Lactoferrin Alleviates Lipopolysaccharide-Induced Infantile Intestinal Immune Barrier Damage by Regulating an ELAVL1-Related Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Pharmacokinetic Study of LF by UPLC
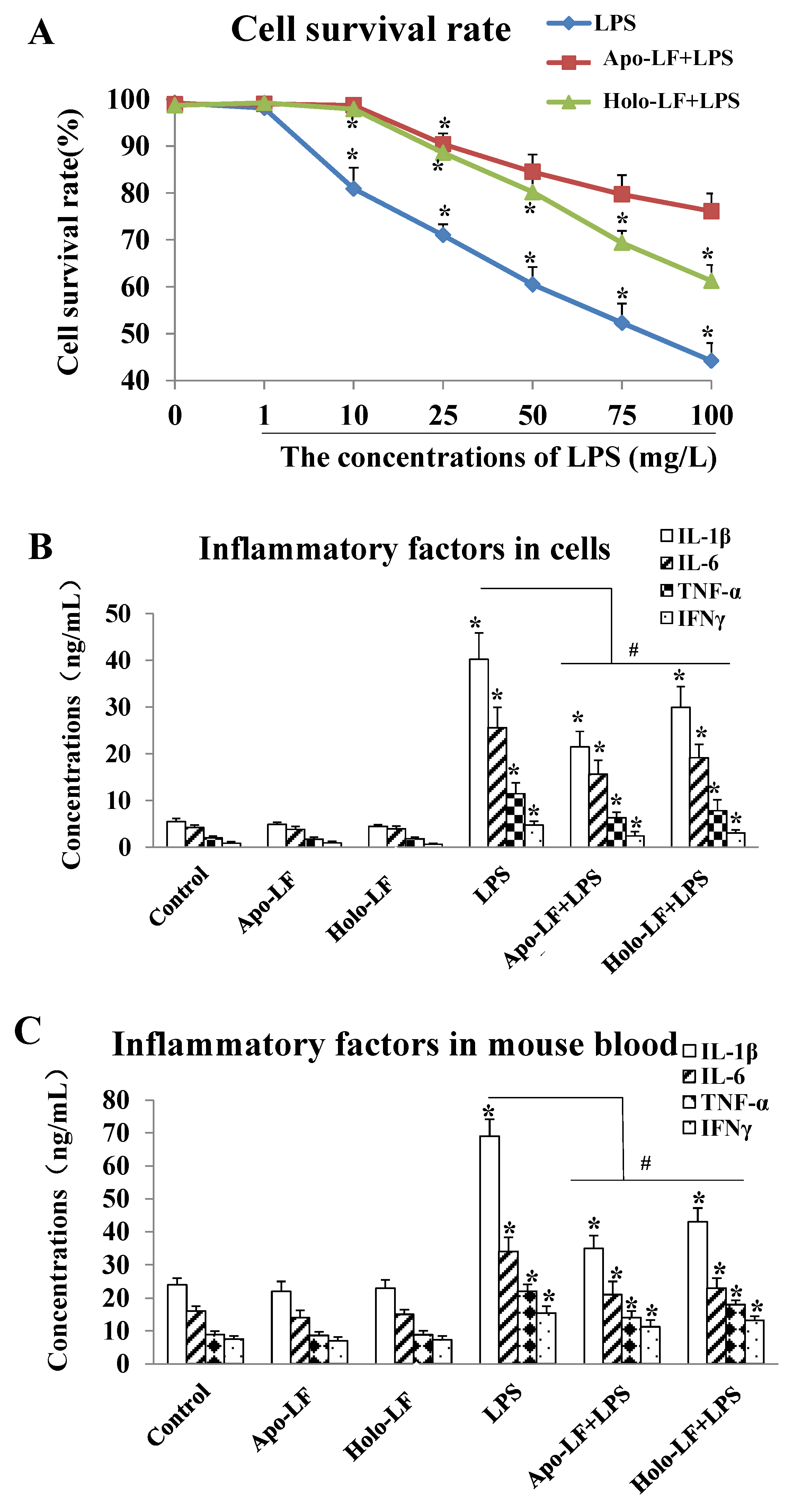
2.2. The Influence of LF with Different Fe3+ Saturation Levels on Iinflammatory Factors In Vitro and In Vivo
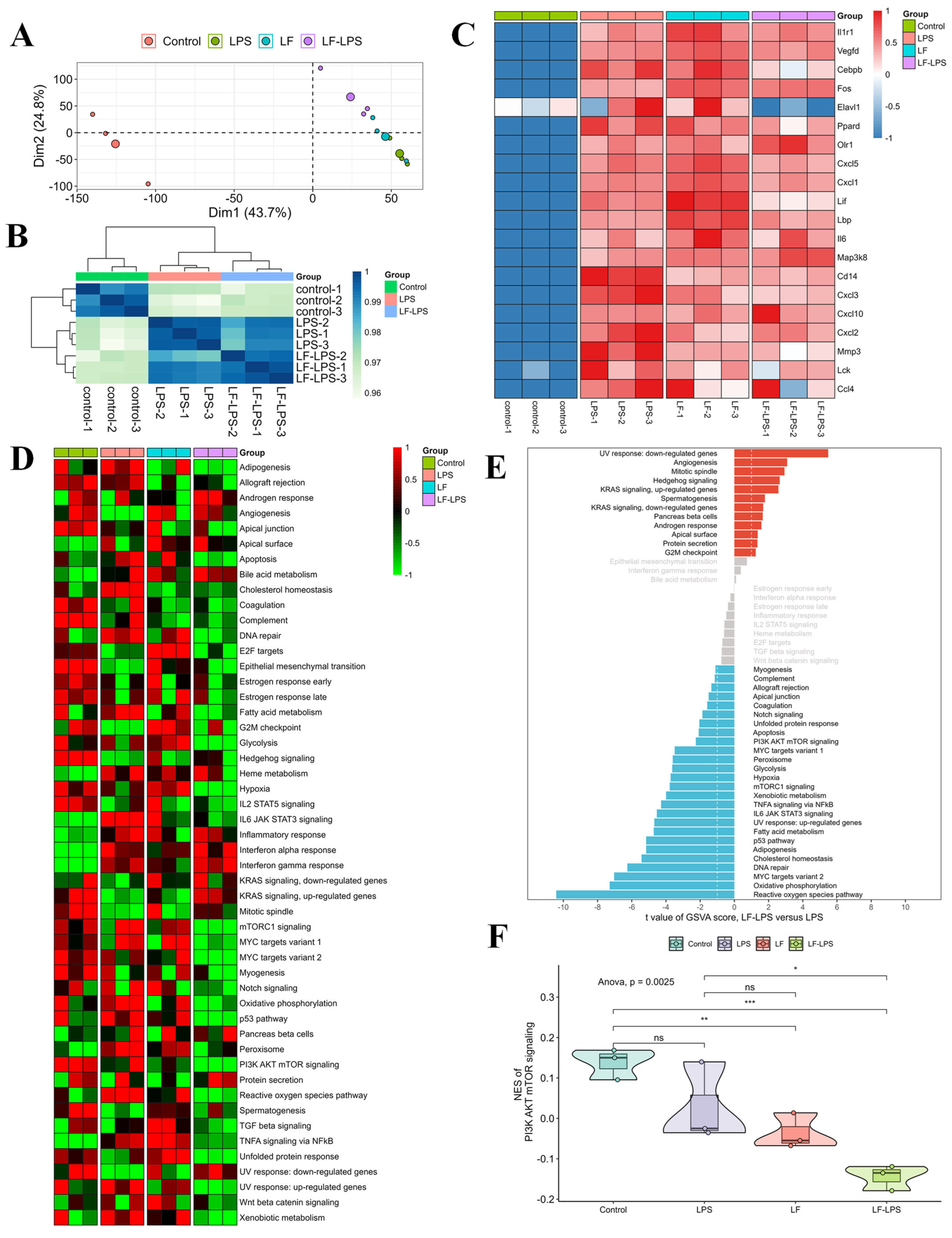
2.3. RNA-Seq Analyses of the LF in the Primary LPS-Induced Primary Intestinal Epithelial Cells of the Mice
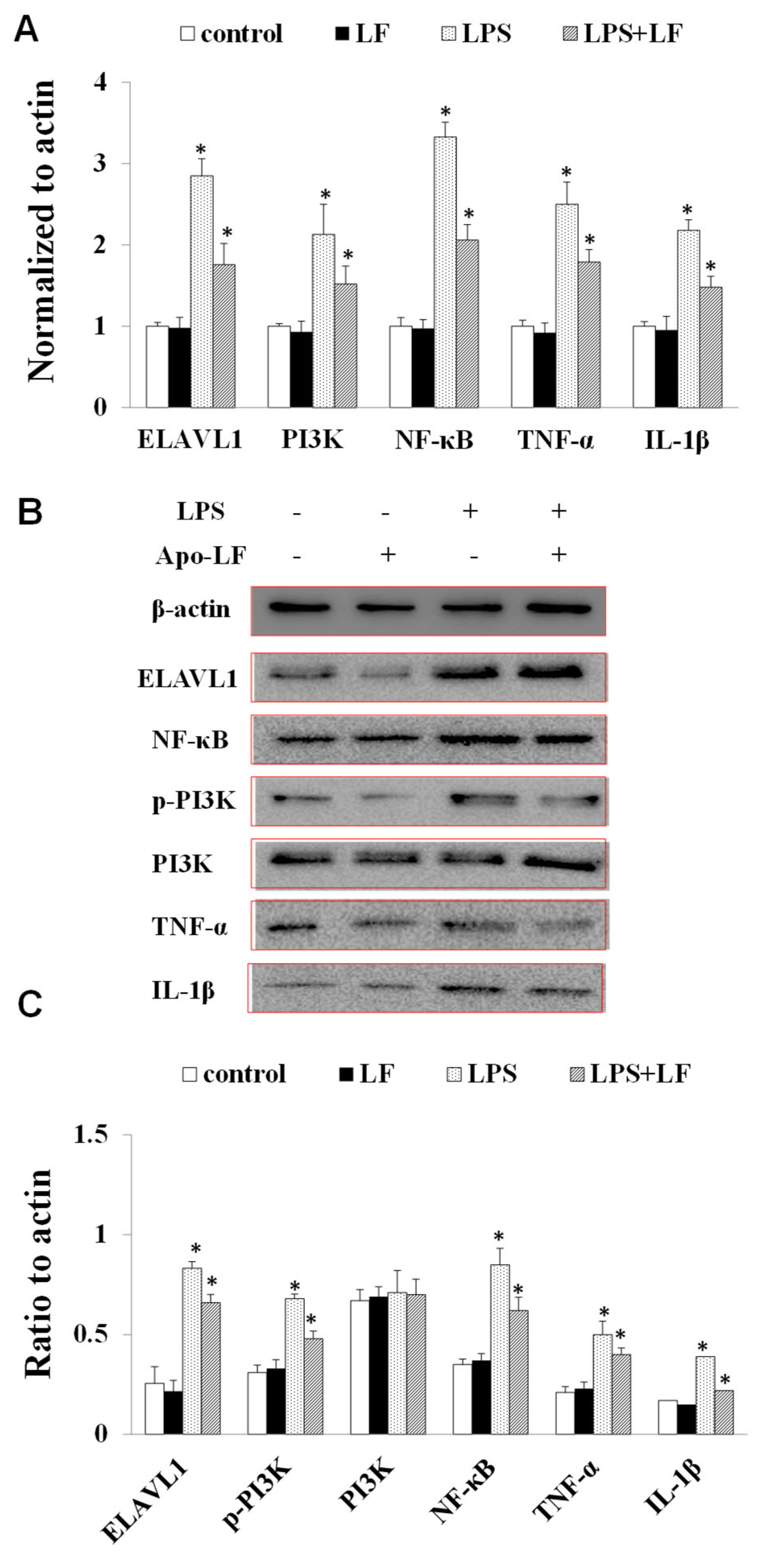
2.4. The Effect of LF on the mRNA and Protein Expression of Immune Indicators In Vitro and In Vivo
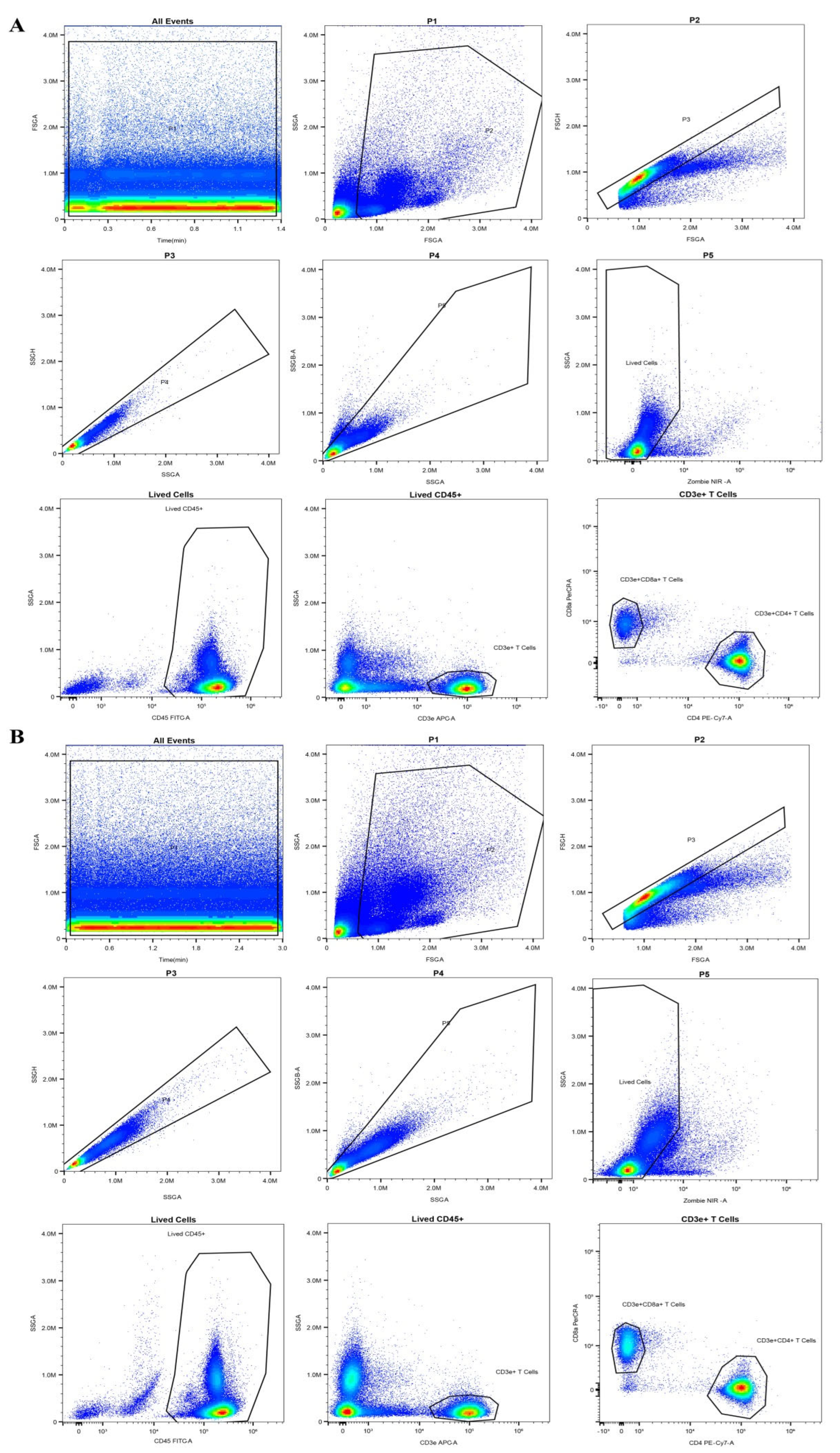
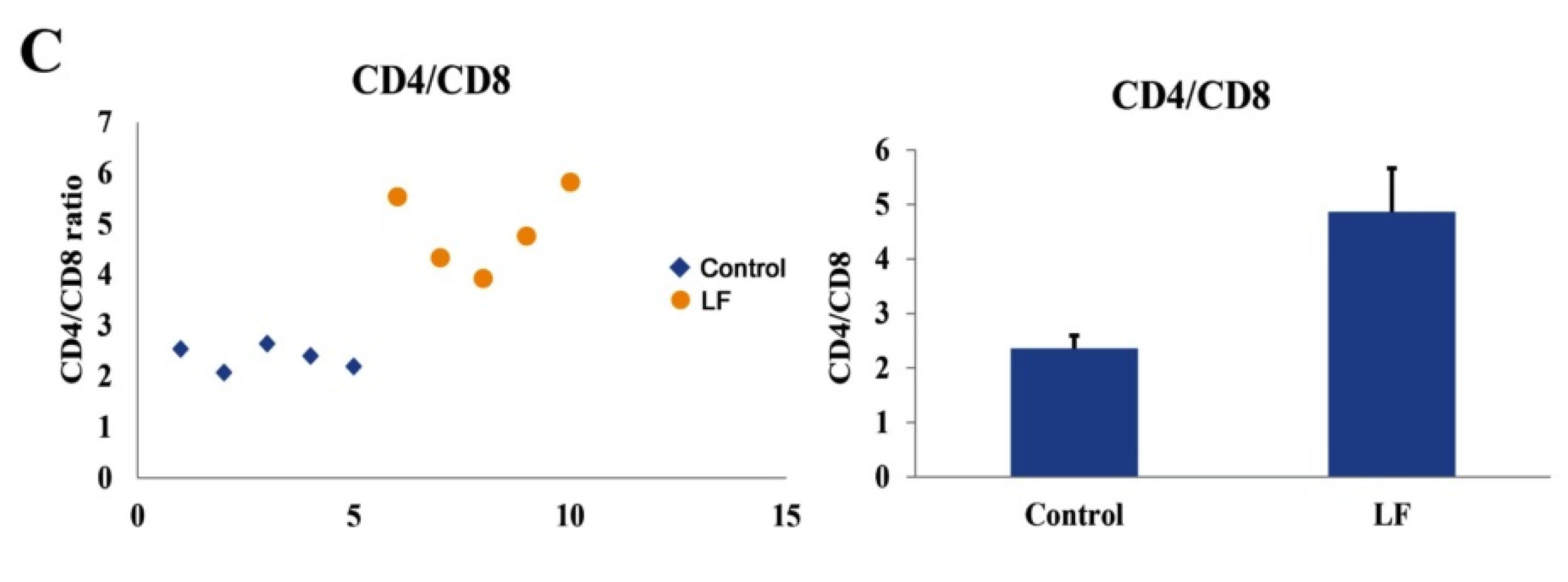
2.5. The Immunomodulatory Functions of LF In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Detection of LF via UPLC
4.4. Separation and Culturing of the Primary Intestinal Epithelial Cells
4.5. Cell Viability Detection Using a Cell-Counting Kit-8 (CCK-8)
4.6. Inflammatory Factors Detection via ELISA
4.7. RNA-Seq Analyses
4.8. Flow Cytometry Analysis of Blood Immune Cells
4.9. RT-PCR
4.10. Western Blotting
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuban, K.C.; O’shea, T.M.; Allred, E.N.; Fichorova, R.N.; Heeren, T.; Paneth, N.; Hirtz, D.; Dammann, O.; Leviton, A. The Breadth and Type of Systemic Inflammation and the Risk of Adverse Neurological Outcomes in Extremely Low Gestation Newborns. Pediatr. Neurol. 2015, 52, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcelroy, S.J.; Frey, M.R.; Torres, B.A.; Maheshwari, A. 72-Innate and Mucosal Immunity in the Developing Gastrointestinal Tract. In Avery’s Diseases of the Newborn, 10th ed.; Gleason, C.A., Juul, S.E., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 1054–1067.e5. [Google Scholar]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Suzuki, H.; Tani, K.; Nishikawa, K.; Irie, K.; Ogura, Y.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Tight junctions. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 2015, 347, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, eaag2770. [Google Scholar] [CrossRef]
- Kucharzik, T.; Lügering, N.; Rautenberg, K.; Lügering, A.; Schmidt, M.A.; Stoll, R.; Domschke, W. Role of M Cells in Intestinal Barrier Function. Ann. N. Y. Acad. Sci. 2000, 915, 171–183. [Google Scholar] [CrossRef]
- Diehl, G.E.; Longman, R.S.; Zhang, J.; Breart, B.; Galan, C.; Cuesta, A.; Schwab, S.R.; Littman, D.R. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 2013, 494, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.O.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.C.; Gong, J.; Nyachoti, M.; Yang, C. Thymol Improves Barrier Function and Attenuates Inflammatory Responses in Porcine Intestinal Epithelial Cells during Lipopolysaccharide (LPS)-Induced Inflammation. J. Agric. Food Chem. 2019, 67, 615–624. [Google Scholar] [CrossRef]
- Bhardwaj, D.K.; Taneja, N.K.; Dp, S.; Chakotiya, A.; Patal, P.; Taneja, P.; Sachdev, D.; Gupta, S.; Sanal, M.G. Phenotypic and genotypic characterization of biofilm forming, antimicrobial resistant, pathogenic Escherichia coli isolated from Indian dairy and meat products. Int. J. Food Microbiol. 2021, 336, 108899. [Google Scholar] [CrossRef]
- Tang, X.; Liu, B.; Wang, X.; Yu, Q.; Fang, R. Epidermal Growth Factor, through Alleviating Oxidative Stress, Protect IPEC-J2 Cells from Lipopolysaccharides-Induced Apoptosis. Int. J. Mol. Sci. 2018, 19, 848. [Google Scholar] [CrossRef] [Green Version]
- Deitch, E.A. Gut-origin sepsis: Evolution of a concept. Surgeon 2012, 10, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Stephens, M.; Von Der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut. Microbes. 2020, 11, 421–432. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, M.; Sorensen, S. The proteins in whey. Compte rendu des Travaux du Laboratoire de Carlsberg, Ser. Chim. 1940, 23, 55–99. [Google Scholar]
- Johanson, B. Isolation of an Iron-Containing Red Protein from Human Milk. J. Acta. Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Lambert, L.A.; Perri, H.; Meehan, T.J. Evolution of duplications in the transferrin family of proteins. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 11–25. [Google Scholar] [CrossRef]
- Gupta, P.M.; Perrine, C.G.; Mei, Z.; Scanlon, K.S. Iron, Anemia, and Iron Deficiency Anemia among Young Children in the United States. Nutrients 2016, 8, 330. [Google Scholar] [CrossRef] [Green Version]
- Anthony, L.; Patrice, C.; Macdougall, I.C.; Biroulrt, L.P. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar]
- Francesca, B.; Ajello, M.; Bosso, P.; Morea, C.; Andrea, P.; Giovanni, A.; Piera, V. Both lactoferrin and iron influence aggregation and biofilm formation in Streptococcus mutans. Biometals 2004, 17, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ostan, N.K.; Yu, R.H.; Ng, D.; Morea, C.; Andrea, P.; Giovanni, A.; Piera, V. Lactoferrin binding protein B-a bi-functional bacterial receptor protein. PLoS Pathog. 2017, 13, e1006244. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Orsi, N. The antimicrobial activity of lactoferrin: Current status and perspectives. Biometals 2004, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Chavez, S.A.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Beljaars, L.; Van Der Strate, B.W.; Bakker, H.I.; Reker Smit, C.; Van Loenen Weemaes, A.M.; Wiegmans, F.C.; Harmsen, M.C.; Molema, G.; Meijer, D.K.F. Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo. Antivir. Res. 2004, 63, 197–208. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M. The role of lactoferrin in the proper development of newborns. Postep. Hig. Med. Dosw. 2005, 59, 421–432. [Google Scholar]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, J.; Xiao, D.; Shu, G.; Gu, L. Bovine Lactoferrin Protects Dextran Sulfate Sodium Salt Mice Against Inflammation and Impairment of Colonic Epithelial Barrier by Regulating Gut Microbial Structure and Metabolites. Front. Nutr. 2021, 8, 660598. [Google Scholar] [CrossRef]
- Hering, N.A.; Luettig, J.; Krug, S.M.; Wiegand, S.; Gross, G.; Van Tol, G.E.; Schulzke, J.D.; Rosenthal, R. Lactoferrin protects against intestinal inflammation and bacteria-induced barrier dysfunction in vitro. Ann. N. Y. Acad. Sci. 2017, 1405, 177–188. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Liu, Y.; Xi, E.; An, J.; Tabys, D.; Liu, N. The In Vitro Protective Role of Bovine Lactoferrin on Intestinal Epithelial Barrier. Molecules 2019, 24, 148. [Google Scholar] [CrossRef]
- Kanyshkova, T.G.; Buneva, V.N.; Nevinsky, G.A. Lactoferrin and its biological functions. Biochemistry 2001, 66, 1–7. [Google Scholar]
- Cutone, A.; Ianiro, G.; Lepanto, M.S.; Rosa, L.; Valenti, P.; Di Patti, M.C.B. Musci, G. Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development. Cancers 2020, 12, 3806. [Google Scholar] [CrossRef]
- Stefanelli, T.; Malesci, A.; Repici, A.; Vetrano, S.; Danese, S. New insights into inflammatory bowel disease pathophysiology: Paving the way for novel therapeutic targets. Curr. Drug Targets 2008, 9, 413–418. [Google Scholar] [CrossRef]
- Bertuccini, L.; Costanzo, M.; Iosi, F.; Tinari, A.; Terruzzi, F.; Stronati, L.; Aloi, M.; Cucchiara, S.; Superti, F. Lactoferrin prevents invasion and inflammatory response following E. coli strain LF82 infection in experimental model of Crohn’s disease. Dig. Liver. Dis. 2014, 46, 496–504. [Google Scholar] [CrossRef]
- Binnewies, M.; Mujal, A.M.; Pollack, J.L.; Combes, A.J.; Hardison, E.A.; Barry, K.C.; Tsui, J.; Ruhland, M.K.; Kersten, K.; Abushawish, M.A.; et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4(+) T Cell Immunity. Cell 2019, 177, 556–571.e16. [Google Scholar] [CrossRef]
- Seung, E.; Xing, Z.; Wu, L.; Rao, E.; Cortez Retamozo, V.; Ospina, B.; Chen, L.; Beil, C.; Song, Z.; Zhang, B.; et al. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. Nature 2022, 603, 328–334. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Metz, L.; Meddings, J.B.; Sharkey, K.A.; Yong, V.W. The intestinal barrier in multiple sclerosis: Implications for pathophysiology and therapeutics. Brain 2018, 141, 1900–1916. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.M.; Gao, Y.; De Groot, N.; Vonk, M.M.; Ulfman, L.; Joost Van Nrreven, R.J. Babies, Bugs, and Barriers: Dietary Modulation of Intestinal Barrier Function in Early Life. Annu. Rev. Nutr. 2022, 42, 165–200. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Sherman, A.; Rogers, G.L.; Liao, G.; Hoffman, B.E.; Leong, K.W.; Terhorst, C.; Daniell, H.; Herzog, R.W. Plant–based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood 2015, 125, 2418–2427. [Google Scholar] [CrossRef] [Green Version]
- Morbe, U.M.; Jorgensen, P.B.; Fenton, T.M.; Von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Bostick, J.W.; Zhou, L. Innate lymphoid cells in intestinal immunity and inflammation. Cell Mol. Life Sci. 2016, 73, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Cheroutre, H.; Lambolez, F.; Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2011, 11, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares-Villagomez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends. Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Persson, E.K.; Scott, C.L.; Mowat, A.M.; Agace, W.W. Dendritic cell subsets in the intestinal lamina propria: Ontogeny and function. Eur. J. Immunol. 2013, 43, 3098–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronke, K.; Kofoed-Nielsen, M.; Diefenbach, A. Isolation and Flow Cytometry Analysis of Innate Lymphoid Cells from the Intestinal Lamina Propria. Methods Mol. Biol. 2017, 1559, 255–265. [Google Scholar]
- Grigg, J.B.; Sonnenberg, G.F. Host-Microbiota Interactions Shape Local and Systemic Inflammatory Diseases. J. Immunol. 2017, 198, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Zietek, T.; Rath, E. Inflammation Meets Metabolic Disease: Gut Feeling Mediated by GLP-1. Front. Immunol. 2016, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Wu, X.; Jin, F. Gut-Brain Psychology: Rethinking Psychology from the Microbiota-Gut-Brain Axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Sorrell, M.F.; Batra, S.K.; Dhawan, P.; Singh, A.B. Gut permeability and mucosal inflammation: Bad, good or context dependent. Mucosal Immunol. 2017, 10, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Rogler, G.; Biedermann, L.; Scharl, M. New insights into the pathophysiology of inflammatory bowel disease: Microbiota, epigenetics and common signalling pathways. Swiss. Med. Wkly. 2018, 148, w14599. [Google Scholar]
- Qin, Z.R.; Zhou, F.F.; Zhang, L. Novel pyroptosis-independent functions of gasdermins. Signal Transduct. Target. Ther. 2022, 5, 1366–1368. [Google Scholar] [CrossRef]
- Lai, Y.J.; Sun, M.; He, Y.; Lei, J.Q.; Han, Y.M.; Wu, Y.Y.; Bai, D.Y.; Guo, Y.M.; Zhang, B. Mycotoxins binder supplementation alleviates aflatoxin B1 toxic effects on the immune response and intestinal barrier function in broilers. Poult. Sci. 2022, 101, 101683. [Google Scholar] [CrossRef]
- Chen, H.M.; Wu, X.H.; Xu, C.J.; Lin, J.; Liu, Z.J. Dichotomous roles of neutrophils in modulating pathogenic and repair processes of inflammatory bowel diseases. Precis. Clin. Med. 2021, 4, 246–257. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Schwerd, T.; Koletzko, S.; Shah, N.; Kammermeier, J.; Elkadir, A.; Ouahed, J.; Wilson, D.C.; Travis, S.P.; Turner, D.; et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014, 147, 990–1007.e3. [Google Scholar] [CrossRef] [Green Version]
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef]
- Kelsen, J.R.; Sullivan, K.E. Inflammatory Bowel Disease in Primary Immunodeficiencies. Curr. Allergy. Asthma Rep. 2017, 17, 57. [Google Scholar] [CrossRef]
- Elizabeth, R.; Michael, P.; Jennifer, P.; Raymond, R.; Pinaki, P.; Lewis, R. Probiotic Supplementation in Very Low Birthweight Infants: Effects on Systemic Immunity and Intestinal Inflammation. Curr. Dev. Nutr. 2022, 6, 706. [Google Scholar]
- Gibson, P.R. Increased gut permeability in Crohn’s disease: Is TNF the link? Gut 2004, 53, 1724–1725. [Google Scholar] [CrossRef] [Green Version]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.L.; Akdis, C.A. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-gamma and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096.e10. [Google Scholar] [CrossRef]
- Hill, D.R.; Newburg, D.S. Clinical applications of bioactive milk components. Nutr. Rev. 2015, 73, 463–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schryvers, A.B. Targeting bacterial transferrin and lactoferrin receptors for vaccines. Trends Microbiol. 2022, 30, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Shau, H.; Kim, A.; Golub, S.H. Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. J. Leukoc. Biol. 1992, 51, 343–349. [Google Scholar] [CrossRef]
- Perdijk, O.; Van Neerven, R.J.J.; Van Den Brink, E.; Savelkoul, H.F.J.; Brugman, S. Bovine Lactoferrin Modulates Dendritic Cell Differentiation and Function. Nutrients 2018, 10, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueiros-Cendon, T.; Arevalo-Gallegos, S.; Iglesias-Figueroa, B.F.; Garcia-Montoya, I.A.; Salazar-Martinez, J.; Rascon-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Ou, A.T.; Zhang, J.X.; Fang, Y.F.; Wang, R.; Tang, X.P.; Zhao, P.F.; Zhao, Y.G.; Zhang, M.; Huang, Y.Z. Disulfiram-loaded lactoferrin nanoparticles for treating inflammatory diseases. Acta Pharmacol. Sin. 2021, 11, 1913–1920. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Lactoferrin attenuates lipopolysaccharide-stimulated inflammatory responses and barrier impairment through the modulation of NF-kappaB/MAPK/Nrf2 pathways in IPEC-J2 cells. Food Funct. 2020, 11, 8516–8526. [Google Scholar] [CrossRef]
- Doursout, M.F.; Horton, H.; Hoang, L.; Liang, Y.; Hwang, S.A.; Boyd, S.; Actor, J.K.; Kruzel, M.L. Lactoferrin moderates LPS-induced hypotensive response and gut injury in rats. Int. Immunopharmacol. 2013, 15, 227–231. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Harari, Y.; Mailman, D.; Actor, J.K.; Zimecki, M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin Exp. Immunol. 2002, 130, 25–31. [Google Scholar] [CrossRef]
- Talukder, M.J.; Harada, E. Bovine lactoferrin protects lipopolysaccharide-induced diarrhea modulating nitric oxide and prostaglandin E2 in mice. Can. J. Physiol. Pharmacol. 2007, 85, 200–208. [Google Scholar] [CrossRef]
- Zong, X.; Hu, W.; Song, D.; Li, Z.; Du, H.; Lu, Z.; Wang, Y. Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response. Biochem. Pharmacol. 2016, 104, 74–82. [Google Scholar] [CrossRef]
- Shimizu, K.; Matsuzawa, H.; Okada, K.; Tazume, S.; Dosako, S.; Kawasaki, Y.; Hashimoto, K.; Koga, Y. Lactoferrin-mediated protection of the host from murine cytomegalovirus infection by a T-cell-dependent augmentation of natural killer cell activity. Arch. Virol. 1996, 141, 1875–1889. [Google Scholar] [CrossRef]
- Yamauchi, K.; Wakabayashi, H.; Hashimoto, S.; Teraguchi, S.; Hayasawa, H.; Tomita, M. Effects of orally administered bovine lactoferrin on the immune system of healthy volunteers. Adv. Exp. Med. Biol. 1998, 443, 261–265. [Google Scholar]
- Broxmeyer, H.E.; Williams, D.E.; Hangoc, G.; Cooper, S.; Gentile, P.; Shen, R.N.; Ralph, P.; Gillis, S.; Bicknell, D.C. The opposing actions in vivo on murine myelopoiesis of purified preparations of lactoferrin and the colony stimulating factors. Blood Cells 1987, 13, 31–48. [Google Scholar]
- Mincheva-Nilsson, L.; Hammarström, S.; Hammarström, M. Activated human gamma delta T lymphocytes express functional lactoferrin receptors. Scand. J. Immunol. 1997, 46, 609–618. [Google Scholar] [CrossRef]
- Kuhara, T.; Yamauchi, K.; Tamura, Y.; Okamura, H. Oral administration of lactoferrin increases NK cell activity in mice via increased production of IL-18 and type I IFN in the small intestine. J. Interferon. Cytokine. Res. 2006, 26, 489–499. [Google Scholar] [CrossRef]
- Lutaty, A.; Soboh, S.; Schif-Zuck, S.; Ariel, A. Resolution-Associated Lactoferrin Peptides Limit LPS Signaling and Cytokine Secretion from Human Macrophages. Int. J. Mol. Sci. 2020, 21, 5166. [Google Scholar] [CrossRef]
- Morshedi, V.; Agh, N.; Noori, F.; Jafari, F. A Ghasemi Growth, body composition, physiological responses and expression of immune-related and growth-related genes of Sobaity seabream (Sparidentex hasta) juvenile fed dietary bovine lactoferrin. Iran. J. Fish. Sci. 2020, 19, 3269–3284. [Google Scholar]
- Fan, L.L.; Yao, Q.Q.; Wu, H.M.; Wen, F.; Wang, J.Q.; Li, H.Y.; Zheng, N. Protective effects of recombinant lactoferrin with different iron saturations on enteritis injury in young mice. J. Dairy Sci. 2022, 105, 4791–4803. [Google Scholar] [CrossRef]
- El-Nasr, I.A.S.; Mahmou, S.A.; Elnaddar, E.M.; Ammar, H.A. Ferrous Sulphate Alone Versus Combination of Ferrous Sulphate and Lactoferrin for The Treatment of Iron Deficiency Anemia during Pregnancy and Their Effect on Neonatal Iron Store: A Randomized Clinical Trial. Egypt. J. Hosp. Med. 2021, 84, 1955–1960. [Google Scholar] [CrossRef]
- Barros, C.A.; Sanches, D.; Carvalho, C.A.M.; Santos, R.A.; Souza, T.L.F.; Leite, V.L.M.; Campos, S.P.C.; Oliverira, A.C.; Goncalves, R.B. Influence of iron binding in the structural stability and cellular internalization of bovine lactoferrin. Heliyon 2021, 7, e08087. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Wen, F.; Zhang, Y.D.; Li, P.; Zheng, N.; Wang, J.Q. Determination of native lactoferrin in milk by HPLC on HiTrapTM Heparin HP column. Food Anal. Methods 2019, 12, 2518–2526. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M. Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Änzelmann, S.; Stelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Liu, X.; Huang, Z.; Zhai, Y.; Li, H.; Wu, J. Lactoferrin Alleviates Lipopolysaccharide-Induced Infantile Intestinal Immune Barrier Damage by Regulating an ELAVL1-Related Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 13719. https://doi.org/10.3390/ijms232213719
Li C, Liu X, Huang Z, Zhai Y, Li H, Wu J. Lactoferrin Alleviates Lipopolysaccharide-Induced Infantile Intestinal Immune Barrier Damage by Regulating an ELAVL1-Related Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(22):13719. https://doi.org/10.3390/ijms232213719
Chicago/Turabian StyleLi, Chaonan, Xinkui Liu, Zhihong Huang, Yiyan Zhai, Huiying Li, and Jiarui Wu. 2022. "Lactoferrin Alleviates Lipopolysaccharide-Induced Infantile Intestinal Immune Barrier Damage by Regulating an ELAVL1-Related Signaling Pathway" International Journal of Molecular Sciences 23, no. 22: 13719. https://doi.org/10.3390/ijms232213719







