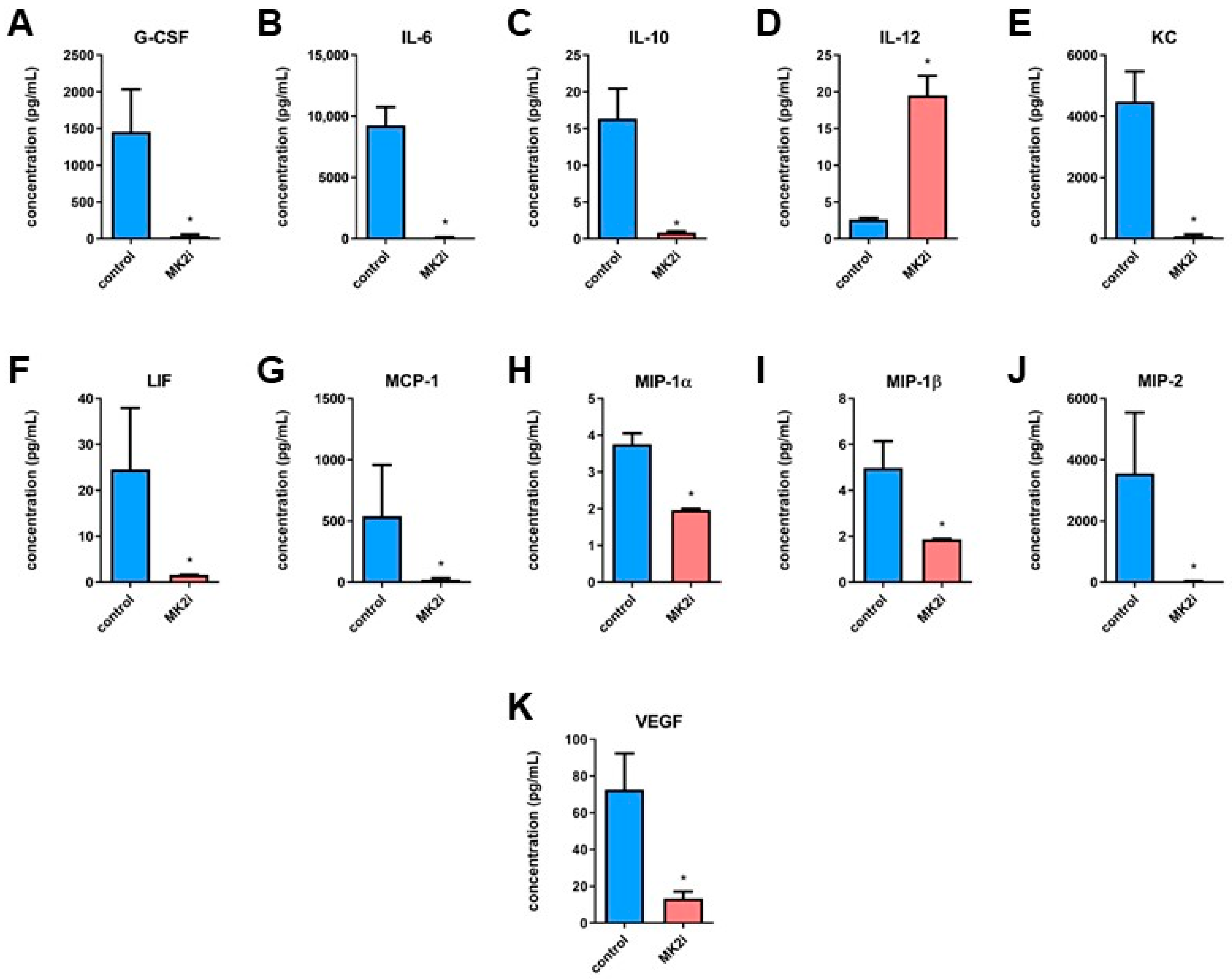
2.2. Inhibition of MK2 Affects Production and Secretion of Pro-Tumorigenic Macrophage-Related Cytokines and Chemokines in Pancreatic Neuroendocrine Tumors
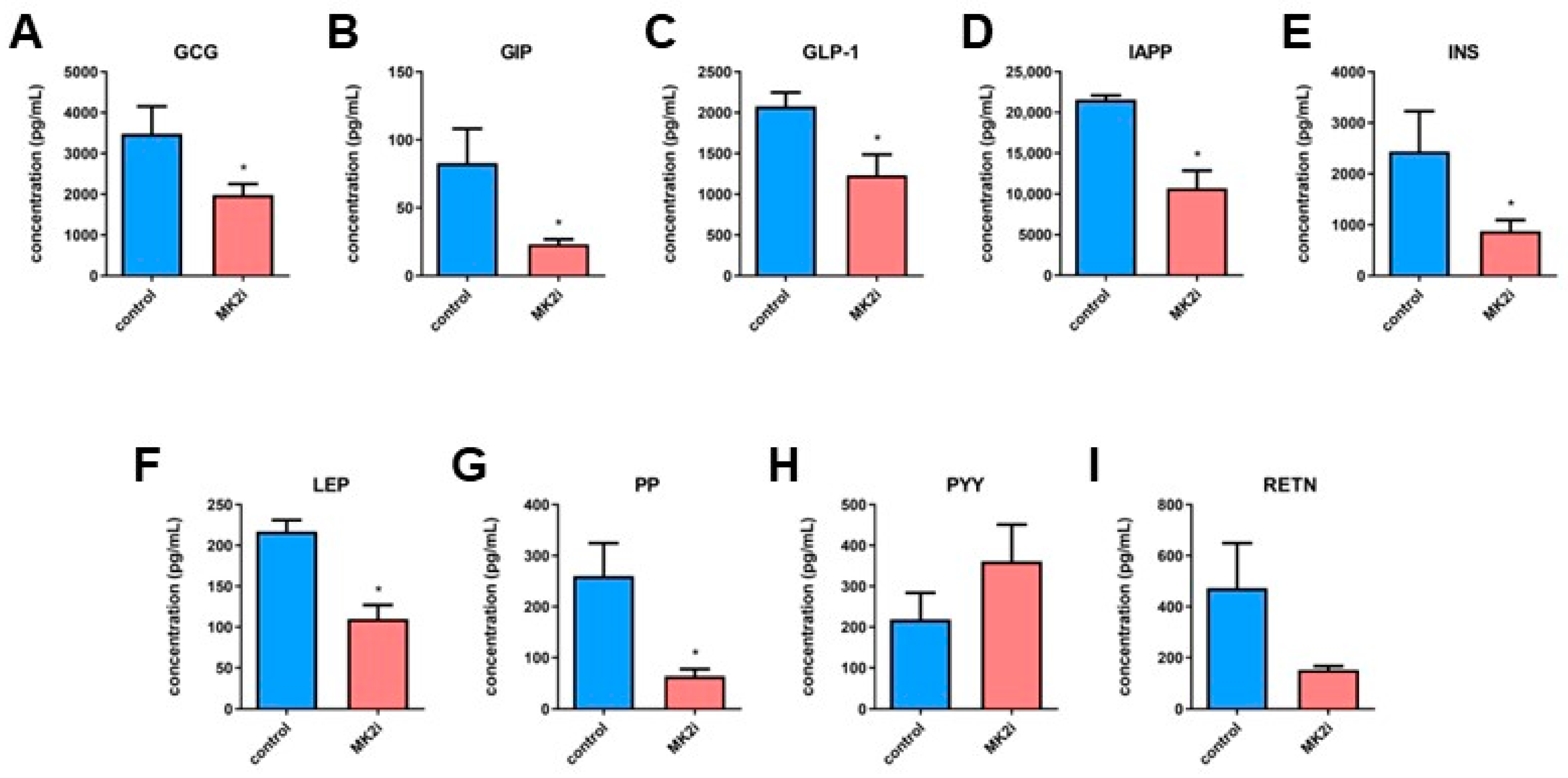
2.3. Inhibition of MK2 Decreases Production and Secretion of Hormones in Pancreatic Neuroendocrine Tumors
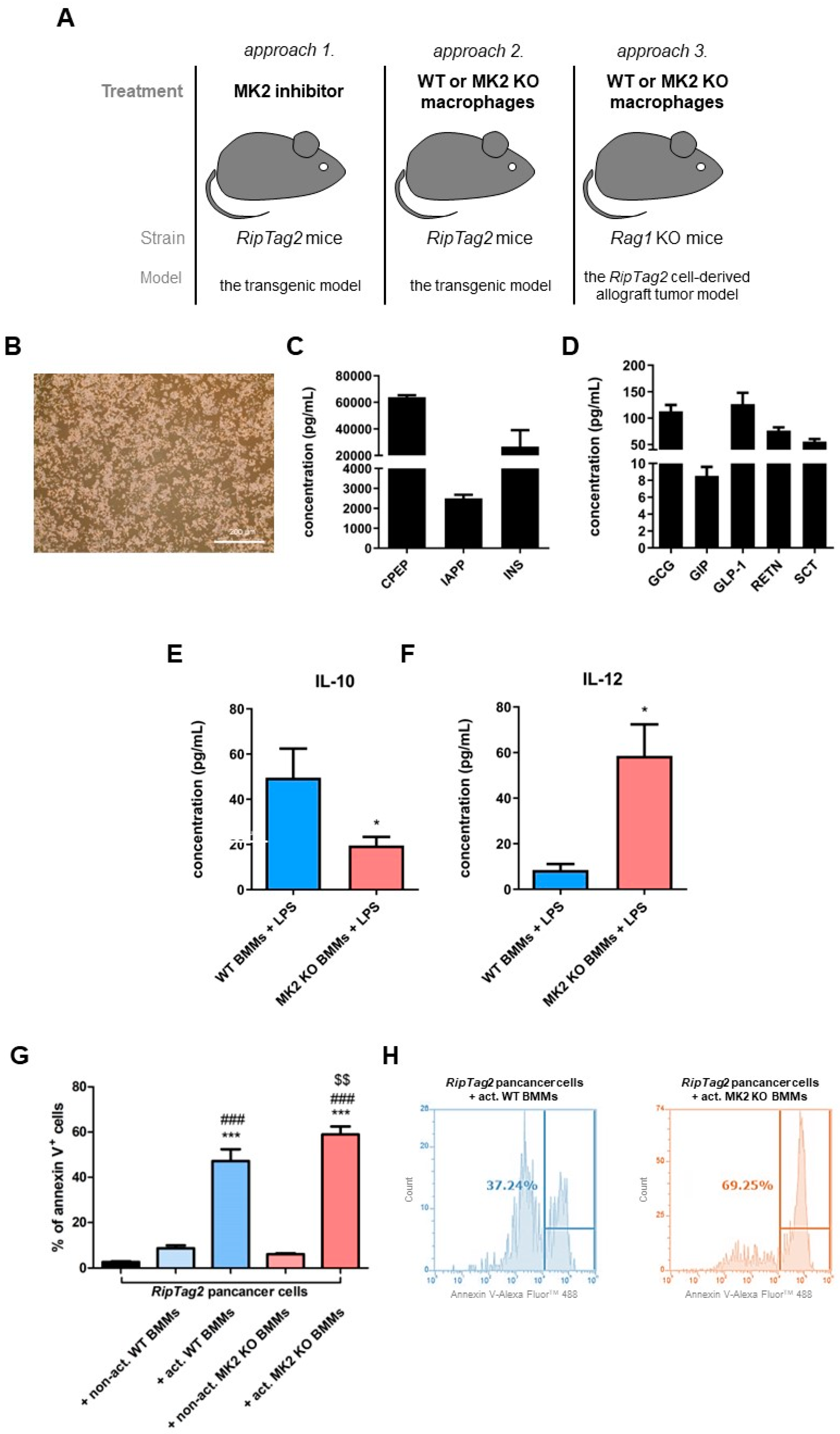
2.4. Primary PNET Cells Produce Metabolic Factors and MK2 Knockout Macrophages Show Enhanced Tumor Cell Killing
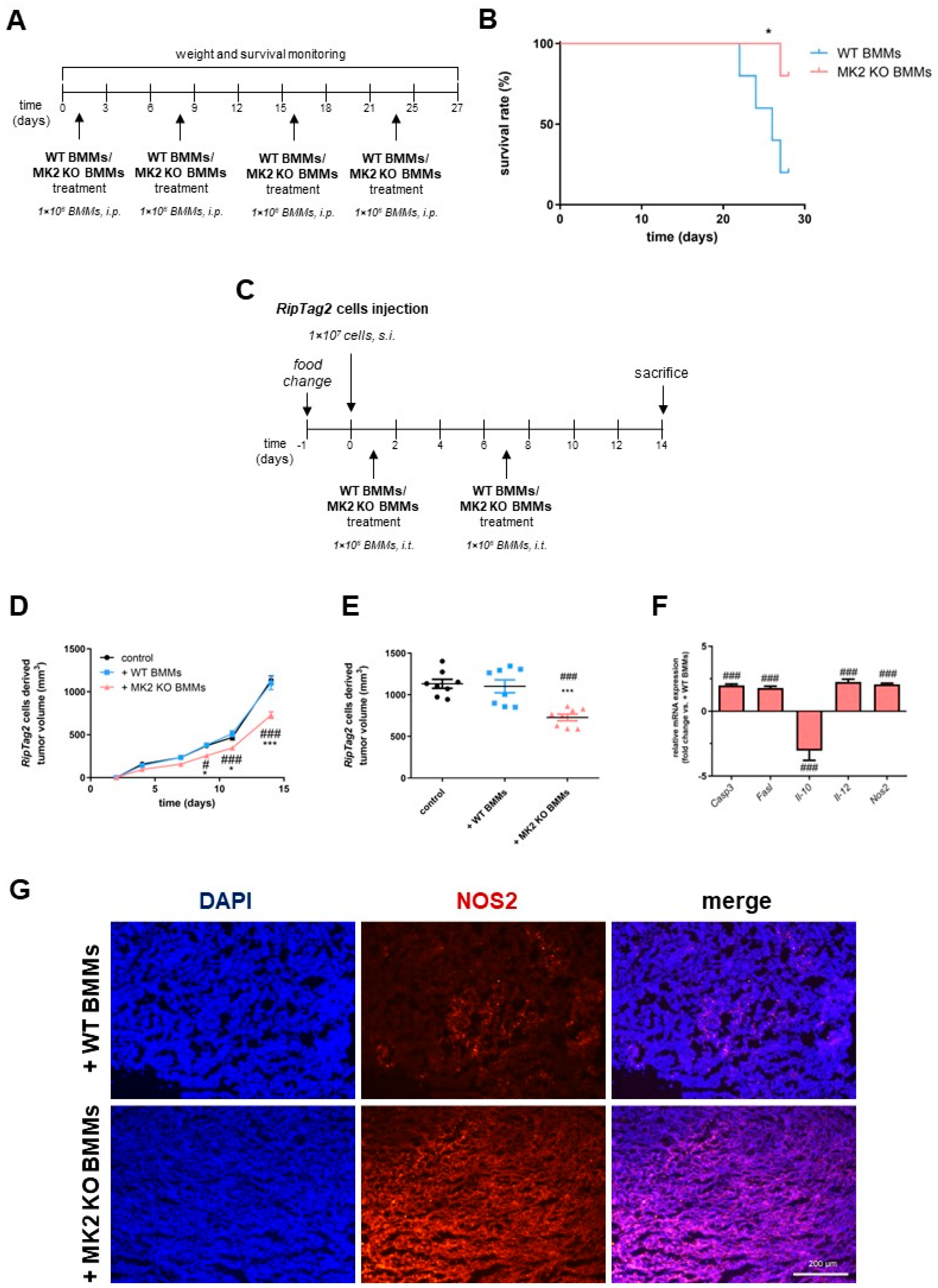
2.5. MK2 Knockout Macrophages Improve Survival and Suppress Growth of Pancreatic Neuroendocrine Tumors
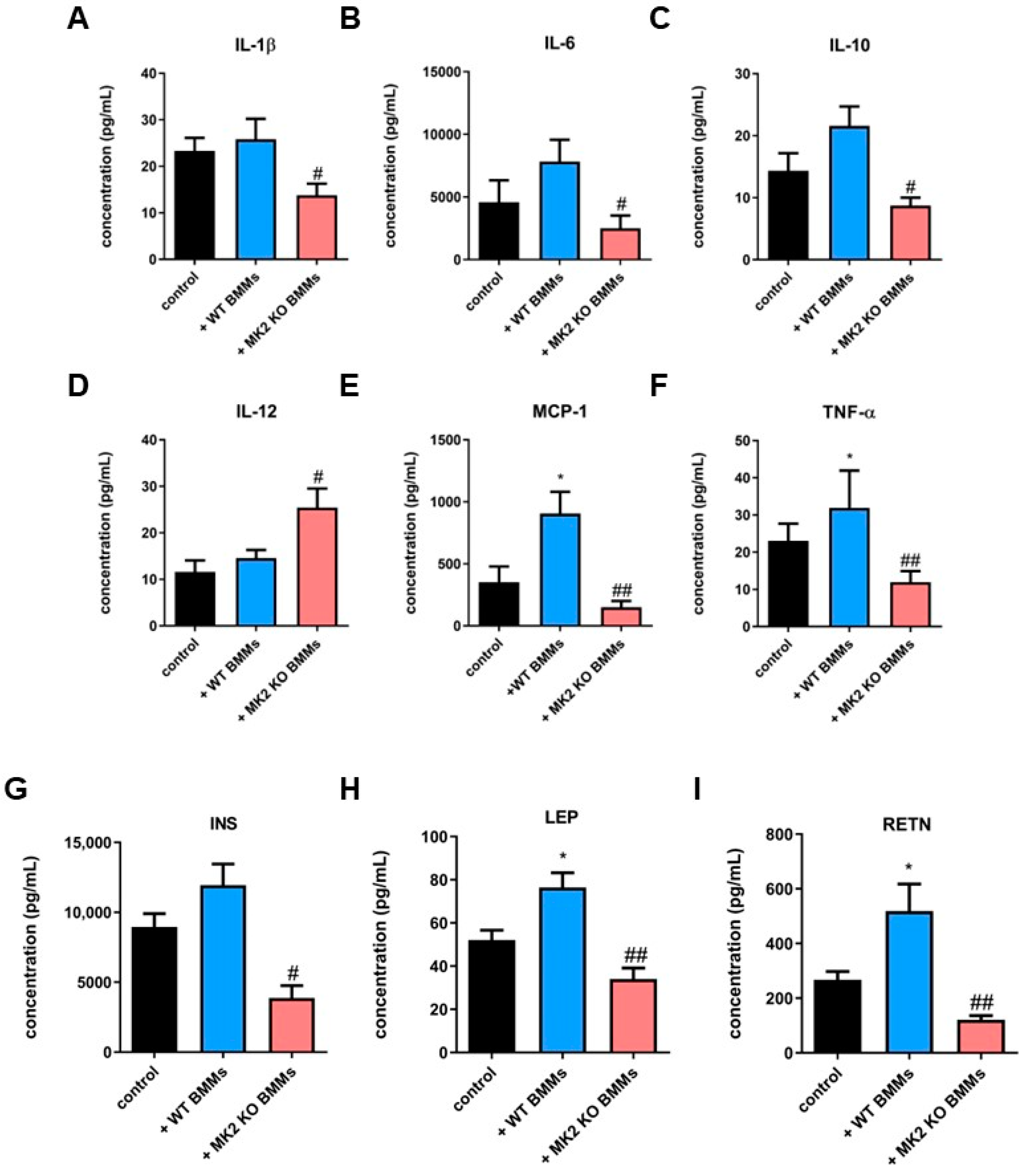
2.6. MK2 Knockout Macrophages Modulate Production and Secretion of Cytokines, Chemokines and Metabolic Factors in Pancreatic Neuroendocrine Tumors
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Bone Marrow-Derived Macrophages and MK2 Inhibition
4.3. Isolation and Culture of Primary RipTag2 Cancer Cells
4.4. Immunofluorescence Analysis
4.5. Murine Allograft Model and Treatments
4.6. Tumor Killing Assay and Flow Cytometry Analysis
4.7. RNA Isolation, Reverse Transcription and Real-Time PCR
4.8. Multiplex Analysis
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shis, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Rabe, K.G.; Rubin, J.; Petersen, G.M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 2008, 19, 1727–1733. [Google Scholar] [CrossRef]
- Egal, E.S.A.; Jacenik, D.; Soares, H.P.; Beswick, E.J. Translational challenges in pancreatic neuroendocrine tumor immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188640. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing but NET: A review of neuroendocrine tumors and carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Jensen, R.T. Pancreatic neuroendocrine tumors: Clinical features, diagnosis and medical treatment: Advances. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 737–753. [Google Scholar] [CrossRef]
- Soni, S.; Anand, P.; Padwad, Y.S. MAPKAPK2: The master regulator of RNA-binding proteins modulates transcript stability and tumor progression. J. Exp. Clin. Cancer Res. 2019, 38, 121. [Google Scholar] [CrossRef]
- Gurgis, F.M.; Ziaziaris, W.; Munoz, L. Mitogen-activated protein kinase-activated protein kinase 2 in neuroinflammation, heat shock protein 27 phosphorylation, and cell cycle: Role and targeting. Mol. Pharmacol. 2014, 85, 345–356. [Google Scholar] [CrossRef]
- Murali, B.; Ren, Q.; Luo, X.; Faget, D.V.; Wang, C.; Johnson, R.M.; Gruosso, T.; Flanagan, K.C.; Fu, Y.; Leahy, K.; et al. Inhibition of the stromal p38MAPK/MK2 pathway limits breast cancer metastases and chemotherapy-induced bone loss. Cancer Res. 2018, 78, 5618–5630. [Google Scholar] [CrossRef]
- Castillo, E.F.; Ray, A.L.; Beswick, E.J. MK2: An unrecognized regulator of tumor promoting macrophages in colorectal cancer? Macrophage (Houst) 2016, 3, e1166. [Google Scholar] [CrossRef][Green Version]
- Suarez-Lopez, L.; Sriram, G.; Kong, Y.W.; Morandell, S.; Merrick, K.A.; Hernandez, Y.; Haigis, K.M.; Yaffe, M.B. MK2 contributes to tumor progression by promoting M2 macrophage polarization and tumor angiogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E4236–E4244. [Google Scholar] [CrossRef] [PubMed]
- Grierson, P.M.; Dodhiawala, P.B.; Cheng, Y.; Chen, T.H.; Khawar, I.A.; Wei, Q.; Zhang, D.; Li, L.; Hernod, J.; Monahan, J.B.; et al. The MK2/Hsp27 axis is a major survival mechanism for pancreatic ductal adenocarcinoma under genotoxic stress. Sci. Transl. Med. 2021, 13, eabb5445. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Sun, D.; Fan, Z.; Yuan, Y.; Shao, M.; Hou, J.; Zhu, Y.; Wei, R.; Zhu, Y.; Qian, J.; et al. Targeting MK2 is a novel approach to interfere in multiple myeloma. Front. Oncol. 2019, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, A.; Neininger, A.; Schubert, C.; Eckert, R.; Birchmeier, C.; Volk, H.D.; Gaestel, M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell Biol. 1999, 1, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Phinney, B.B.; Ray, A.L.; Peretti, A.S.; Jerman, S.J.; Grim, C.; Pinchuk, I.V.; Beswick, E.J. MK2 regulates macrophage chemokine activity and recruitment to promote colon tumor growth. Front. Immunol. 2018, 9, 1857. [Google Scholar] [CrossRef]
- Kobayashi, S.; Contractor, T.; Vosburgh, E.; Du, Y.N.; Tang, L.H.; Clausen, R.; Charris, C.R. Alleles of Insm1 determine whether RIP1-Tag2 mice produce insulinomas or nonfunctioning pancreatic neuroendocrine tumors. Oncogenesis 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Dhall, D.; Nissen, N.N.; Zhou, C.; Ren, S.G. Pancreatic neuroendocrine tumors in glucagon receptor-deficient mice. PLoS ONE 2011, 6, e23397. [Google Scholar] [CrossRef]
- Sun, W.; Liu, D.B.; Li, W.W.; Zhang, L.L.; Long, G.X.; Wang, J.F.; Mei, Q.; Hu, G.-H. Interleukin-6 promotes the migration and invasion of nasopharyngeal carcinoma cell lines and upregulates the expression of MMP-2 and MMP-9. Int. J. Oncol. 2014, 44, 1551–1560. [Google Scholar] [CrossRef]
- Guo, R.; Qin, Y.; Shi, P.; Xie, J.; Chou, M.; Chen, Y. IL-1β promotes proliferation and migration of gallbladder cancer cells via Twist activation. Oncol. Lett. 2016, 12, 4749–4755. [Google Scholar] [CrossRef]
- Jourdan, M.; Tarte, K.; Legouffe, E.; Brochier, J.; Rossi, J.F.; Klein, B. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur. Cytokine Netw. 1999, 10, 65–70. [Google Scholar]
- Tripsianis, G.; Papadopoulou, E.; Anagnostopoulos, K.; Botaitis, S.; Katotomichelakis, M.; Romanidis, K.; Kontomanolis, E.; Tentes, I.; Kortsaris, A. Coexpression of IL-6 and TNF-α: Prognostic significance on breast cancer outcome. Neoplasma 2014, 61, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakamura, T.; Iino, T.; Hagi, T.; Kita, K.; Asanuma, K.; Sudo, A. Expression of Interleukin-6 and the Interleukin-6 Receptor Predicts the Clinical Outcomes of Patients with Soft Tissue Sarcomas. Cancers 2020, 12, 585. [Google Scholar] [CrossRef]
- Ray, A.L.; Berggren, K.L.; Restrepo Cruz, S.; Gan, G.N.; Beswick, E.J. Inhibition of MK2 suppresses IL-1β, IL-6, and TNF-α-dependent colorectal cancer growth. Int. J. Cancer 2018, 142, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Tietz, A.B.; Malo, A.; Diebold, J.; Kotlyarov, A.; Herbst, A.; Kolligs, F.T.; Brandt-Nedelev, B.; Halangk, W.; Gaestel, M.; Göke, B.; et al. Gene deletion of MK2 inhibits TNF-alpha and IL-6 and protects against cerulein-induced pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1298–G1306. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, C.; Ronkina, N.; Tehrani, M.; Dhamija, S.; Laass, K.; Holtmann, H.; Kotlyarov, A.; Gaestel, M. The p38/MK2-driven exchange between tristetraprolin and HuR regulates AU-rich element-dependent translation. PLoS Genet. 2012, 8, e1002977. [Google Scholar] [CrossRef]
- Ray, A.L.; Castillo, E.F.; Morris, K.T.; Nofchissey, R.A.; Weston, L.L.; Samedi, V.G.; Hanson, J.A.; Gaestel, M.; Pinchuk, I.V.; Beswick, E.J. Blockade of MK2 is protective in inflammation-associated colorectal cancer development. Int. J. Cancer 2016, 138, 770–775. [Google Scholar] [CrossRef]
- Qeadan, F.; Bansal, P.; Hanson, J.A.; Beswick, E.J. The MK2 pathway is linked to G-CSF, cytokine production and metastasis in gastric cancer: A novel intercorrelation analysis approach. J. Transl. Med. 2020, 18, 137. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, Z.; Li, Q.; Wang, L.; Rashid, A.; Zhu, Z.; Evans, D.B.; Vauthey, J.-N.; Xie, K.; Jao, J.C. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 2007, 109, 1478–1486. [Google Scholar] [CrossRef]
- Hansel, D.E.; Rahman, A.; Hermans, J.; de Krijger, R.R.; Ashfaq, R.; Yeo, C.J.; Cameron, J.L.; Maita, A. Liver metastases arising from well-differentiated pancreatic endocrine neoplasms demonstrate increased VEGF-C expression. Mod. Pathol. 2003, 16, 652–659. [Google Scholar] [CrossRef][Green Version]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef]
- Liu, J.F.; Chen, P.C.; Chang, T.M.; Hou, C.H. Monocyte Chemoattractant Protein-1 promotes cancer cell migration via c-Raf/MAPK/AP-1 pathway and MMP-9 production in osteosarcoma. J. Exp. Clin. Cancer Res. 2020, 39, 254. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, Y.; Zhu, X.; Guo, W.; Zhang, M.; Che, Y.; Tang, L.; Yang, X.; You, Q.; Liu, Z. IL-10 secreted by cancer-associated macrophages regulates proliferation and invasion in gastric cancer cells via c-Met/STAT3 signaling. Oncol. Rep. 2019, 42, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, M.; Zhang, J.; Chen, S.; Luo, X.; Qin, Y.; Chen, H. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J. Exp. Clin. Cancer Res. 2011, 30, 62. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Huang, R.R.; Wen, D.R.; Paul, E.; Wünsch, P.H.; Cochran, A.J. IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod. Pathol. 2011, 24, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Fontaine, C.; Deforet, M.; Akkari, L.; Thompson, C.B.; Joyce, J.A.; Xavier, J.B. Metabolic origins of spatial organization in the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2017, 114, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Crona, J.; Norlén, O.; Antonodimitrakis, P.; Welin, S.; Stålberg, P.; Eriksson, B. Multiple and secondary hormone secretion in patients with metastatic pancreatic neuroendocrine tumours. J. Clin. Endocrinol. Metab. 2016, 101, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Bao, Q.; Enders, G. The altered metabolic molecular signatures contribute to the RAD001 resistance in gastric neuroendocrine tumor. Front. Oncol. 2020, 10, 546. [Google Scholar] [CrossRef]
- Tuveson, D.; Hanahan, D. Translational medicine: Cancer lessons from mice to humans. Nature 2011, 471, 316–317. [Google Scholar] [CrossRef]
- Yagi, T.; Kubota, E.; Koyama, H.; Tanaka, T.; Kataoka, H.; Imaeda, K.; Joh, T. Glucagon promotes colon cancer cell growth via regulating AMPK and MAPK pathways. Oncotarget 2018, 9, 10650–10664. [Google Scholar] [CrossRef][Green Version]
- Lee, J.O.; Kim, N.; Lee, H.J.; Lee, Y.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci. Rep. 2016, 6, 18923. [Google Scholar] [CrossRef] [PubMed]
- Farilla, L.; Hui, H.; Bertolotto, C.; Kang, E.; Bulotta, A.; Di Mario, U.; Perfetti, R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 2002, 143, 4397–4408. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.R.; Cherfils, S.; Escobar, M.; Yoo, J.H.; Carino, C.; Styer, A.K.; Sullivan, B.T.; Sakamoto, H.; Olawaiye, A.; Serikawa, T.; et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J. Biol. Chem. 2006, 281, 26320–26328. [Google Scholar] [CrossRef]
- Cao, R.; Brakenhielm, E.; Wahlestedt, C.; Thyberg, J.; Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 2001, 98, 6390–6395. [Google Scholar] [CrossRef]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 2016, 7, 2346–2359. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Deshmukh, B.; Ramteke, P.; Bhati, F.K.; Bhat, M.K. Resistin: A journey from metabolism to cancer. Transl. Oncol. 2021, 14, 101178. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Glucagon-like peptide-1 and the islet beta-cell: Augmentation of cell proliferation and inhibition of apoptosis. Endocrinology 2003, 144, 5145–5148. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Vona-Davis, L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr. Relat. Cancer 2012, 19, R225–R241. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, S.; Hong, B.J.; Lee, C.J.; Kim, Y.E.; Bok, S.; Oh, J.M.; Gwak, S.H.; Yoo, M.Y.; Lee, M.S.; et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res. 2019, 79, 795–806. [Google Scholar] [CrossRef]
- Lin, S.; Sun, L.; Lyu, X.; Ai, X.; Du, D.; Su, N.; Li, H.; Zhang, L.; Yu, J.; Yuan, S. Lactate-activated macrophages induced aerobic glycolysis and epithelial-mesenchymal transition in breast cancer by regulation of CCL5-CCR5 axis: A positive metabolic feedback loop. Oncotarget 2017, 8, 110426–110443. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Q.; Zheng, S.; Li, G.; Lin, Q.; Wei, L.; Fu, Z.; Zhang, B.; Liu, Y.; Li, Z.; et al. Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, G.; Chu, H.; Wang, X.; Xiong, L.; Cai, G.; Liu, R.; Gao, H.; Tao, B.; Li, W.; et al. Macrophage-associated PGK1 phosphorylation promotes aerobic glycolysis and tumorigenesis. Mol. Cell 2018, 71, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Blank, A.; Cui, C.; Schoenfelt, K.Q.; Zhou, G.; Xu, Y.; Khramtsova, G.; Olopade, F.; Shah, A.M.; Khan, S.A.; et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J. Exp. Med. 2019, 216, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Coats, B.R.; Schoenfelt, K.Q.; Barbosa-Lorenzi, V.C.; Peris, E.; Cui, C.; Hoffman, A.; Zhou, G.; Fernandez, S.; Zhai, L.; Hall, B.A.; et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017, 20, 3149–3161. [Google Scholar] [CrossRef] [PubMed]
- Kratz, M.; Coats, B.R.; Hisert, K.B.; Hagman, D.; Mutskov, V.; Peris, E.; Schoenfelt, K.Q.; Kuzma, J.N.; Larson, I.; Billing, P.S.; et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014, 20, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, I.; de Santana Van Vilet, E.; Said Abu Egal, E.; Phinney, B.; Jacenik, D.; Prossnitz, E.R.; Beswick, E.J. G-CSF and G-CSFR induce a pro-tumorigenic macrophage phenotype to promote colon and pancreas tumor growth. Cancers 2020, 12, 2868. [Google Scholar] [CrossRef]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008, 14, 14-1. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacenik, D.; Lebish, E.J.; Beswick, E.J. MK2 Promotes the Development and Progression of Pancreatic Neuroendocrine Tumors Mediated by Macrophages and Metabolomic Factors. Int. J. Mol. Sci. 2022, 23, 13561. https://doi.org/10.3390/ijms232113561
Jacenik D, Lebish EJ, Beswick EJ. MK2 Promotes the Development and Progression of Pancreatic Neuroendocrine Tumors Mediated by Macrophages and Metabolomic Factors. International Journal of Molecular Sciences. 2022; 23(21):13561. https://doi.org/10.3390/ijms232113561
Chicago/Turabian StyleJacenik, Damian, Eric J. Lebish, and Ellen J. Beswick. 2022. "MK2 Promotes the Development and Progression of Pancreatic Neuroendocrine Tumors Mediated by Macrophages and Metabolomic Factors" International Journal of Molecular Sciences 23, no. 21: 13561. https://doi.org/10.3390/ijms232113561
APA StyleJacenik, D., Lebish, E. J., & Beswick, E. J. (2022). MK2 Promotes the Development and Progression of Pancreatic Neuroendocrine Tumors Mediated by Macrophages and Metabolomic Factors. International Journal of Molecular Sciences, 23(21), 13561. https://doi.org/10.3390/ijms232113561






