Effects of Two Bacillus Velezensis Microbial Inoculants on the Growth and Rhizosphere Soil Environment of Prunus davidiana
Abstract
:1. Introduction
2. Results
2.1. Effect of Inoculation on Peach Tree Growth
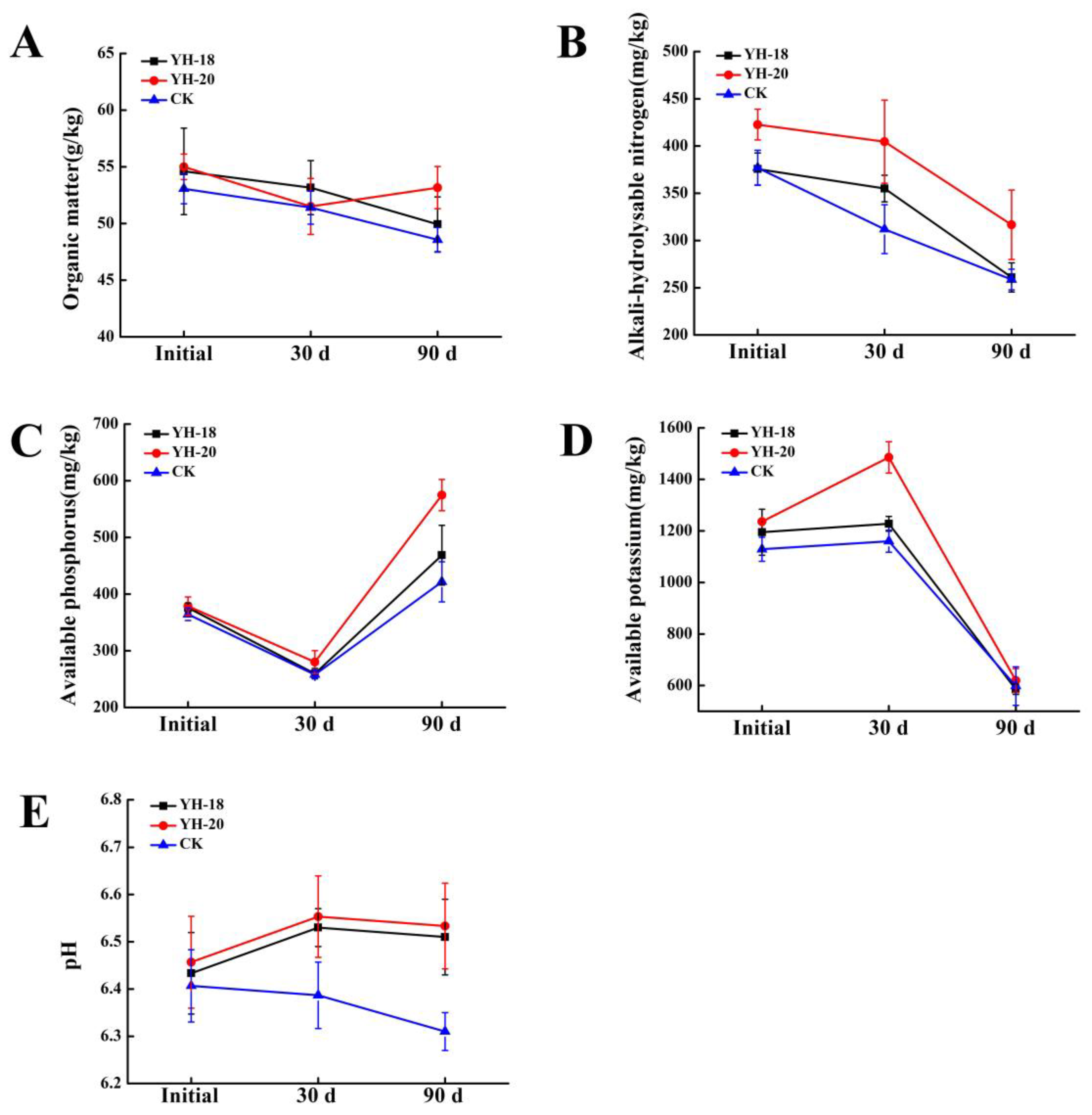
2.2. Effect of Inoculated Microbial Agents on the Soil Nutrient Content
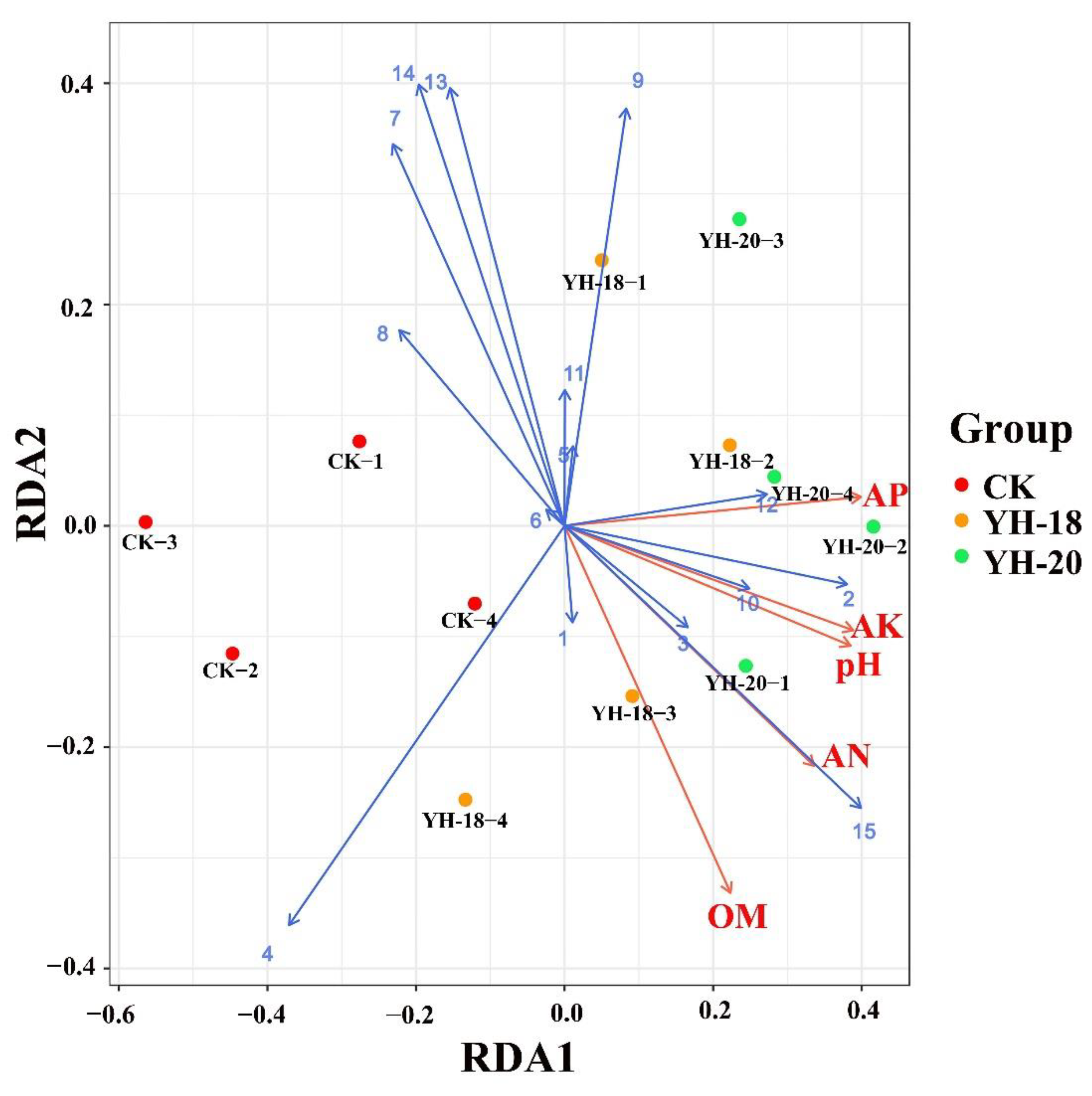
2.3. Correlation Analysis of Seedling Growth and Soil Environmental Parameters
2.4. Study of a Diversity and Its Correlation with Soil Properties after Inoculation
2.5. Bacterial Community Composition at the Taxonomic Level after Inoculation
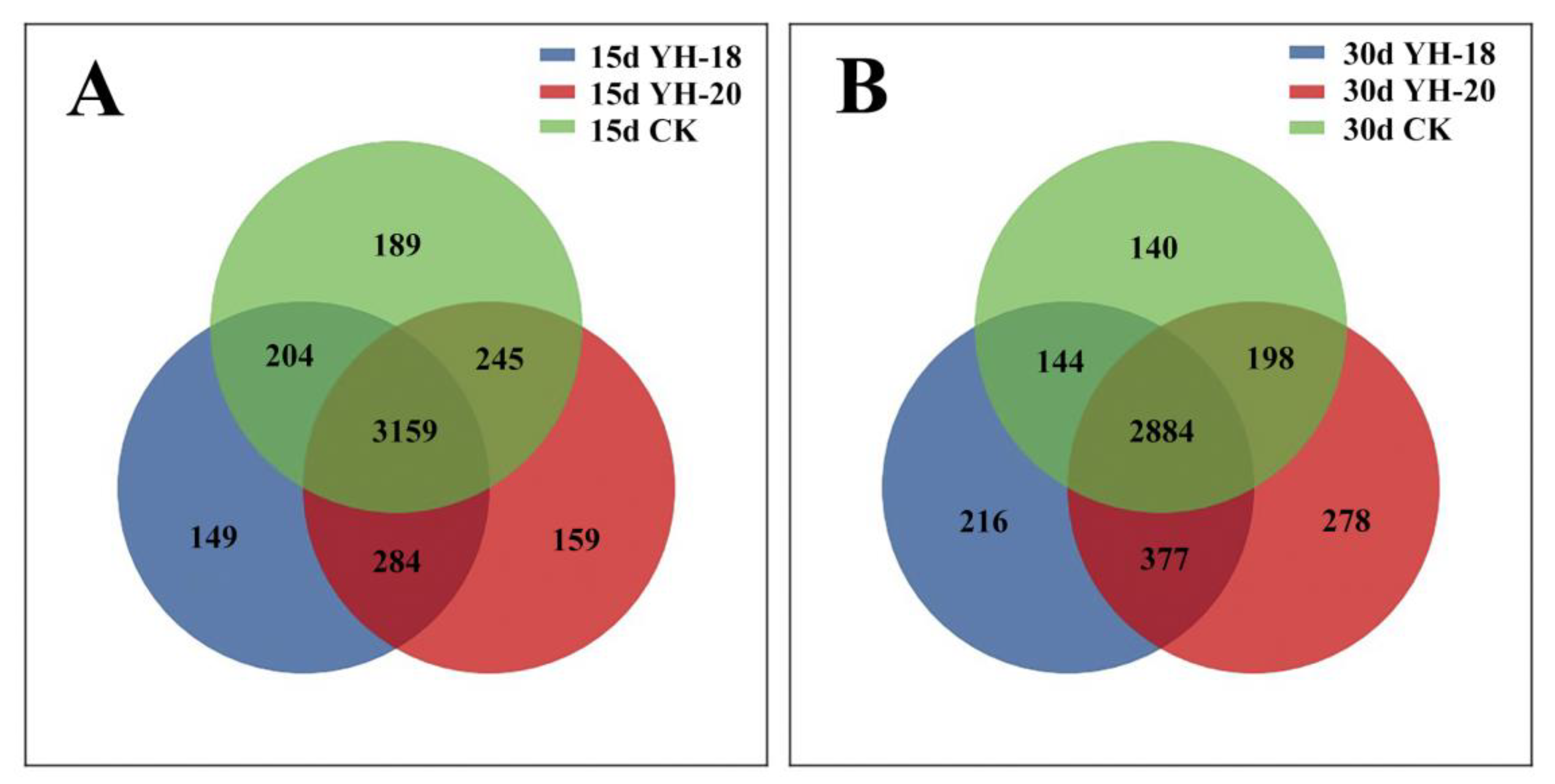
2.6. Community Composition at the OTU Level after Inoculation
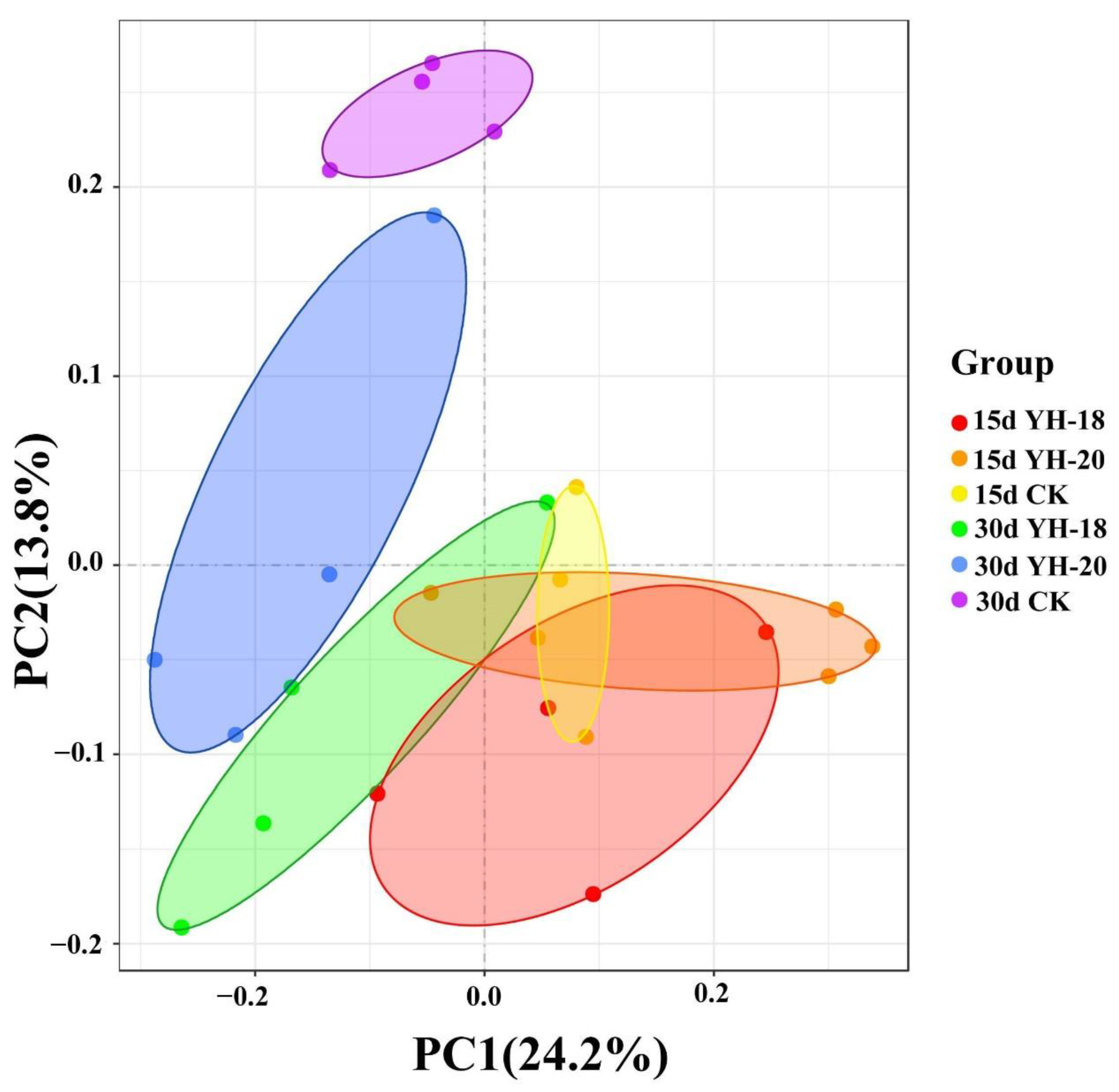
2.7. A PCoA Plot Based on Bray–Curtis Distances Provided a Visualization of the Microbial
2.8. Correlation between Environmental Parameters and Bacterial Community Composition at the Genus Level
3. Discussion
4. Materials and Methods
4.1. Overview of the Potted Seedling Test Site
4.2. Experimental Materials
4.3. Pot Experiment
4.4. Seedling Height and Ground Diameter
4.5. Leaf Area and Chlorophyll Content
4.6. Soil Sample Collection
4.7. Determination of Soil Properties
4.8. Total Bacterial DNA Extraction, Polymerase Chain Reaction (PCR) and 16S rRNA Amplification Based on High-Throughput Sequencing
4.9. Bioinformatics Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Liu, Y.Q.; Jing, L.I.; Sun, S.X.; Wang, Y.Q. Effects of alkali stress on Prunus davidiana (Carr.) leaf morphological structure and photosynthetic characteristics. Southwest China J. Agri. Sci. 2017, 30, 327–332. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wang, J.Y.; Hu, Y.L.; Wang, Y.S.; Zhu, S.H.; Mao, Z.Q. Root lifespan and root respiration of fruit of fruit tree species. Acta Hortic. 2008, 772, 269. [Google Scholar] [CrossRef]
- Xu, D.; Li, H.; Lin, L.; Liao, M.; Xia, H. Effects of carboxymethyl chitosan on the growth and nutrient uptake in Prunus davidiana seedlings. Physiol. Mol. Biol. Plants 2020, 26, 661–668. [Google Scholar] [CrossRef]
- Giorgi, M.; Capocasa, F.; Scalzo, J.; Murri, G.; Battino, M.; Mezzetti, B. The rootstock effects on plant adaptability, production, fruit quality, and nutrition in the peach (cv. “Suncrest”). Sci Hortic. 2005, 107, 36–42. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, P.; Li, Y.; Ma, L.; Alva, A.; Dou, Z.; Chen, Q.; Zhang, F. Phosphorus in China’s Intensive vegetable production systems: Overfertilization, soil enrichment, and environmental implications. J. Environ. Qual. 2013, 42, 982–989. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Niu, M.; Fan, H.; Li, J.; Lin, W. Responses of physiological ecology and quality evaluation of Rehmannia gltinosa in continuous cropping. Zhongguo Zhong Yao Za Zhi 2011, 36, 1133–1136. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Yang, Q.; Chi, X.; Pan, L.; Chen, N.; Yang, Z.; Wang, T.; Wang, M.; Yu, S. Soil eukaryotic microorganism succession as affected by continuous cropping of peanut--pathogenic and beneficial fungi were selected. PLoS ONE 2012, 7, e40659. [Google Scholar] [CrossRef] [Green Version]
- Albornoz, F. Crop responses to nitrogen overfertilization: A review. Sci Hortic. 2016, 205, 79–83. [Google Scholar] [CrossRef]
- Sahu, P.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Abhilash, P.C. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecol. Indic. 2019, 105, 601–612. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Ayangbenro, A.S.; Babalola, O.O. Elucidating the rhizosphere associated bacteria for environmental sustainability. Agriculture 2021, 11, 75. [Google Scholar] [CrossRef]
- Huang, Z.; Ruan, S.; Sun, Y.; Cheng, X.; Dai, J.; Gui, P.; Yu, M.; Zhong, Z.; Wu, J. Bacterial inoculants improved the growth and nitrogen use efficiency of Pyrus betulifolia under nitrogen-limited conditions by affecting the native soil bacterial communities. Appl. Soil Ecol. 2022, 170, 104285. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, H.; Zhang, B.; Geng, M.; Cai, X.; Wang, J.; Wang, Y. Investigating the effect of microbial inoculants Frankia F1 on growth-promotion, rhizosphere soil physicochemical properties, and bacterial community of ginseng. Appl. Soil Ecol. 2022, 172, 104369. [Google Scholar] [CrossRef]
- Xiao, X.; Zhu, Y.; Gao, C.; Zhang, Y.; Gao, Y.; Zhao, Y. Microbial inoculations improved rice yields by altering the presence of soil rare bacteria. Microbiol. Res. 2022, 254, 126910. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, S.; Liu, N.; He, H.; Cao, X.; Lv, C.; Zhang, K.; Dai, J. Effects of different types of microbial inoculants on available nitrogen and phosphorus, soil microbial community, and wheat growth in high-P soil. Environ. Sci. Pollut. Res. 2021, 28, 23036–23047. [Google Scholar] [CrossRef]
- Wang, X.; Ji, C.; Song, X.; Liu, Z.; Liu, Y.; Li, H.; Gao, Q.; Li, C.; Zheng, R.; Han, X.; et al. Biocontrol of two bacterial inoculant strains and their effects on the rhizosphere microbial community of field-grown wheat. BioMed Res. Int. 2021, 2021, 8835275. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. Effects of microbial inoculants on phosphorus and potassium availability, bacterial community composition, and chili pepper growth in a calcareous soil: A greenhouse study. J. Soil Sediments 2019, 19, 3597–3607. [Google Scholar] [CrossRef]
- Wang, H.W.; Zhu, Y.X.; Xu, M.; Cai, X.Y.; Tian, F. Co-application of spent mushroom substrate and PGPR alleviates tomato continuous cropping obstacle by regulating soil microbial properties. Rhizosphere 2022, 23, 100563. [Google Scholar] [CrossRef]
- Meng, Q.; Jiang, H.; Hao, J.J. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol. Control 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Jiang, C.H.; Liao, M.J.; Wang, H.K.; Zheng, M.Z.; Xu, J.J.; Guo, J.H. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, Y.; Cheon, W.; Park, J.; Kwon, H.T.; Balaraju, K.; Kim, J.; Yoon, Y.J.; Jeon, Y. Characterization of Bacillus velezensis AK-0 as a biocontrol agent against apple bitter rot caused by Colletotrichum gloeosporioides. Sci. Rep. 2021, 11, 626. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Liang, W.; Liu, M. Biocontrol potential of endophytic Bacillus velezensis strain QSE-21 against postharvest grey mould of fruit. Biol. Control 2021, 161, 104711. [Google Scholar] [CrossRef]
- Tian, X.L.; Zhao, X.M.; Zhao, S.Y.; Zhao, J.L.; Mao, Z.C. The biocontrol functions of Bacillus velezensis strain Bv-25 against Meloidogyne incognita. Front. Microbiol. 2022, 13, 843041. [Google Scholar] [CrossRef] [PubMed]
- Schisler, D.A.; Slininger, P.J.; Behle, R.W.; Jackson, M.A. Formulation of Bacillus spp. for biological control of plant diseases. Phytopathology 2004, 94, 1267–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Tang, X.; Yang, R.; Zhang, H.; Li, F.; Tao, F.; Li, F.; Wang, Z. Characteristics and application of a novel species of Bacillus: Bacillus velezensis. ACS Chem. Biol. 2018, 13, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.M. Isolation and Identification about Several Strains of Endophytic Bacteria. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2016. [Google Scholar]
- Wei, Q. Application and Research of Several Excellent Endophytic Bacteria in Shanghai Ecological Forest. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2017. [Google Scholar]
- Ding, S.; Ye, J.R. Preliminary study on antibacterial properties of Bacillus velezensis YH-20 fermentation broth. J. West. China For. Sci. 2019, 48, 81–88. [Google Scholar] [CrossRef]
- Khan, M.S.; Gao, J.; Chen, X.; Zhang, M.; Yang, F.; Du, Y.; Moe, T.S.; Munir, I.; Xue, J.; Zhang, X. The endophytic bacteria Bacillus velezensis Lle-9, isolated from Lilium leucanthum, harbors antifungal activity and plant growth-promoting effects. J. Microbiol. Biotechnol. 2020, 30, 668–680. [Google Scholar] [CrossRef]
- Xue, L.; Sun, B.; Yang, Y.; Jin, B.; Zhuang, G.; Bai, Z.; Zhuang, X. Efficiency and mechanism of reducing ammonia volatilization in alkaline farmland soil using Bacillus amyloliquefaciens biofertilizer. Environ. Res. 2021, 202, 111672. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, M.; Huang, G.; Yuan, Y.; Fu, C.; Yu, L. Coculture with two Bacillus velezensis strains enhances the growth of Anoectochilus plants via promoting nutrient assimilation and regulating rhizosphere microbial community. Ind. Crop. Prod. 2020, 154, 112697. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Ayilara, M.S.; Ayangbenro, A.S.; Babalola, O.O. Genome mining of three plant growth-promoting Bacillus species from maize rhizosphere. Appl. Biochem. Biotechnol. 2021, 193, 3949–3969. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Marzec-Grządziel, A.; Pecio, M.; Stuczyński, T. Testing combined effect of amendments and inoculation with bacteria for improving phytostabilisation of smelter waste extremely contaminated with trace elements. Agronomy 2021, 11, 2064. [Google Scholar] [CrossRef]
- Shi, J.W.; Lu, L.X.; Shi, H.M.; Ye, J.R. Effects of plant growth-promoting rhizobacteria on the growth and soil microbial community of Carya illinoinensis. Curr. Microbiol. 2022, 79, 352. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.; Yadav, A.N.; Suman, A.; Ahluwalia, A.S.; Saxena, A.K. Minerals solubilizing and mobilizing microbiomes: A sustainable approach for managing minerals’ deficiency in agricultural soil. J. Appl. Microbiol. 2022, 133, 1245–1272. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Daraz, U.; Li, Y.; Sun, Q.; Zhang, M.; Ahmad, I. Inoculation of Bacillus spp. Modulate the soil bacterial communities and available nutrients in the rhizosphere of vetiver plant irrigated with acid mine drainage. Chemosphere 2021, 263, 128345. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, H.S.; Sang, M.K.; Song, J.; Weon, H.Y. Effect of Bacillus mesonae H20-5 treatment on rhizospheric bacterial community of tomato plants under salinity stress. Plant Pathol. J. 2021, 37, 662–672. [Google Scholar] [CrossRef]
- Ren, H.; Huang, B.L.; Fernandez-Garcia, V.; Miesel, J.; Yan, L.; Lv, C.Q. Biochar and rhizobacteria amendments improve several soil properties and bacterial diversity. Microorganisms 2020, 8, 502. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, H.; Liu, J.; Wang, Q.; Shen, T.; Guo, W.; Wang, R. Shifts in microbial community function and structure along the successional gradient of coastal wetlands in Yellow River Estuary. Eur. J. Soil Biol. 2012, 49, 12–21. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef]
- Sait, M.; Davis, K.E.R.; Janssen, P.H. Effect of pH on isolation and distribution of members of subdivision 1 of the phylum Acidobacteria occurring in soil. Appl. Environ. Microbiol. 2006, 72, 1852–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plyuta, V.; Lipasova, V.; Popova, A.; Koksharova, O.; Kuznetsov, A.; Szegedi, E.; Chernin, L.; Khmel, I. Influence of volatile organic compounds emitted by Pseudomonas and Serratia strains on Agrobacterium tumefaciens biofilms. APMIS 2016, 124, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, H.; Yao, T.; Su, M.; Ran, F.; Han, B.; Li, J.; Lan, X.; Zhang, Y.; Yang, X.; et al. Microbial inoculation influences bacterial community succession and physicochemical characteristics during pig manure composting with corn straw. Bioresour. Technol. 2019, 289, 121653. [Google Scholar] [CrossRef]
- Jin, L.; Kim, K.; An, K.G.; Oh, H.M.; Lee, S.T. Arenimonas daejeonensis sp. nov., isolated from compost. Int. J. Syst. Evol. Microbiol. 2011, 62, 1674–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huy, H.; Jin, L.; Lee, Y.K.; Lee, K.C.; Lee, J.S.; Yoon, J.H.; Ahn, C.Y.; Oh, H.M. Arenimonas daechungensis sp. nov., isolated from the sediment of a eutrophic reservoir. Int. J. Syst. Evol. Microbiol. 2013, 63, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Makk, J.; Homonnay, Z.G.; Kéki, Z.; Nemes-Barnás, K.; Márialigeti, K.; Schumann, P.; Tóth, E.M. Arenimonas subflava sp. nov., isolated from a drinking water network, and emended description of the genus Arenimonas. Int. J. Syst. Evol. Microbiol. 2015, 65, 1915–1921. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Cao, Y.; Li, X.; Wang, G. High quality draft genomic sequence of Arenimonas donghaensis DSM 18148T. Stand. Genom. Sci. 2015, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef] [Green Version]
- Buckley, D.H.; Schmidt, T.M. Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environ. Microbiol. 2003, 5, 441–452. [Google Scholar] [CrossRef]
- Wang, B.; Wan, C.-X.; Zeng, H. Colonization on Cotton plants with a GFP labeled srain of Bacillus axarquiensis. Curr. Microbiol. 2020, 77, 3085–3094. [Google Scholar] [CrossRef]
- Ambrosini, A.; de Souza, R.; Passaglia, L.M.P. Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant Soil 2016, 400, 193–207. [Google Scholar] [CrossRef]
- Chen, L.; Hao, Z.; Li, K.; Sha, Y.; Wang, E.T.; Sui, X.; Mi, G.; Tian, C.; Chen, W. Effectsof growth-promoting rhizobacteria on maize growth and rhizosphere microbial community under conservation tillage in Northeast China. Microb. Biotechnol. 2020, 14, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Shen, M.; Wang, H.; Zhao, Q. A possible mechanism of action of plant growth-promoting rhizobacteria (PGPR) strain Bacillus pumilus WP8 via regulation of soil bacterial community structure. J. Gen. Appl. Microbiol. 2013, 59, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, S.; Yang, R.; Zhao, W.; Zhu, D.; Zhang, X.; Huang, Z. Bacillus amyloliquefaciens FH-1 significantly affects cucumber seedlings and the rhizosphere bacterial community but not soil. Sci Rep. 2021, 11, 12055. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2017, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Trabelsi, D.; Mhamdi, R. Microbial inoculants and their impact on soil microbial communities: A review. BioMed Res. Int. 2013, 2013, 863240. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Bao, J.; Chu, G.; Peng, F. Soil type influences rhizosphere bacterial community assemblies of pecan plantations, a case study of eastern China. Forests 2022, 13, 363. [Google Scholar] [CrossRef]
- Wang, F.P.; Wang, X.C.; Yao, B.Q.; Zhang, Z.H.; Shi, G.X.; Ma, Z.; Chen, Z.; Zhou, H. Effects of land-use types on soil organic carbon stocks: A case study across an altitudinal gradient within a farm-pastoral area on the eastern Qinghai-Tibetan Plateau, China. J. Mt. Sci. 2018, 15, 2693–2702. [Google Scholar] [CrossRef]
- Kowalenko, C.G.; Babuin, D. Interference problems with phosphoantimonylmolybdenum colorimetric measurement of phosphorus in soil and plant materials. Commun. Soil Sci. Plant Anal. 2007, 38, 1299–1316. [Google Scholar] [CrossRef]
- Havre, G.N. The fame photometric determination of sodium, potassium and calcium in plant extracts with special reference to interference efects. Anal. Chim. Acta 1961, 25, 557–566. [Google Scholar] [CrossRef]
- Wu, J.; Lin, H.; Meng, C.; Jiang, P.; Fu, W. Effects of intercropping grasses on soil organic carbon and microbial community functional diversity under Chinese hickory (Carya cathayensis Sarg.) stands. Soil Res. 2014, 52, 575–583. [Google Scholar] [CrossRef]
- Nossa, C.W.; Oberdorf, W.E.; Aas, J.A.; Paster, B.J.; DeSantis, T.Z.; Brodie, E.L.; Malamud, D.; Poles, M.A.; Pei, Z. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 2010, 16, 4135–4144. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina Sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyon, D.; Tsai, S.Q.; Khayter, C.; Foden, J.A.; Sander, J.D.; Joung, J.K. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 2012, 30, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
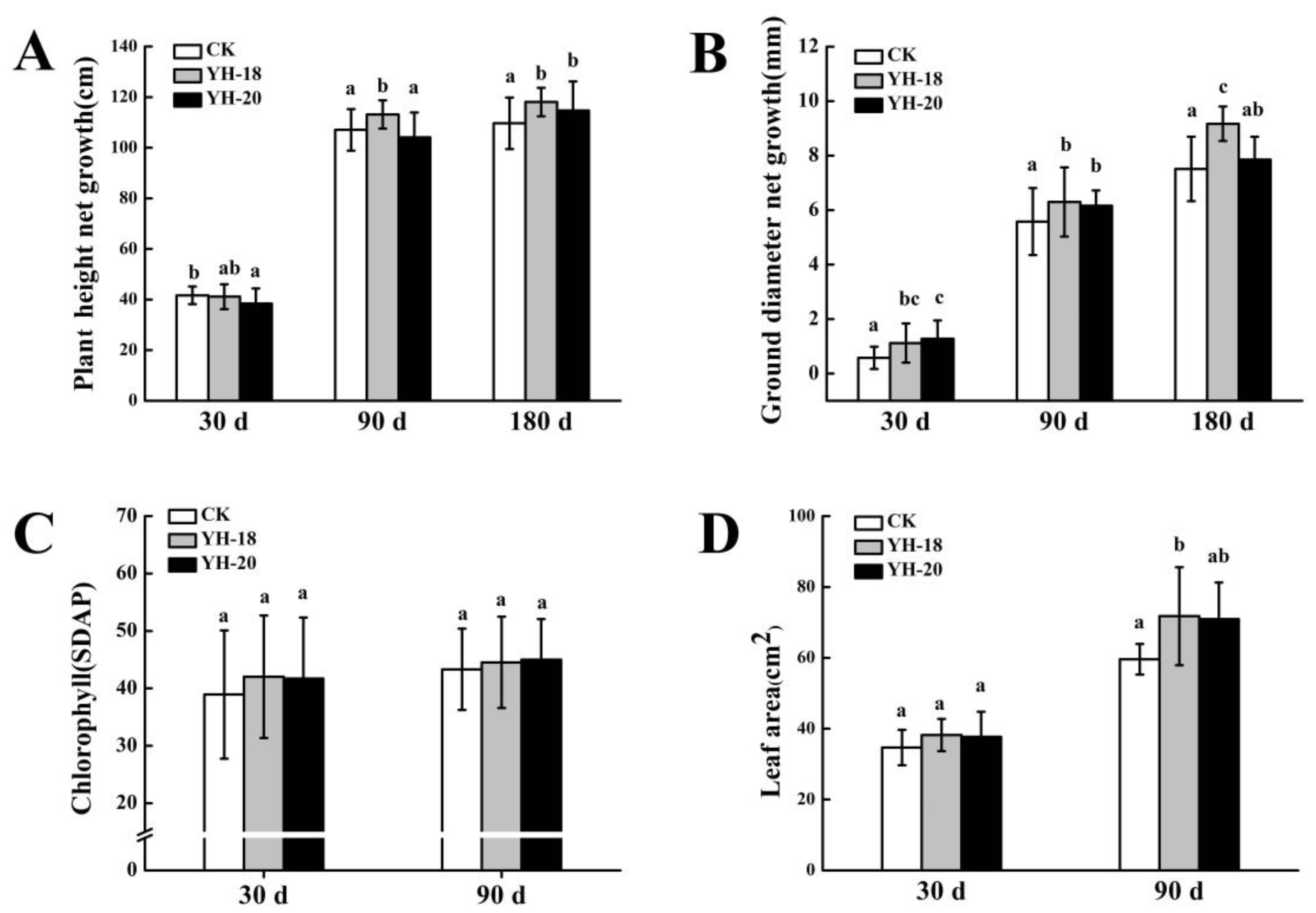


| Ground Net Diameter Growth | Plant Height Net Growth | Leaf Area | Chlorophyll Content | |
|---|---|---|---|---|
| OM | 0.198 | 0.718 * | 0.471 | 0.211 |
| AN | 0.629 | −0.224 | 0.547 | 0.476 |
| AK | 0.747 * | −0.244 | 0.614 | 0.627 |
| AP | 0.421 | 0.356 | 0.531 | 0.38 |
| pH | 0.832 ** | 0.028 | 0.861 ** | 0.785 * |
| Treatment | Ace | Chao1 | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|
| 15-YH-18 | 3278.88 ± 52.11 b | 3327.92 ± 70.43 b | 9.65 ± 0.07 ab | 0.9963 ± 0.001 a | 0.9753 ± 0.0005 a |
| 15-YH-20 | 3325.81 ± 149.77 b | 3362.47 ± 150.70 b | 9.75 ± 0.16 b | 0.9967 ± 0.001 a | 0.975 ± 0.0008 a |
| 15-CK | 3290.15 ± 82.13 ab | 3307.20 ± 75.65 b | 9.66 ± 0.08 ab | 0.9967 ± 0.001 a | 0.975 ± 0.0008 a |
| 30-YH-18 | 3085.22 ± 161.97 b | 3125.67 ± 157.64 b | 9.53 ± 0.16 ab | 0.9963 ± 0.001 a | 0.977 ± 0.0008 bc |
| 30-YH-20 | 3187.30 ± 281.74 b | 3189.75 ± 285.43 b | 9.60 ± 0.34 ab | 0.9968 ± 0.001 a | 0.9757 ± 0.0017 ab |
| 30-CK | 2861.84 ± 132.61 a | 2867.85 ± 134.50 a | 9.38 ± 0.16 a | 0.996 ± 0.001 a | 0.9785 ± 0.0013 c |
| Treatment | Ace | Chao1 | Shannon | Simpson |
|---|---|---|---|---|
| OM | 0.595 * | 0.590 * | 0.668 * | 0.328 |
| AN | 0.692 * | 0.658 * | 0.545 | 0.219 |
| AK | 0.681 * | 0.635 * | 0.615 * | 0.514 |
| AP | 0.532 | 0.504 | 0.633 * | 0.637 * |
| pH | 0.679 * | 0.679 * | 0.594 * | 0.362 |
| Treatment | Salt Tolerance | Alkali Tolerance | Phosphate Solubilization Capacity (μg/mL) | Potassium Solubilization Capacity (μg/mL) | IAA Production (μg/mL) | Siderophore Production | Nitrogenase Activity |
|---|---|---|---|---|---|---|---|
| YH-18 | 9% | 10 | 143.07 | 14.63 | 33.59 | + | + |
| YH-20 | 9% | 9 | 157.75 | 12.50 | 17.40 | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Lu, L.; Ye, J.; Shi, L. Effects of Two Bacillus Velezensis Microbial Inoculants on the Growth and Rhizosphere Soil Environment of Prunus davidiana. Int. J. Mol. Sci. 2022, 23, 13639. https://doi.org/10.3390/ijms232113639
Shi H, Lu L, Ye J, Shi L. Effects of Two Bacillus Velezensis Microbial Inoculants on the Growth and Rhizosphere Soil Environment of Prunus davidiana. International Journal of Molecular Sciences. 2022; 23(21):13639. https://doi.org/10.3390/ijms232113639
Chicago/Turabian StyleShi, Huimin, Lanxiang Lu, Jianren Ye, and Lina Shi. 2022. "Effects of Two Bacillus Velezensis Microbial Inoculants on the Growth and Rhizosphere Soil Environment of Prunus davidiana" International Journal of Molecular Sciences 23, no. 21: 13639. https://doi.org/10.3390/ijms232113639





