Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Participants
4.2. GPCR-AAbs
4.3. OCT-A
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Novel Coronavirus (2019-nCoV) Situation Report-1. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 (accessed on 18 February 2022).
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Salzberger, B.; Buder, F.; Lampl, B.; Ehrenstein, B.; Hitzenbichler, F.; Holzmann, T.; Schmidt, B.; Hanses, F. Epidemiology of SARS-CoV-2. Infection 2021, 49, 233–239. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. WHO Coronavirus (COVID-19) Dashbord. Available online: https://covid19.who.int/ (accessed on 18 February 2022).
- Szewczykowski, C.; Mardin, C.; Lucio, M.; Wallukat, G.; Hoffmanns, J.; Schroder, T.; Raith, F.; Rogge, L.; Heltmann, F.; Moritz, M.; et al. Long COVID: Association of Functional Autoantibodies against G-Protein-Coupled Receptors with an Impaired Retinal Microcirculation. Int. J. Mol. Sci. 2022, 23, 7209. [Google Scholar] [CrossRef] [PubMed]
- AWMF online, Portal der wissenschaftlichen Medizin. S1-Leitlinie Post-COVID/Long-COVID. 2022. Available online: https://www.awmf.org/uploads/tx_szleitlinien/020-027l_S1_Post_COVID_Long_COVID_2022-08.pdf (accessed on 17 August 2022).
- Fernández-de-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef] [PubMed]
- Campen, C.; Rowe, P.C.; Visser, F.C. Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Medicina 2021, 58, 28. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Hopkinson, N.S.; Jenkins, G.; Hart, N. COVID-19 and what comes after? Thorax 2021, 76, 324–325. Available online: https://pubmed.ncbi.nlm.nih.gov/33589513/ (accessed on 26 September 2022). [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Hohberger, B.; Harrer, T.; Mardin, C.; Kruse, F.; Hoffmanns, J.; Rogge, L.; Heltmann, F.; Moritz, M.; Szewczykowski, C.; Schottenhamml, J.; et al. Case Report: Neutralization of Autoantibodies Targeting G-Protein-Coupled Receptors Improves Capillary Impairment and Fatigue Symptoms After COVID-19 Infection. Front. Med. 2021, 8, 754667. [Google Scholar] [CrossRef]
- Hohberger, B.; Hosari, S.; Wallukat, G.; Kunze, R.; Krebs, J.; Muller, M.; Hennig, T.; Lammer, R.; Horn, F.; Munoz, L.E.; et al. Agonistic autoantibodies against beta2-adrenergic receptor influence retinal microcirculation in glaucoma suspects and patients. PLoS ONE 2021, 16, e0249202. [Google Scholar] [CrossRef]
- Lukitsch, I.; Kehr, J.; Chaykovska, L.; Wallukat, G.; Nieminen-Kelha, M.; Batuman, V.; Dragun, D.; Gollasch, M. Renal ischemia and transplantation predispose to vascular constriction mediated by angiotensin II type 1 receptor-activating antibodies. Transplantation 2012, 94, 8–13. [Google Scholar] [CrossRef]
- Wallukat, G.; Wollenberger, A. Involvement of β2-Adrenergic Receptors in the Potentaion of the Chronotropic Action of Isoprenaline Evoked in Rocker-Cultured Neonatal Rat Heart Cells by Pyruvate and L (+) Lactate; Martinius Nijhoff Publishing: Boston, MA, USA, 1987. [Google Scholar]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021, 75, e13746. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, B.; Mardin, C.Y. OCT Angiography as an Interdisciplinary Diagnostic Tool for Systemic Diseases. Klin. Mon. Augenheilkd. 2021, 238, 1294–1298. [Google Scholar] [CrossRef]
- Hohberger, B.; Lucio, M.; Schlick, S.; Wollborn, A.; Hosari, S.; Mardin, C. OCT-angiography: Regional reduced macula microcirculation in ocular hypertensive and pre-perimetric glaucoma patients. PLoS ONE 2021, 16, e0246469. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A review of optical coherence tomography angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef]
- Hohberger, B.; Muller, M.; Hosari, S.; Mardin, C.Y. OCT-Angiography: Mydriatic phenylephrine and tropicamide do not influence retinal microvasculature in macula and peripapillary region. PLoS ONE 2019, 14, e0221395. [Google Scholar] [CrossRef]
- Vanichkachorn, G.; Newcomb, R.; Cowl, C.T.; Murad, M.H.; Breeher, L.; Miller, S.; Trenary, M.; Neveau, D.; Higgins, S. Post-COVID-19 Syndrome (Long Haul Syndrome): Description of a Multidisciplinary Clinic at Mayo Clinic and Characteristics of the Initial Patient Cohort. Mayo Clin. Proc. 2021, 96, 1782–1791. [Google Scholar] [CrossRef]
- Park, R.; Chidharla, A.; Mehta, K.; Sun, W.; Wulff-Burchfield, E.; Kasi, A. Sex-bias in COVID-19-associated illness severity and mortality in cancer patients: A systematic review and meta-analysis. EClinicalMedicine 2020, 26, 100519. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Was ist Long COVID?—Deutsche Gesellschaft für ME/CFS. 2022. Available online: https://www.mecfs.de/longcovid (accessed on 26 September 2022).
- Sandler, C.X.; Wyller, V.B.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and Post-infective Fatigue Syndrome: A Review. Open Forum Infect. Dis. 2021, 8, ofab440. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L.; Bateman, L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front. Med. 2020, 7, 606824. [Google Scholar] [CrossRef]
- Friedman, K.J.; Murovska, M.; Pheby, D.F.H.; Zalewski, P. Our Evolving Understanding of ME/CFS. Medicina 2021, 57, 200. [Google Scholar] [CrossRef]
- Wong, T.L.; Weitzer, D.J. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 5, 418. [Google Scholar] [CrossRef]
- Töpfner, N.; Alberer, M.; Ankermann, T.; Bender, S.; Berner, R.; de Laffolie, J.; Dingemann, J.; Heinicke, D.; Haas, J.P.; Hufnagel, M.; et al. Recommendation for standardized medical care for children and adolescents with long COVID. Mon. Kinderheilkd 2022, 170, 539–547. [Google Scholar] [CrossRef]
- Tomas, C.; Newton, J.; Watson, S. A review of hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. ISRN Neurosci. 2013, 2013, 784520. [Google Scholar] [CrossRef]
- Bhui, K.S.; Dinos, S.; Ashby, D.; Nazroo, J.; Wessely, S.; White, P.D. Chronic fatigue syndrome in an ethnically diverse population: The influence of psychosocial adversity and physical inactivity. BMC Med. 2011, 9, 26. [Google Scholar] [CrossRef]
- Jason, L.A.; Islam, M.; Conroy, K.; Cotler, J.; Torres, C.; Johnson, M.; Mabie, B. COVID-19 Symptoms Over Time: Comparing Long-Haulers to ME/CFS. Fatigue 2021, 9, 59–68. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; Cha, D.S.; Mansur, R.B.; McIntyre, R.S. Inflamed moods: A review of the interactions between inflammation and mood disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 23–34. [Google Scholar] [CrossRef]
- Sonneville, R.; Klein, I.; de Broucker, T.; Wolff, M. Post-infectious encephalitis in adults: Diagnosis and management. J. Infect. 2009, 58, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Pont, C.; Ascaso, F.J.; Grzybowski, A.; Huerva, V. Corneal endothelial cell density during diabetes mellitus and ocular diabetes complications treatment. J. Fr. Ophtalmol. 2020, 43, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Urrets-Zavalía, J.A.; Espósito, E.; Garay, I.; Monti, R.; Ruiz-Lascano, A.; Correa, L.; Serra, H.M.; Grzybowski, A. The eye and the skin in endocrine metabolic diseases. Clin. Dermatol. 2016, 34, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Mulè, G.; Vadalà, M.; Carollo, C.; Cottone, S.; Agabiti Rosei, C.; De Ciuceis, C.; Rizzoni, D.; Ferri, C.; Muiesan, M.L. Arterial Hypertension and the Hidden Disease of the Eye: Diagnostic Tools and Therapeutic Strategies. Nutrients 2022, 14, 2200. [Google Scholar] [CrossRef] [PubMed]
- Dziedziak, J.; Zaleska-Żmijewska, A.; Szaflik, J.P.; Cudnoch-Jędrzejewska, A. Impact of Arterial Hypertension on the Eye: A Review of the Pathogenesis, Diagnostic Methods, and Treatment of Hypertensive Retinopathy. Med. Sci. Monit. 2022, 28, e935135. [Google Scholar] [CrossRef]
- Vasiliou, V.; Thompson, D.C.; Smith, C.; Fujita, M.; Chen, Y. Aldehyde dehydrogenases: From eye crystallins to metabolic disease and cancer stem cells. Chem. Biol. Interact. 2013, 202, 2–10. [Google Scholar] [CrossRef]
- Østergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Huertas, A.; Montani, D.; Savale, L.; Pichon, J.; Tu, L.; Parent, F.; Guignabert, C.; Humbert, M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020, 56, 2001634. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Lauritano, D.; Ronconi, G.; Calvisi, V.; Conti, P. Antibodies for COVID-19-which, when and how long? J. Biol. Regul. Homeost. Agents 2021, 35, 417–422. [Google Scholar] [CrossRef]
- Wallukat, G.; Wollenberger, A. Effects of the serum gamma globulin fraction of patients with allergic asthma and dilated cardiomyopathy on chronotropic beta adrenoceptor function in cultured neonatal rat heart myocytes. Biomed Biochim. Acta 1987, 46, S634–S639. [Google Scholar]
- Wenzel, K.; Schulze-Rothe, S.; Haberland, A.; Müller, J.; Wallukat, G.; Davideit, H. Performance and in-house validation of a bioassay for the determination of beta1-autoantibodies found in patients with cardiomyopathy. Heliyon 2017, 3, e00362. [Google Scholar] [CrossRef]
- Wallukat, G.; Wenzel, K.; Schimke, I. Analytics of Functional Autoantibodies in Patients with Chagas Disease. Methods Mol. Biol. 2019, 1955, 247–261. [Google Scholar] [CrossRef]
- Hosari, S.; Hohberger, B.; Theelke, L.; Sari, H.; Lucio, M.; Mardin, C.Y. OCT Angiography: Measurement of Retinal Macular Microvasculature with Spectralis II OCT Angiography—Reliability and Reproducibility. Ophthalmologica. J. Int. D’ophtalmologie. Int. J. Ophthalmol. Z. Augenheilkd. 2020, 243, 75–84. [Google Scholar] [CrossRef]


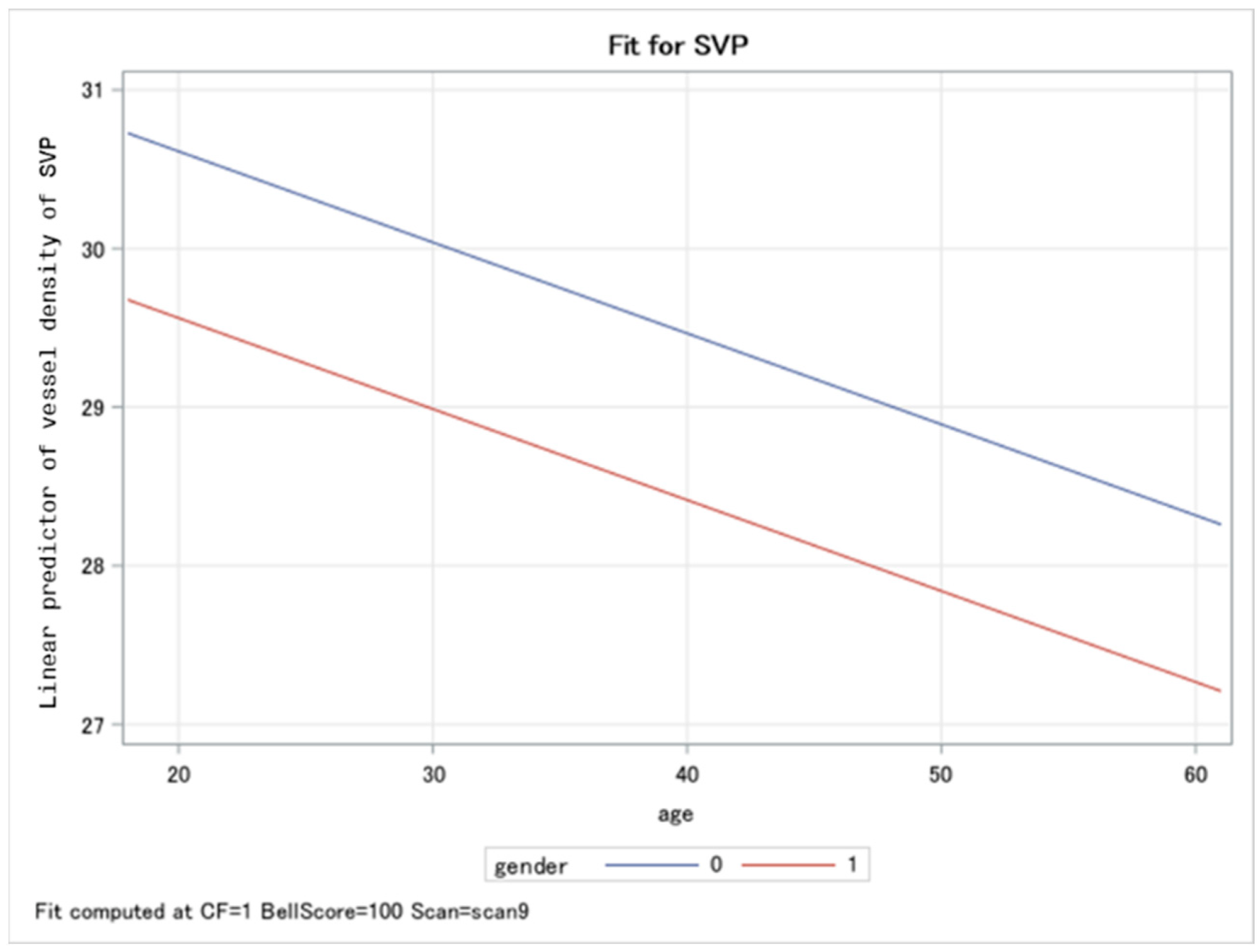
| Differences in Least Squares Means of Vessel Densities in Patients with PCS Compared to Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Controls | _PCS | Estimate | SE | t-Value | Pr > |t| | Lower | Upper | |
| SVP | Post-COVID-19 | 0 | 1 | 0.16 | 0.2 | 0.81 | 0.42 | −0.23 | 0.55 |
| ICP | Post-COVID-19 | 0 | 1 | 0.66 | 0.2 | 3.84 | 0.0001 | 0.32 | 0.1 |
| DCP | Post-COVID-19 | 0 | 1 | 0.11 | 0.2 | 0.54 | 0.59 | −0.2855 | 0.5 |
| Differences in Least Square Means of Vessel Density in PCS patients with CF (1) and without CF (0) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | CF | _CF | Estimate | SE | t-Value | Pr > |t| | Lower | Upper | |
| SVP | Chronic Fatigue | 0 | 1 | −2.71 | 0.91 | −2.98 | 0.0033 | −4.51 | −0.92 |
| ICP | Chronic Fatigue | 0 | 1 | −0.31 | 0.84 | −0.37 | 0.71 | −1.97 | 1.35 |
| DCP | Chronic Fatigue | 0 | 1 | 0.61 | 0.8 | 0.76 | 0.45 | −0.97 | 2.19 |
| Differences in Least Square Means of Vessel Density in the SVP in PCS Patients Considering GPCR-AAb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect | GPCR-AAb (0) | GPCR-AAb (1) | Estimate | SE | t-Value | Pr > |t| | Lower | Upper | Adj. Lower | Adj. Upper |
| Noci-AAb | 0 | 1 | 0.69 | 0.44 | 1.58 | 0.1146 | −0.17 | 1.55 | −0.17 | 1.55 |
| β2-AAb | 0 | 1 | −1.85 | 0.69 | −2.7 | 0.0075 | −3.20 | −0.50 | −3.20 | −0.50 |
| AT1-AAb | 0 | 1 | 0.75 | 0.87 | 0.86 | 0.3914 | −0.97 | 2.46 | −0.97 | 2.46 |
| α1-AAb | 0 | 1 | 0.15 | 0.36 | 0.42 | 0.678 | −0.55 | 0.85 | −0.55 | 0.85 |
| MAS-AAb | 0 | 1 | −0.42 | 0.91 | −0.46 | 0.6439 | −2.20 | 1.37 | −2.20 | 1.37 |
| M2-AAb | 0 | 1 | 0.61 | 0.46 | 1.33 | 0.1833 | −0.29 | 1.52 | −0.29 | 1.52 |
| ETA-AAb | 0 | 1 | −0.03 | 0.91 | −0.03 | 0.9764 | −1.82 | 1.77 | −1.82 | 1.77 |
| Differences in Least Square Means of Vessel Density in the ICP in PCS Patients Considering GPCR-AAb | ||||||||||
| Effect | GPCR-AAb (0) | GPCR-AAb (1) | Estimate | SE | t-Value | Pr > |t| | Lower | Upper | Adj. Lower | Adj. Upper |
| Noci-AAb | 0 | 1 | 0.88 | 0.41 | 2.13 | 0.0344 | 0.07 | 1.70 | 0.07 | 1.70 |
| β2-AAb | 0 | 1 | −1.66 | 0.65 | −2.56 | 0.0112 | −2.94 | −0.38 | −2.94 | −0.38 |
| AT1-AAb | 0 | 1 | 1.27 | 0.90 | 1.4 | 0.1624 | −0.51 | 3.05 | −0.51 | 3.05 |
| α1-AAb | 0 | 1 | 0.36 | 0.34 | 1.07 | 0.2848 | −0.30 | 1.03 | −0.30 | 1.03 |
| MAS-AAb | 0 | 1 | −0.85 | 0.92 | −0.93 | 0.3553 | −2.67 | 0.96 | −2.67 | 0.96 |
| M2-AAb | 0 | 1 | 0.58 | 0.44 | 1.34 | 0.1826 | −0.28 | 1.44 | −0.28 | 1.44 |
| ETA-AAb | 0 | 1 | −1.93 | 0.86 | −2.24 | 0.0261 | −3.63 | −0.23 | −3.63 | −0.23 |
| Differences in Least Square Means of Vessel Density in the DCP in PCS Patients Considering GPCR-AAb | ||||||||||
| Effect | GPCR-AAb (0) | GPCR-AAb (1) | Estimate | SE | t-Value | Pr > |t| | Lower | Upper | Adj. Lower | Adj. Upper |
| Noci-AAb | 0 | 1 | 0.87 | 0.45 | 1.93 | 0.055 | −0.02 | 1.75 | −0.02 | 1.75 |
| β2-AAb | 0 | 1 | −1.08 | 0.70 | −1.53 | 0.1271 | −2.47 | 0.31 | −2.47 | 0.31 |
| AT1-AAb | 0 | 1 | 0.50 | 0.89 | 0.56 | 0.5788 | −1.26 | 2.26 | −1.26 | 2.26 |
| α1-AAb | 0 | 1 | −0.09 | 0.37 | −0.26 | 0.7973 | −0.81 | 0.63 | −0.81 | 0.63 |
| MAS-AAb | 0 | 1 | −0.16 | 0.93 | −0.18 | 0.8595 | −2.00 | 1.67 | −2.00 | 1.67 |
| M2-AAb | 0 | 1 | 0.40 | 0.47 | 0.85 | 0.3976 | −0.53 | 1.33 | −0.53 | 1.33 |
| ETA-AAb | 0 | 1 | −1.83 | 0.94 | −1.96 | 0.0516 | −3.67 | 0.01 | −3.67 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlick, S.; Lucio, M.; Wallukat, G.; Bartsch, A.; Skornia, A.; Hoffmanns, J.; Szewczykowski, C.; Schröder, T.; Raith, F.; Rogge, L.; et al. Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue. Int. J. Mol. Sci. 2022, 23, 13683. https://doi.org/10.3390/ijms232213683
Schlick S, Lucio M, Wallukat G, Bartsch A, Skornia A, Hoffmanns J, Szewczykowski C, Schröder T, Raith F, Rogge L, et al. Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue. International Journal of Molecular Sciences. 2022; 23(22):13683. https://doi.org/10.3390/ijms232213683
Chicago/Turabian StyleSchlick, Sarah, Marianna Lucio, Gerd Wallukat, Alexander Bartsch, Adam Skornia, Jakob Hoffmanns, Charlotte Szewczykowski, Thora Schröder, Franziska Raith, Lennart Rogge, and et al. 2022. "Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue" International Journal of Molecular Sciences 23, no. 22: 13683. https://doi.org/10.3390/ijms232213683
APA StyleSchlick, S., Lucio, M., Wallukat, G., Bartsch, A., Skornia, A., Hoffmanns, J., Szewczykowski, C., Schröder, T., Raith, F., Rogge, L., Heltmann, F., Moritz, M., Beitlich, L., Schottenhamml, J., Herrmann, M., Harrer, T., Ganslmayer, M., Kruse, F. E., Lämmer, R., ... Hohberger, B. (2022). Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue. International Journal of Molecular Sciences, 23(22), 13683. https://doi.org/10.3390/ijms232213683








