The Cognitive Improvement and Alleviation of Brain Hypermetabolism Caused by FFAR3 Ablation in Tg2576 Mice Is Persistent under Diet-Induced Obesity
Abstract
:1. Introduction
2. Results
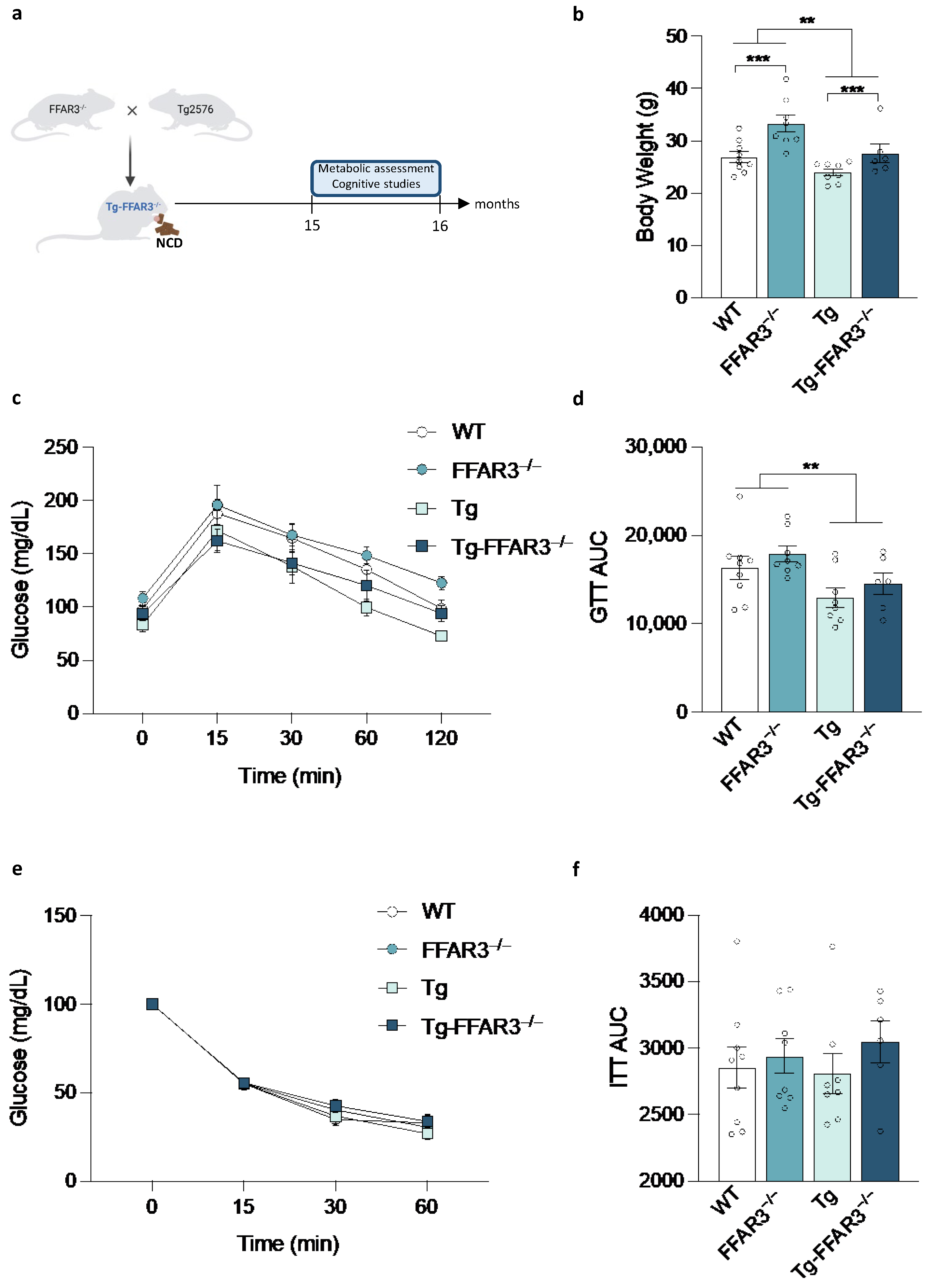
2.1. FFAR3 Ablation-Induced Systemic Glucose Homeostasis Impairment Is Reverted in Tg2576 Mice
2.2. FFAR3 Ablation Decreases the Brain Glucose Hypermetabolism Observed in Tg2576 Mice
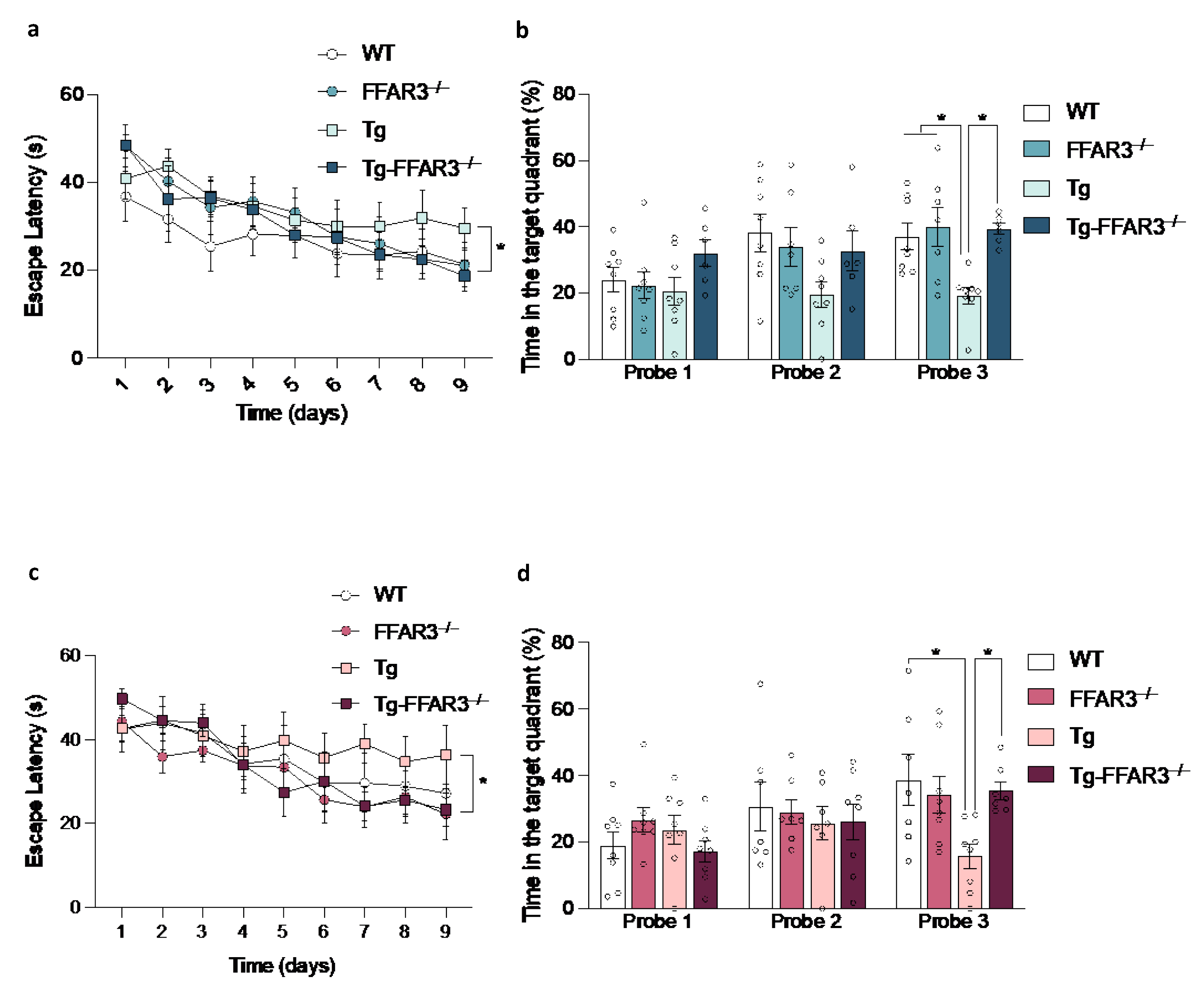
2.3. FFAR3 Ablation Restores the Cognitive Impairment of Tg2576 Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Genotyping of Mouse Models
4.3. Metabolic Studies
4.3.1. Body Weight
4.3.2. Glucose Tolerance Test
4.3.3. Insulin Tolerance Test
4.4. Morris Water Maze
4.5. 18F-FDG-PET
4.5.1. PET Acquisition
4.5.2. PET Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2022 Alzheimer’s Disease Facts and Figures. Available online: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf (accessed on 29 September 2022).
- Zetterberg, H.; Mattsson, N. Understanding the Cause of Sporadic Alzheimer’s Disease. Expert Rev. Neurother. 2014, 14, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Ngandu, T.; Laatikainen, T.; Winblad, B.; Soininen, H.; Tuomilehto, J. Risk Score for the Prediction of Dementia Risk in 20 Years among Middle Aged People: A Longitudinal, Population-Based Study. Lancet Neurol. 2006, 5, 735–741. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes Mellitus and Risk of Alzheimer Disease and Decline in Cognitive Function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.; Hofman, A.; Breteler, M.M. Diabetes Mellitus and the Risk of Dementia: The Rotterdam Study. Neurology 1999, 53, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Strachan, M.W.J.; Visseren, F.L.J.; Kappelle, L.J.; Whitmer, R.A. Dementia and Cognitive Decline in Type 2 Diabetes and Prediabetic Stages: Towards Targeted Interventions. Lancet Diabetes Endocrinol. 2014, 2, 246–255. [Google Scholar] [CrossRef]
- Gudala, K.; Bansal, D.; Schifano, F.; Bhansali, A. Diabetes Mellitus and Risk of Dementia: A Meta-Analysis of Prospective Observational Studies. J. Diabetes Investig. 2013, 4, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Barbiellini Amidei, C.; Fayosse, A.; Dumurgier, J.; Machado-Fragua, M.D.; Tabak, A.G.; van Sloten, T.; Kivimäki, M.; Dugravot, A.; Sabia, S.; Singh-Manoux, A. Association Between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA 2021, 325, 1640–1649. [Google Scholar] [CrossRef]
- Craft, S.; Peskind, E.; Schwartz, M.W.; Schellenberg, G.D.; Raskind, M.; Porte, D. Cerebrospinal Fluid and Plasma Insulin Levels in Alzheimer’s Disease: Relationship to Severity of Dementia and Apolipoprotein E Genotype. Neurology 1998, 50, 164–168. [Google Scholar] [CrossRef]
- Neth, B.J.; Craft, S. Insulin Resistance and Alzheimer’s Disease: Bioenergetic Linkages. Front. Aging Neurosci. 2017, 9, 345. [Google Scholar] [CrossRef] [Green Version]
- Luchsinger, J.A.; Tang, M.-X.; Shea, S.; Mayeux, R. Hyperinsulinemia and Risk of Alzheimer Disease. Neurology 2004, 63, 1187–1192. [Google Scholar] [CrossRef]
- Biessels, G.J.; Reagan, L.P. Hippocampal Insulin Resistance and Cognitive Dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. [Google Scholar] [CrossRef]
- De Felice, F.G.; Gonçalves, R.A.; Ferreira, S.T. Impaired Insulin Signalling and Allostatic Load in Alzheimer Disease. Nat. Rev. Neurosci. 2022, 23, 215–230. [Google Scholar] [CrossRef]
- Kellar, D.; Craft, S. Brain Insulin Resistance in Alzheimer’s Disease and Related Disorders: Mechanisms and Therapeutic Approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Nugent, S.; Potvin, O.; Cunnane, S.C.; Chen, T.-H.; Duchesne, S. Associating Type 2 Diabetes Risk Factor Genes and FDG-PET Brain Metabolism in Normal Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 580633. [Google Scholar] [CrossRef]
- Képes, Z.; Aranyi, C.; Forgács, A.; Nagy, F.; Kukuts, K.; Hascsi, Z.; Esze, R.; Somodi, S.; Káplár, M.; Varga, J.; et al. Glucose-Level Dependent Brain Hypometabolism in Type 2 Diabetes Mellitus and Obesity. Eur. J. Hybrid Imaging 2021, 5, 3. [Google Scholar] [CrossRef]
- Roberts, R.O.; Knopman, D.S.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; Baertlein, L.; Boeve, B.F.; Tangalos, E.G.; Ivnik, R.J.; Mielke, M.M.; et al. Association of Diabetes with Amnestic and Nonamnestic Mild Cognitive Impairment. Alzheimer’s Dement. 2014, 10, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Mosconi, L.; Mistur, R.; Switalski, R.; Tsui, W.H.; Glodzik, L.; Li, Y.; Pirraglia, E.; De Santi, S.; Reisberg, B.; Wisniewski, T.; et al. FDG-PET Changes in Brain Glucose Metabolism from Normal Cognition to Pathologically Verified Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Mosconi, L.; De Santi, S.; Li, J.; Tsui, W.H.; Li, Y.; Boppana, M.; Laska, E.; Rusinek, H.; de Leon, M.J. Hippocampal Hypometabolism Predicts Cognitive Decline from Normal Aging. Neurobiol. Aging 2008, 29, 676–692. [Google Scholar] [CrossRef] [Green Version]
- Protas, H.D.; Chen, K.; Langbaum, J.B.S.; Fleisher, A.S.; Alexander, G.E.; Lee, W.; Bandy, D.; de Leon, M.J.; Mosconi, L.; Buckley, S.; et al. Posterior Cingulate Glucose Metabolism, Hippocampal Glucose Metabolism, and Hippocampal Volume in Cognitively Normal, Late-Middle-Aged Persons at 3 Levels of Genetic Risk for Alzheimer Disease. JAMA Neurol. 2013, 70, 320–325. [Google Scholar] [CrossRef]
- Rubinski, A.; Franzmeier, N.; Neitzel, J.; Ewers, M. FDG-PET Hypermetabolism Is Associated with Higher Tau-PET in Mild Cognitive Impairment at Low Amyloid-PET Levels. Alzheimer’s Res. Ther. 2020, 12, 133. [Google Scholar] [CrossRef]
- Gupta, V.; Booth, S.; Ko, J.H. Hypermetabolic Cerebellar Connectome in Alzheimer’s Disease. Brain Connect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Fan, Z.; Brooks, D.J.; Edison, P. Cortical Hypermetabolism in MCI Subjects: A Compensatory Mechanism? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Gunderson, E.P.; Barrett-Connor, E.; Quesenberry, C.P.; Yaffe, K. Obesity in Middle Age and Future Risk of Dementia: A 27 Year Longitudinal Population Based Study. BMJ 2005, 330, 1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitmer, R.A.; Gustafson, D.R.; Barrett-Connor, E.; Haan, M.N.; Gunderson, E.P.; Yaffe, K. Central Obesity and Increased Risk of Dementia More than Three Decades Later. Neurology 2008, 71, 1057–1064. [Google Scholar] [CrossRef]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kåreholt, I.; Winblad, B.; Helkala, E.-L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and Vascular Risk Factors at Midlife and the Risk of Dementia and Alzheimer Disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef] [Green Version]
- Luchsinger, J.A.; Gustafson, D.R. Adiposity, Type 2 Diabetes, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 16, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The Glucose Fatty-Acid Cycle. Its Role in Insulin Sensitivity and the Metabolic Disturbances of Diabetes Mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Björntorp, P.; Bergman, H.; Varnauskas, E. Plasma Free Fatty Acid Turnover Rate in Obesity. Acta Med. Scand. 1969, 185, 351–356. [Google Scholar] [CrossRef]
- Boden, G. Obesity and Free Fatty Acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- Jensen, M.D.; Haymond, M.W.; Rizza, R.A.; Cryer, P.E.; Miles, J.M. Influence of Body Fat Distribution on Free Fatty Acid Metabolism in Obesity. J. Clin. Investig. 1989, 83, 1168–1173. [Google Scholar] [CrossRef]
- Zhao, L.; Ni, Y.; Ma, X.; Zhao, A.; Bao, Y.; Liu, J.; Chen, T.; Xie, G.; Panee, J.; Su, M.; et al. A Panel of Free Fatty Acid Ratios to Predict the Development of Metabolic Abnormalities in Healthy Obese Individuals. Sci. Rep. 2016, 6, 28418. [Google Scholar] [CrossRef] [Green Version]
- Bergman, R.N.; Ader, M. Free Fatty Acids and Pathogenesis of Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2000, 11, 351–356. [Google Scholar] [CrossRef]
- Kim, J.Y.; Bacha, F.; Tfayli, H.; Michaliszyn, S.F.; Yousuf, S.; Arslanian, S. Adipose Tissue Insulin Resistance in Youth on the Spectrum from Normal Weight to Obese and From Normal Glucose Tolerance to Impaired Glucose Tolerance to Type 2 Diabetes. Diabetes Care 2019, 42, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Gastaldelli, A.; Gaggini, M.; DeFronzo, R.A. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results from the San Antonio Metabolism Study. Diabetes 2017, 66, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Mahendran, Y.; Cederberg, H.; Vangipurapu, J.; Kangas, A.J.; Soininen, P.; Kuusisto, J.; Uusitupa, M.; Ala-Korpela, M.; Laakso, M. Glycerol and Fatty Acids in Serum Predict the Development of Hyperglycemia and Type 2 Diabetes in Finnish Men. Diabetes Care 2013, 36, 3732–3738. [Google Scholar] [CrossRef] [Green Version]
- Frohnert, B.I.; Jacobs, D.R.; Steinberger, J.; Moran, A.; Steffen, L.M.; Sinaiko, A.R. Relation between Serum Free Fatty Acids and Adiposity, Insulin Resistance, and Cardiovascular Risk Factors from Adolescence to Adulthood. Diabetes 2013, 62, 3163–3169. [Google Scholar] [CrossRef] [Green Version]
- Pankow, J.S.; Duncan, B.B.; Schmidt, M.I.; Ballantyne, C.M.; Couper, D.J.; Hoogeveen, R.C.; Golden, S.H. Atherosclerosis Risk in Communities Study Fasting Plasma Free Fatty Acids and Risk of Type 2 Diabetes: The Atherosclerosis Risk in Communities Study. Diabetes Care 2004, 27, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Paolisso, G.; Tataranni, P.A.; Foley, J.E.; Bogardus, C.; Howard, B.V.; Ravussin, E. A High Concentration of Fasting Plasma Non-Esterified Fatty Acids Is a Risk Factor for the Development of NIDDM. Diabetologia 1995, 38, 1213–1217. [Google Scholar] [CrossRef]
- Fryk, E.; Olausson, J.; Mossberg, K.; Strindberg, L.; Schmelz, M.; Brogren, H.; Gan, L.-M.; Piazza, S.; Provenzani, A.; Becattini, B.; et al. Hyperinsulinemia and Insulin Resistance in the Obese May Develop as Part of a Homeostatic Response to Elevated Free Fatty Acids: A Mechanistic Case-Control and a Population-Based Cohort Study. EBioMedicine 2021, 65, 103264. [Google Scholar] [CrossRef]
- Offermanns, S. Free Fatty Acid (FFA) and Hydroxy Carboxylic Acid (HCA) Receptors. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 407–434. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Everard, A.; Duparc, T. Gut Microbiota, Enteroendocrine Functions and Metabolism. Curr. Opin. Pharmacol. 2013, 13, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Poulsen, S.S.; Han, S.; et al. GPR41/FFAR3 and GPR43/FFAR2 as Cosensors for Short-Chain Fatty Acids in Enteroendocrine Cells vs. FFAR3 in Enteric Neurons and FFAR2 in Enteric Leukocytes. Endocrinology 2013, 154, 3552–3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjursell, M.; Admyre, T.; Göransson, M.; Marley, A.E.; Smith, D.M.; Oscarsson, J.; Bohlooly-Y, M. Improved Glucose Control and Reduced Body Fat Mass in Free Fatty Acid Receptor 2-Deficient Mice Fed a High-Fat Diet. Am. J. Physiol.-Endocrinol. Metab. 2011, 300, E211–E220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The Gut Microbiota Suppresses Insulin-Mediated Fat Accumulation via the Short-Chain Fatty Acid Receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.H.; Smith, D.M.; Arch, J.R.S. Roles of GPR41 and GPR43 in Leptin Secretory Responses of Murine Adipocytes to Short Chain Fatty Acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [Green Version]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the Gut Microbiota on Host Adiposity Are Modulated by the Short-Chain Fatty-Acid Binding G Protein-Coupled Receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-Chain Fatty Acids and Ketones Directly Regulate Sympathetic Nervous System via G Protein-Coupled Receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [Green Version]
- Zamarbide, M.; Martinez-Pinilla, E.; Gil-Bea, F.; Yanagisawa, M.; Franco, R.; Perez-Mediavilla, A. Genetic Inactivation of Free Fatty Acid Receptor 3 Impedes Behavioral Deficits and Pathological Hallmarks in the APPswe Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2022, 23, 3533. [Google Scholar] [CrossRef]
- Luo, F.; Rustay, N.R.; Ebert, U.; Hradil, V.P.; Cole, T.B.; Llano, D.A.; Mudd, S.R.; Zhang, Y.; Fox, G.B.; Day, M. Characterization of 7- and 19-Month-Old Tg2576 Mice Using Multimodal in Vivo Imaging: Limitations as a Translatable Model of Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 933–944. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common Neurodegenerative Pathways in Obesity, Diabetes, and Alzheimer’s Disease. Biochim. Biophys. Acta 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- Monda, V.; La Marra, M.; Perrella, R.; Caviglia, G.; Iavarone, A.; Chieffi, S.; Messina, G.; Carotenuto, M.; Monda, M.; Messina, A. Obesity and Brain Illness: From Cognitive and Psychological Evidences to Obesity Paradox. Diabetes Metab. Syndr. Obes. 2017, 10, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Cho, H.; Kim, J.; Lee, D.-W.; Kim, G.H.; Hong, Y.S.; Moon, S.; Park, S.; Lee, S.; Lee, S.; et al. Brain Changes in Overweight/Obese and Normal-Weight Adults with Type 2 Diabetes Mellitus. Diabetologia 2017, 60, 1207–1217. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.V.; Sadler, R.K.; Llovera, G.; Singh, V.; Roth, S.; Heindl, S.; Sebastian Monasor, L.; Verhoeven, A.; Peters, F.; Parhizkar, S.; et al. Microbiota-Derived Short Chain Fatty Acids Modulate Microglia and Promote Aβ Plaque Deposition. eLife 2021, 10, e59826. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-Chain Fatty Acids: Microbial Metabolites That Alleviate Stress-Induced Brain-Gut Axis Alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, e00632-19. [Google Scholar] [CrossRef] [Green Version]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr. Physiol. 2018, 8, 1091–1115. [Google Scholar] [CrossRef] [PubMed]
- Bellahcene, M.; O’Dowd, J.F.; Wargent, E.T.; Zaibi, M.S.; Hislop, D.C.; Ngala, R.A.; Smith, D.M.; Cawthorne, M.A.; Stocker, C.J.; Arch, J.R.S. Male Mice That Lack the G-Protein-Coupled Receptor GPR41 Have Low Energy Expenditure and Increased Body Fat Content. Br. J. Nutr. 2013, 109, 1755–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Cook, T.M.; Gavini, C.K.; Jesse, J.; Aubert, G.; Gornick, E.; Bonomo, R.; Gautron, L.; Layden, B.T.; Mansuy-Aubert, V. Vagal Neuron Expression of the Microbiota-Derived Metabolite Receptor, Free Fatty Acid Receptor (FFAR3), Is Necessary for Normal Feeding Behavior. Mol. Metab. 2021, 54, 101350. [Google Scholar] [CrossRef]
- Veprik, A.; Laufer, D.; Weiss, S.; Rubins, N.; Walker, M.D. GPR41 Modulates Insulin Secretion and Gene Expression in Pancreatic β-Cells and Modifies Metabolic Homeostasis in Fed and Fasting States. FASEB J. 2016, 30, 3860–3869. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Ahmed, K.; Gille, A.; Lu, S.; Gröne, H.-J.; Tunaru, S.; Offermanns, S. Loss of FFA2 and FFA3 Increases Insulin Secretion and Improves Glucose Tolerance in Type 2 Diabetes. Nat. Med. 2015, 21, 173–177. [Google Scholar] [CrossRef]
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.-I.; Kuwahara, A. Roles of Short-Chain Fatty Acids Receptors, GPR41 and GPR43 on Colonic Functions. J. Physiol. Pharmacol. 2008, 59 (Suppl. 2), 251–262. [Google Scholar]
- Girard, J. The Incretins: From the Concept to Their Use in the Treatment of Type 2 Diabetes. Part A: Incretins: Concept and Physiological Functions. Diabetes Metab. 2008, 34, 550–559. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [Green Version]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Acetate and Propionate Short Chain Fatty Acids Stimulate Adipogenesis via GPCR43-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16123168/ (accessed on 26 September 2022).
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-Chain Fatty Acids Stimulate Leptin Production in Adipocytes through the G Protein-Coupled Receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [Green Version]
- Ardanaz, C.G.; Ramírez, M.J.; Solas, M. Brain Metabolic Alterations in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 3785. [Google Scholar] [CrossRef]
- Talbot, K. Brain Insulin Resistance in Alzheimer’s Disease and Its Potential Treatment with GLP-1 Analogs. Neurodegener. Dis. Manag. 2014, 4, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain Insulin Resistance in Type 2 Diabetes and Alzheimer Disease: Concepts and Conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Riederer, P.; Korczyn, A.D.; Ali, S.S.; Bajenaru, O.; Choi, M.S.; Chopp, M.; Dermanovic-Dobrota, V.; Grünblatt, E.; Jellinger, K.A.; Kamal, M.A.; et al. The Diabetic Brain and Cognition. J. Neural Transm. 2017, 124, 1431–1454. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, K.; Chapman, P.; Nilsen, S.; Eckman, C.; Harigaya, Y.; Younkin, S.; Yang, F.; Cole, G. Correlative Memory Deficits, Abeta Elevation, and Amyloid Plaques in Transgenic Mice. Science 1996, 274, 99–102. [Google Scholar] [CrossRef]
- Pedersen, W.A.; Flynn, E.R. Insulin Resistance Contributes to Aberrant Stress Responses in the Tg2576 Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2004, 17, 500–506. [Google Scholar] [CrossRef]
- Rodriguez-Rivera, J.; Denner, L.; Dineley, K.T. Rosiglitazone Reversal of Tg2576 Cognitive Deficits Is Independent of Peripheral Gluco-Regulatory Status. Behav. Brain Res. 2011, 216, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagust, W. Positron Emission Tomography and Magnetic Resonance Imaging in the Diagnosis and Prediction of Dementia. Alzheimer’s Dement. 2006, 2, 36–42. [Google Scholar] [CrossRef]
- Kuntner, C.; Kesner, A.L.; Bauer, M.; Kremslehner, R.; Wanek, T.; Mandler, M.; Karch, R.; Stanek, J.; Wolf, T.; Müller, M.; et al. Limitations of Small Animal PET Imaging with [18F]FDDNP and FDG for Quantitative Studies in a Transgenic Mouse Model of Alzheimer’s Disease. Mol. Imaging Biol. 2009, 11, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Poisnel, G.; Hérard, A.-S.; El Tannir El Tayara, N.; Bourrin, E.; Volk, A.; Kober, F.; Delatour, B.; Delzescaux, T.; Debeir, T.; Rooney, T.; et al. Increased Regional Cerebral Glucose Uptake in an APP/PS1 Model of Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 1995–2005. [Google Scholar] [CrossRef] [Green Version]
- Rojas, S.; Herance, J.R.; Gispert, J.D.; Abad, S.; Torrent, E.; Jiménez, X.; Pareto, D.; Perpiña, U.; Sarroca, S.; Rodríguez, E.; et al. In Vivo Evaluation of Amyloid Deposition and Brain Glucose Metabolism of 5XFAD Mice Using Positron Emission Tomography. Neurobiol. Aging 2013, 34, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Brendel, M.; Probst, F.; Jaworska, A.; Overhoff, F.; Korzhova, V.; Albert, N.L.; Beck, R.; Lindner, S.; Gildehaus, F.-J.; Baumann, K.; et al. Glial Activation and Glucose Metabolism in a Transgenic Amyloid Mouse Model: A Triple-Tracer PET Study. J. Nucl. Med. 2016, 57, 954–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-Y.; Men, W.-W.; Zhu, H.; Lei, J.-F.; Zuo, F.-X.; Wang, Z.-J.; Zhu, Z.-H.; Bao, X.-J.; Wang, R.-Z. Age- and Brain Region-Specific Changes of Glucose Metabolic Disorder, Learning, and Memory Dysfunction in Early Alzheimer’s Disease Assessed in APP/PS1 Transgenic Mice Using 18F-FDG-PET. Int. J. Mol. Sci. 2016, 17, 1707. [Google Scholar] [CrossRef]
- Takkinen, J.S.; López-Picón, F.R.; Al Majidi, R.; Eskola, O.; Krzyczmonik, A.; Keller, T.; Löyttyniemi, E.; Solin, O.; Rinne, J.O.; Haaparanta-Solin, M. Brain Energy Metabolism and Neuroinflammation in Ageing APP/PS1-21 Mice Using Longitudinal 18F-FDG and 18F-DPA-714 PET Imaging. J. Cereb. Blood Flow Metab. 2017, 37, 2870–2882. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, R.M.; Kusne, Y.; Nowak, L.A.; LaFerla, F.M.; Reiman, E.M.; Valla, J. Regional Cerebral Glucose Uptake in the 3xTG Model of Alzheimer’s Disease Highlights Common Regional Vulnerability across AD Mouse Models. Brain Res. 2010, 1347, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Pedrós, I.; Petrov, D.; Allgaier, M.; Sureda, F.; Barroso, E.; Beas-Zarate, C.; Auladell, C.; Pallàs, M.; Vázquez-Carrera, M.; Casadesús, G.; et al. Early Alterations in Energy Metabolism in the Hippocampus of APPswe/PS1dE9 Mouse Model of Alzheimer’s Disease. Biochim. Biophys. Acta 2014, 1842, 1556–1566. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, I.R.; DeBay, D.R.; Reid, G.A.; O’Leary, T.P.; Jollymore, C.T.; Mawko, G.; Burrell, S.; Martin, E.; Bowen, C.V.; Brown, R.E.; et al. Early Detection of Cerebral Glucose Uptake Changes in the 5XFAD Mouse. Curr. Alzheimer Res. 2014, 11, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Waldron, A.-M.; Wyffels, L.; Verhaeghe, J.; Bottelbergs, A.; Richardson, J.; Kelley, J.; Schmidt, M.; Stroobants, S.; Langlois, X.; Staelens, S. Quantitative ΜPET Imaging of Cerebral Glucose Metabolism and Amyloidosis in the TASTPM Double Transgenic Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2015, 12, 694–703. [Google Scholar] [CrossRef]
- Waldron, A.-M.; Wyffels, L.; Verhaeghe, J.; Richardson, J.C.; Schmidt, M.; Stroobants, S.; Langlois, X.; Staelens, S. Longitudinal Characterization of [18F]-FDG and [18F]-AV45 Uptake in the Double Transgenic TASTPM Mouse Model. J. Alzheimer’s Dis. 2017, 55, 1537–1548. [Google Scholar] [CrossRef]
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.-Q.; Kreitzer, A.; et al. Aberrant Excitatory Neuronal Activity and Compensatory Remodeling of Inhibitory Hippocampal Circuits in Mouse Models of Alzheimer’s Disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Brendel, M.; Focke, C.; Blume, T.; Peters, F.; Deussing, M.; Probst, F.; Jaworska, A.; Overhoff, F.; Albert, N.; Lindner, S.; et al. Time Courses of Cortical Glucose Metabolism and Microglial Activity Across the Life Span of Wild-Type Mice: A PET Study. J. Nucl. Med. 2017, 58, 1984–1990. [Google Scholar] [CrossRef]
- Deleye, S.; Waldron, A.-M.; Richardson, J.C.; Schmidt, M.; Langlois, X.; Stroobants, S.; Staelens, S. The Effects of Physiological and Methodological Determinants on 18F-FDG Mouse Brain Imaging Exemplified in a Double Transgenic Alzheimer Model. Mol. Imaging 2016, 15, 1536012115624919. [Google Scholar] [CrossRef] [Green Version]
- Kayed, R.; Sokolov, Y.; Edmonds, B.; McIntire, T.M.; Milton, S.C.; Hall, J.E.; Glabe, C.G. Permeabilization of Lipid Bilayers Is a Common Conformation-Dependent Activity of Soluble Amyloid Oligomers in Protein Misfolding Diseases. J. Biol. Chem. 2004, 279, 46363–46366. [Google Scholar] [CrossRef] [Green Version]
- Atamna, H.; Frey, W.H. Mechanisms of Mitochondrial Dysfunction and Energy Deficiency in Alzheimer’s Disease. Mitochondrion 2007, 7, 297–310. [Google Scholar] [CrossRef]
- Etcheberrigaray, R.; Tan, M.; Dewachter, I.; Kuipéri, C.; Van der Auwera, I.; Wera, S.; Qiao, L.; Bank, B.; Nelson, T.J.; Kozikowski, A.P.; et al. Therapeutic Effects of PKC Activators in Alzheimer’s Disease Transgenic Mice. Proc. Natl. Acad. Sci. USA 2004, 101, 11141–11146. [Google Scholar] [CrossRef] [Green Version]
- Etschmaier, K.; Becker, T.; Eichmann, T.O.; Schweinzer, C.; Scholler, M.; Tam-Amersdorfer, C.; Poeckl, M.; Schuligoi, R.; Kober, A.; Chirackal Manavalan, A.P.; et al. Adipose Triglyceride Lipase Affects Triacylglycerol Metabolism at Brain Barriers. J. Neurochem. 2011, 119, 1016–1028. [Google Scholar] [CrossRef]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a Free Fatty Acid Receptor, FFA2R, Expressed on Leukocytes and Activated by Short-Chain Fatty Acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solas, M.; Zamarbide, M.; Ardanaz, C.G.; Ramírez, M.J.; Pérez-Mediavilla, A. The Cognitive Improvement and Alleviation of Brain Hypermetabolism Caused by FFAR3 Ablation in Tg2576 Mice Is Persistent under Diet-Induced Obesity. Int. J. Mol. Sci. 2022, 23, 13591. https://doi.org/10.3390/ijms232113591
Solas M, Zamarbide M, Ardanaz CG, Ramírez MJ, Pérez-Mediavilla A. The Cognitive Improvement and Alleviation of Brain Hypermetabolism Caused by FFAR3 Ablation in Tg2576 Mice Is Persistent under Diet-Induced Obesity. International Journal of Molecular Sciences. 2022; 23(21):13591. https://doi.org/10.3390/ijms232113591
Chicago/Turabian StyleSolas, Maite, Marta Zamarbide, Carlos G. Ardanaz, María J. Ramírez, and Alberto Pérez-Mediavilla. 2022. "The Cognitive Improvement and Alleviation of Brain Hypermetabolism Caused by FFAR3 Ablation in Tg2576 Mice Is Persistent under Diet-Induced Obesity" International Journal of Molecular Sciences 23, no. 21: 13591. https://doi.org/10.3390/ijms232113591
APA StyleSolas, M., Zamarbide, M., Ardanaz, C. G., Ramírez, M. J., & Pérez-Mediavilla, A. (2022). The Cognitive Improvement and Alleviation of Brain Hypermetabolism Caused by FFAR3 Ablation in Tg2576 Mice Is Persistent under Diet-Induced Obesity. International Journal of Molecular Sciences, 23(21), 13591. https://doi.org/10.3390/ijms232113591






