SNPs in 3′UTR miRNA Target Sequences Associated with Individual Drug Susceptibility
Abstract
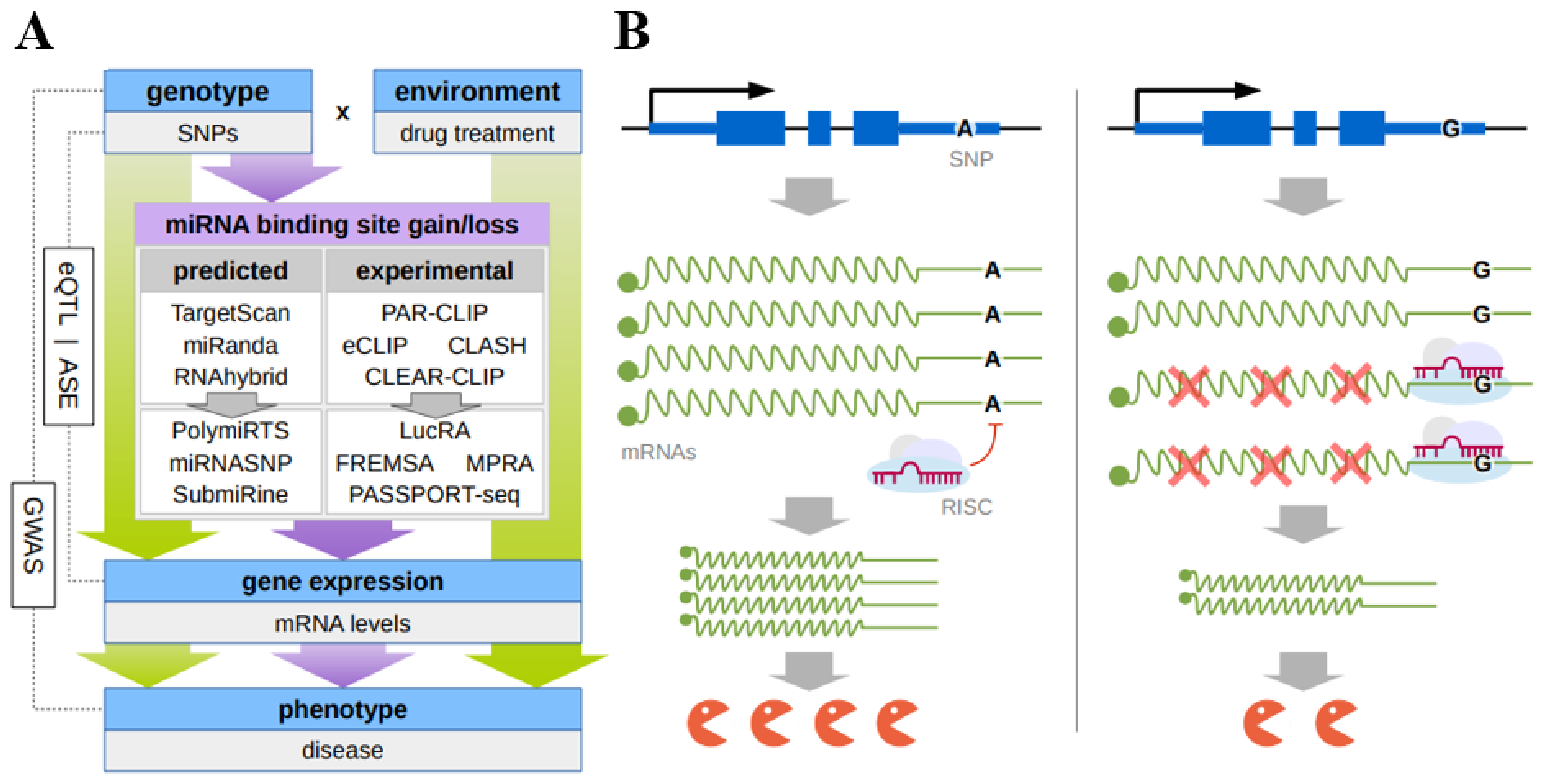
1. Introduction
2. Modern Approaches to Identify Functional SNPs in 3′UTR miRNA Target Sequences
3. Variation in 3′UTR miRNA Target Sequences of the Genes Involved in Drug Metabolism
3.1. Phase I Enzymes
3.1.1. CYP2E1
3.1.2. CYP3A5
3.1.3. CYP2B6
3.1.4. CYP3A7
3.2. Phase II Enzymes
3.2.1. SULT1A1
3.2.2. UGT1A
3.3. Transporters
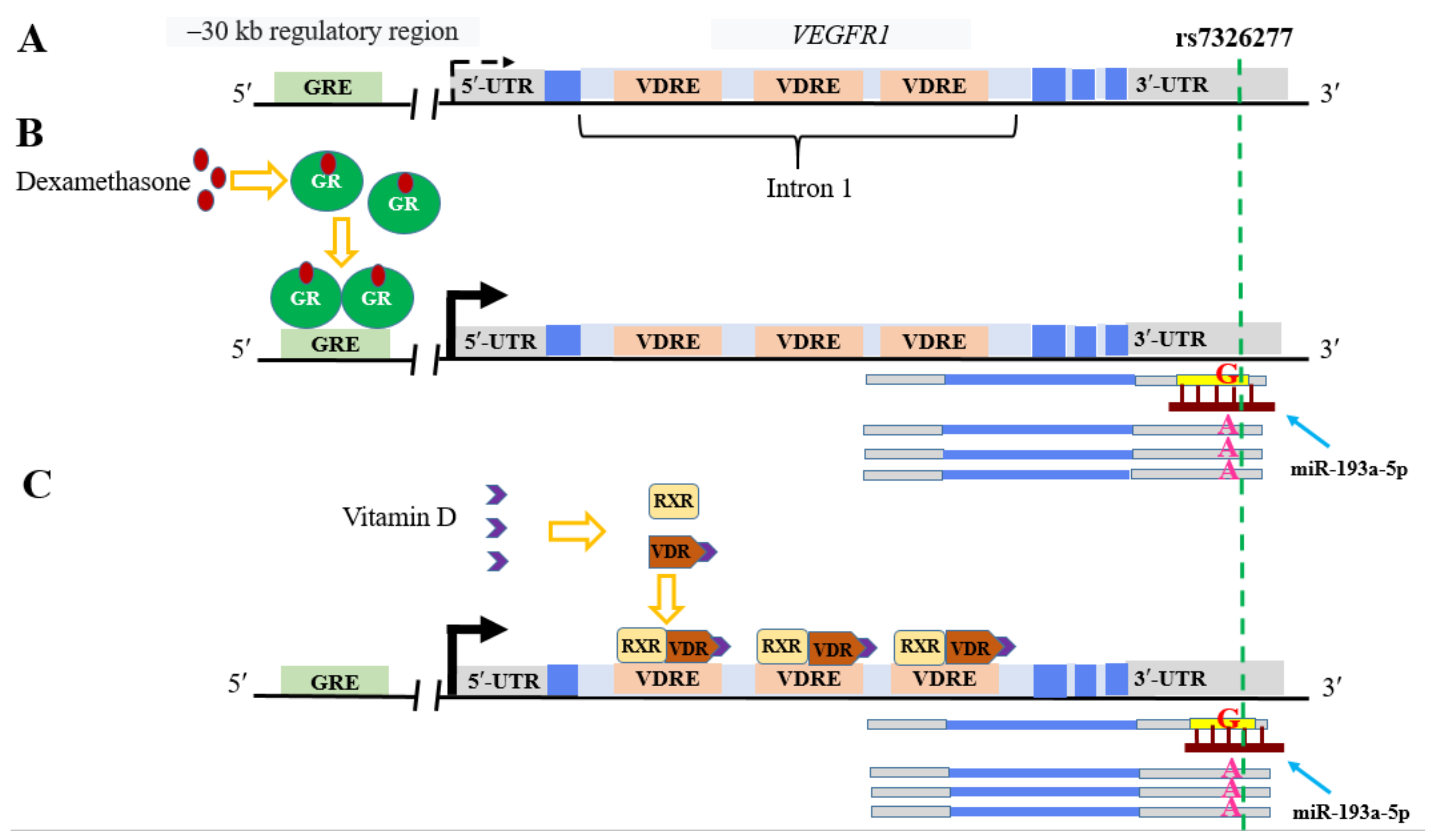
3.4. Transcription Regulators
3.4.1. PXR
3.4.2. GATA4
4. Variation in 3′UTR miRNA Target Sequences of the Genes Not Belonging to Conventional Drug-Metabolizing System
4.1. RPA1
4.2. ADAR
4.3. CTNNBIP1
5. Unbiased Genome-Wide Approaches Appropriate for Detecting the SNPs That Influence the Interaction of miRNAs with Their Target Sites and Associated with Individual Drug Response
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and Limitations of Genome-Wide Association Studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of Published Genome-Wide Association Studies, Targeted Arrays and Summary Statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef] [PubMed]
- Claussnitzer, M.; Cho, J.H.; Collins, R.; Cox, N.J.; Dermitzakis, E.T.; Hurles, M.E.; Kathiresan, S.; Kenny, E.E.; Lindgren, C.M.; MacArthur, D.G.; et al. A Brief History of Human Disease Genetics. Nature 2020, 577, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Hindorff, L.A.; Sethupathy, P.; Junkins, H.A.; Ramos, E.M.; Mehta, J.P.; Collins, F.S.; Manolio, T.A. Potential Etiologic and Functional Implications of Genome-Wide Association Loci for Human Diseases and Traits. Proc. Natl. Acad. Sci. USA 2009, 106, 9362–9367. [Google Scholar] [CrossRef] [PubMed]
- Maurano, M.T.; Humbert, R.; Rynes, E.; Thurman, R.E.; Haugen, E.; Wang, H.; Reynolds, A.P.; Sandstrom, R.; Qu, H.; Brody, J.; et al. Systematic Localization of Common Disease-Associated Variation in Regulatory DNA. Science 2012, 337, 1190–1195. [Google Scholar] [CrossRef]
- Huang, Q. Genetic Study of Complex Diseases in the Post-GWAS Era. J. Genet. Genom. 2015, 42, 87–98. [Google Scholar] [CrossRef]
- Degtyareva, A.O.; Antontseva, E.V.; Merkulova, T.I. Regulatory SNPs: Altered Transcription Factor Binding Sites Implicated in Complex Traits and Diseases. Int. J. Mol. Sci. 2021, 22, 6454. [Google Scholar] [CrossRef]
- Jin, Y.; Jiang, J.; Wang, R.; Qin, Z.S. Systematic Evaluation of DNA Sequence Variations on in Vivo Transcription Factor Binding Affinity. Front. Genet. 2021, 12, 667866. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Wong, M.-C.; Liao, W.-T.; Chen, C.-J.; Lee, S.-C.; Yen, J.-H.; Chang, S.-J. Genetic Variants in Transcription Factor Binding Sites in Humans: Triggered by Natural Selection and Triggers of Diseases. Int. J. Mol. Sci. 2021, 22, 4187. [Google Scholar] [CrossRef]
- Dufner-Almeida, L.G.; do Carmo, R.T.; Masotti, C.; Haddad, L.A. Understanding Human DNA Variants Affecting Pre-MRNA Splicing in the NGS Era. Adv. Genet. 2019, 103, 39–90. [Google Scholar] [CrossRef]
- Li, B.; Dong, J.; Yu, J.; Fan, Y.; Shang, L.; Zhou, X.; Bai, Y. Pinpointing MiRNA and Genes Enrichment over Trait-Relevant Tissue Network in Genome-Wide Association Studies. BMC Med. Genom. 2020, 13, 191. [Google Scholar] [CrossRef]
- Chhichholiya, Y.; Suryan, A.K.; Suman, P.; Munshi, A.; Singh, S. SNPs in MiRNAs and Target Sequences: Role in Cancer and Diabetes. Front. Genet. 2021, 12, 793523. [Google Scholar] [CrossRef]
- Moszyńska, A.; Gebert, M.; Collawn, J.F.; Bartoszewski, R. SNPs in MicroRNA Target Sites and Their Potential Role in Human Disease. Open Biol. 2017, 7, 170019. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of MRNA Translation and Stability by MicroRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Wilczynska, A.; Bushell, M. The Complexity of MiRNA-Mediated Repression. Cell Death. Differ. 2015, 22, 22–33. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Nakano, M.; Iwakami, C.; Fukami, T.; Nakajima, M. Identification of MiRNAs That Regulate Human CYP2B6 Expression. Drug Metab. Pharmacokinet. 2021, 38, 100388. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. MiRNA-Based Biomarkers, Therapies, and Resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Therapeutic Advances of MiRNAs: A Preclinical and Clinical Update. J. Adv. Res. 2021, 28, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Reyes, P.R.; Garza, B.S.; Sharma, A. MicroRNAs and Child Neuropsychiatric Disorders: A Brief Review. Neurochem. Res. 2020, 45, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Bravo Vázquez, L.A.; Uribe, S.P.; Manzanero Cárdenas, L.A.; Ruíz Aguilar, M.F.; Chakraborty, S.; Sharma, A. Roles of MicroRNAs in Carbohydrate and Lipid Metabolism Disorders and Their Therapeutic Potential. Biochimie 2021, 187, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Swart, M.; Dandara, C. Genetic Variation in the 3’-UTR of CYP1A2, CYP2B6, CYP2D6, CYP3A4, NR1I2, and UGT2B7: Potential Effects on Regulation by MicroRNA and Pharmacogenomics Relevance. Front. Genet. 2014, 5, 167. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-P.; Hu, Y.-D.; Hu, X.-L.; Zhang, Y.-J.; Yang, Y.-L.; Jiang, C.; Tang, J.; Chen, X.-P. MiRNAs and MiRNA Polymorphisms Modify Drug Response. Int. J. Environ. Res. Public Health 2016, 13, 1096. [Google Scholar] [CrossRef]
- Zanger, U.M.; Klein, K.; Kugler, N.; Petrikat, T.; Ryu, C.S. Epigenetics and MicroRNAs in Pharmacogenetics. Adv. Pharmacol. 2018, 83, 33–64. [Google Scholar] [CrossRef]
- Knox, B.; Wang, Y.; Rogers, L.J.; Xuan, J.; Yu, D.; Guan, H.; Chen, J.; Shi, T.; Ning, B.; Kadlubar, S.A.; et al. A Functional SNP in the 3′-UTR of TAP2 Gene Interacts with MicroRNA Hsa-miR-1270 to Suppress the Gene Expression. Environ. Mol. Mutagen. 2018, 59, 134–143. [Google Scholar] [CrossRef]
- Yu, X.; Dhakal, I.B.; Beggs, M.; Edavana, V.K.; Williams, S.; Zhang, X.; Mercer, K.; Ning, B.; Lang, N.P.; Kadlubar, F.F.; et al. Functional Genetic Variants in the 3′-Untranslated Region of Sulfotransferase Isoform 1A1 (SULT1A1) and Their Effect on Enzymatic Activity. Toxicol. Sci. 2010, 118, 391–403. [Google Scholar] [CrossRef]
- Omariba, G.; Xu, F.; Wang, M.; Li, K.; Zhou, Y.; Xiao, J. Genome-Wide Analysis of MicroRNA-Related Single Nucleotide Polymorphisms (SNPs) in Mouse Genome. Sci. Rep. 2020, 10, 5789. [Google Scholar] [CrossRef]
- Zheng, Z.; Xue, F.; Wang, H.; He, Y.; Zhang, L.; Ma, W.; Zhang, C.; Guan, Y.; Ye, F.; Wen, Y.; et al. A Single Nucleotide Polymorphism-Based Formula to Predict the Risk of Propofol TCI Concentration Being over 4 µg ML−1 at the Time of Loss of Consciousness. Pharmacogenom. J. 2022, 22, 109–116. [Google Scholar] [CrossRef]
- Clément, T.; Salone, V.; Rederstorff, M. Dual Luciferase Gene Reporter Assays to Study MiRNA Function. Methods Mol. Biol. 2015, 1296, 187–198. [Google Scholar] [CrossRef]
- Li, S.; Xu, K.; Gu, D.; He, L.; Xie, L.; Chen, Z.; Fan, Z.; Zhu, L.; Du, M.; Chu, H.; et al. Genetic Variants in RPA1 Associated with the Response to Oxaliplatin-Based Chemotherapy in Colorectal Cancer. J. Gastroenterol. 2019, 54, 939–949. [Google Scholar] [CrossRef]
- Hua, H.; Zeng, J.; Xing, H.; He, Y.; Han, L.; Zhang, N.; Yang, M. The RNA Editing Enzyme ADAR Modulated by the Rs1127317 Genetic Variant Diminishes EGFR-TKIs Efficiency in Advanced Lung Adenocarcinoma. Life Sci. 2022, 296, 120408. [Google Scholar] [CrossRef]
- Papageorgiou, I.; Court, M.H. Identification and Validation of the MicroRNA Response Elements in the 3′-Untranslated Region of the UDP Glucuronosyltransferase (UGT) 2B7 and 2B15 Genes by a Functional Genomics Approach. Biochem. Pharmacol. 2017, 146, 199–213. [Google Scholar] [CrossRef]
- Papageorgiou, I.; Court, M.H. Identification and Validation of MicroRNAs Directly Regulating the UDP-Glucuronosyltransferase 1A Subfamily Enzymes by a Functional Genomics Approach. Biochem. Pharmacol. 2017, 137, 93–106. [Google Scholar] [CrossRef]
- Nakano, M.; Mohri, T.; Fukami, T.; Takamiya, M.; Aoki, Y.; McLeod, H.L.; Nakajima, M. Single-Nucleotide Polymorphisms in Cytochrome P450 2E1 (CYP2E1) 3′-Untranslated Region Affect the Regulation of CYP2E1 by MiR-570. Drug Metab. Dispos. 2015, 43, 1450–1457. [Google Scholar] [CrossRef]
- Tomasello, L.; Cluts, L.; Croce, C.M. Experimental Validation of MicroRNA Targets: Mutagenesis of Binding Regions. Methods Mol. Biol. 2019, 1970, 331–339. [Google Scholar] [CrossRef]
- Ipe, J.; Collins, K.S.; Hao, Y.; Gao, H.; Bhatia, P.; Gaedigk, A.; Liu, Y.; Skaar, T.C. PASSPORT-Seq: A Novel High-Throughput Bioassay to Functionally Test Polymorphisms in Micro-RNA Target Sites. Front Genet. 2018, 9, 219. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, Y.; Wang, Y.; Yu, L.-R.; Knox, B.; Chen, J.; Shi, T.; Chen, S.; Ren, Z.; Guo, L.; et al. MicroRNA Hsa-MiR-370-3p Suppresses the Expression and Induction of CYP2D6 by Facilitating MRNA Degradation. Biochem. Pharmacol. 2017, 140, 139–149. [Google Scholar] [CrossRef]
- Yu, D.; Chen, S.; Li, D.; Knox, B.; Guo, L.; Ning, B. FREMSA: A Method That Provides Direct Evidence of the Interaction between MicroRNA and MRNA. Methods Mol. Biol. 2020, 2102, 557–566. [Google Scholar] [CrossRef]
- Wei, R.; Yang, F.; Urban, T.J.; Li, L.; Chalasani, N.; Flockhart, D.A.; Liu, W. Impact of the Interaction between 3′-UTR SNPs and MicroRNA on the Expression of Human Xenobiotic Metabolism Enzyme and Transporter Genes. Front. Genet. 2012, 3, 248. [Google Scholar] [CrossRef] [PubMed]
- Kheradpour, P.; Ernst, J.; Melnikov, A.; Rogov, P.; Wang, L.; Zhang, X.; Alston, J.; Mikkelsen, T.S.; Kellis, M. Systematic Dissection of Regulatory Motifs in 2000 Predicted Human Enhancers Using a Massively Parallel Reporter Assay. Genome Res. 2013, 23, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, M.; Hong, H.; Tong, W.; Ning, B. Integrative Approaches for Studying the Role of Noncoding RNAs in Influencing Drug Efficacy and Toxicity. Expert. Opin. Drug Metab. Toxicol. 2022, 18, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.R.; Zhao, H.; Ipe, J.; Liu, Y.; Skaar, T.C. Mapping the MiRNA-mRNA Interactome in Human Hepatocytes and Identification of Functional MirSNPs in Pharmacogenes. Clin. Pharmacol. Ther. 2021, 110, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Helwak, A.; Kudla, G.; Dudnakova, T.; Tollervey, D. Mapping the Human MiRNA Interactome by CLASH Reveals Frequent Noncanonical Binding. Cell 2013, 153, 654–665. [Google Scholar] [CrossRef]
- Offer, S.M.; Butterfield, G.L.; Jerde, C.R.; Fossum, C.C.; Wegner, N.J.; Diasio, R.B. MicroRNAs MiR-27a and MiR-27b Directly Regulate Liver Dihydropyrimidine Dehydrogenase Expression through Two Conserved Binding Sites. Mol. Cancer Ther. 2014, 13, 742–751. [Google Scholar] [CrossRef]
- Ning, B.; Yu, D.; Yu, A.-M. Advances and Challenges in Studying Noncoding RNA Regulation of Drug Metabolism and Development of RNA Therapeutics. Biochem. Pharmacol. 2019, 169, 113638. [Google Scholar] [CrossRef]
- Gaedigk, A.; Whirl-Carrillo, M.; Pratt, V.M.; Miller, N.A.; Klein, T.E. PharmVar and the Landscape of Pharmacogenetic Resources. Clin. Pharmacol. Ther. 2020, 107, 43–46. [Google Scholar] [CrossRef]
- Mohri, T.; Nakajima, M.; Fukami, T.; Takamiya, M.; Aoki, Y.; Yokoi, T. Human CYP2E1 Is Regulated by MiR-378. Biochem. Pharmacol. 2010, 79, 1045–1052. [Google Scholar] [CrossRef]
- Maillard, M.; Chevreau, C.; le Louedec, F.; Cassou, M.; Delmas, C.; Gourdain, L.; Blay, J.-Y.; Cupissol, D.; Bompas, E.; Italiano, A.; et al. Pharmacogenetic Study of Trabectedin-Induced Severe Hepatotoxicity in Patients with Advanced Soft Tissue Sarcoma. Cancers 2020, 12, 3647. [Google Scholar] [CrossRef]
- Meng, H.-Y.; Li, X.; Jin, W.-L.; Yan, C.-K.; Dong, X.-H.; Xu, Q.; Peng, Y.-Y.; Li, Z.-B.; Li, Y.; Luo, Z.-H.; et al. Multiple Genetic Factors Affecting the Pharmacokinetic and Pharmacodynamic Processes of Tacrolimus in Chinese Myasthenia Gravis Patients. Eur. J. Clin. Pharmacol. 2020, 76, 659–671. [Google Scholar] [CrossRef]
- Tamashiro, E.Y.; Felipe, C.R.; Genvigir, F.D.V.; Rodrigues, A.C.; Campos, A.B.; Hirata, R.D.C.; Tedesco-Silva, H.; Medina-Pestana, J.O. Influence of CYP3A4 and CYP3A5 Polymorphisms on Tacrolimus and Sirolimus Exposure in Stable Kidney Transplant Recipients. Drug Metab. Pers. Ther. 2017, 32, 89–95. [Google Scholar] [CrossRef]
- Liu, J.; Feng, D.; Kan, X.; Zheng, M.; Zhang, X.; Wang, Z.; Sun, L.; Chen, H.; Gao, X.; Lu, T.; et al. Polymorphisms in the CYP3A5 Gene Significantly Affect the Pharmacokinetics of Sirolimus after Kidney Transplantation. Pharmacogenomics 2021, 22, 903–912. [Google Scholar] [CrossRef]
- Burgess, K.S.; Ipe, J.; Swart, M.; Metzger, I.F.; Lu, J.; Gufford, B.T.; Thong, N.; Desta, Z.; Gaedigk, R.; Pearce, R.E.; et al. Variants in the CYP2B6 3′UTR Alter In Vitro and In Vivo CYP2B6 Activity: Potential Role of MicroRNAs. Clin. Pharmacol. Ther. 2018, 104, 130–138. [Google Scholar] [CrossRef]
- Li, S.; Shao, W.; Wang, C.; Wang, L.; Xia, R.; Yao, S.; Du, M.; Ji, X.; Chu, H.; Zhang, Z.; et al. Identification of Common Genetic Variants Associated with Serum Concentrations of p, P′-DDE in Non-Occupational Populations in Eastern China. Environ. Int. 2021, 152, 106507. [Google Scholar] [CrossRef]
- Ramli, F.F. Pharmacogenomics Biomarkers for Personalized Methadone Maintenance Treatment: The Mechanism and Its Potential Use. Bosn. J. Basic Med. Sci. 2020, 21, 145–154. [Google Scholar] [CrossRef]
- Wang, S.-C.; Ho, I.-K.; Tsou, H.-H.; Tian, J.-N.; Hsiao, C.-F.; Chen, C.-H.; Tan, H.K.-L.; Lin, L.; Wu, C.-S.; Su, L.-W.; et al. CYP2B6 Polymorphisms Influence the Plasma Concentration and Clearance of the Methadone S-Enantiomer. J. Clin. Psychopharmacol. 2011, 31, 463–469. [Google Scholar] [CrossRef]
- Packiasabapathy, S.; Aruldhas, B.W.; Zhang, P.; Overholser, B.R.; Quinney, S.K.; Sadhasivam, S. Novel Associations between CYP2B6 Polymorphisms, Perioperative Methadone Metabolism and Clinical Outcomes in Children. Pharmacogenomics 2021, 22, 591–602. [Google Scholar] [CrossRef]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, Q.; Li, R.; Tao, Y.; Zhang, Q.; Li, J.; Ma, Z.; Shen, C.; Zhong, M.; Wang, Z.; et al. CYP3A7, CYP3A4, and CYP3A5 Genetic Polymorphisms in Recipients Rather than Donors Influence Tacrolimus Concentrations in the Early Stages after Liver Transplantation. Gene 2022, 809, 146007. [Google Scholar] [CrossRef]
- Sohn, M.; Kim, M.G.; Han, N.; Kim, I.-W.; Gim, J.; Min, S.-I.; Song, E.Y.; Kim, Y.S.; Jung, H.S.; Shin, Y.K.; et al. Whole Exome Sequencing for the Identification of CYP3A7 Variants Associated with Tacrolimus Concentrations in Kidney Transplant Patients. Sci. Rep. 2018, 8, 18064. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Rysz, J.; Gluba-Brzózka, A. Pharmacogenetics of Drugs Used in the Treatment of Cancers. Genes 2022, 13, 311. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Spitman, A.B.; Dezentjé, V.O.; Swen, J.J.; Moes, D.J.A.R.; Gelderblom, H.; Guchelaar, H.-J. Genetic Polymorphisms of 3′-Untranslated Region of SULT1A1 and Their Impact on Tamoxifen Metabolism and Efficacy. Breast Cancer Res. Treat. 2018, 172, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, R.; Herrmann, K.; Engst, W.; Meinl, W.; Klein, K.; Glatt, H.; Zanger, U.M. Methyleugenol DNA Adducts in Human Liver Are Associated with SULT1A1 Copy Number Variations and Expression Levels. Arch. Toxicol. 2017, 91, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Helland, T.; Alsomairy, S.; Lin, C.; Søiland, H.; Mellgren, G.; Hertz, D.L. Generating a Precision Endoxifen Prediction Algorithm to Advance Personalized Tamoxifen Treatment in Patients with Breast Cancer. J. Pers. Med. 2021, 11, 201. [Google Scholar] [CrossRef]
- Guillemette, C.; Lévesque, É.; Rouleau, M. Pharmacogenomics of Human Uridine Diphospho-Glucuronosyltransferases and Clinical Implications. Clin. Pharmacol. Ther. 2014, 96, 324–339. [Google Scholar] [CrossRef]
- Mehboob, H.; Tahir, I.M.; Iqbal, T.; Saleem, S.; Perveen, S.; Farooqi, A. Effect of UDP-Glucuronosyltransferase (UGT) 1A Polymorphism (Rs8330 and Rs10929303) on Glucuronidation Status of Acetaminophen. Dose-Response 2017, 15, 155932581772373. [Google Scholar] [CrossRef]
- Court, M.H.; Freytsis, M.; Wang, X.; Peter, I.; Guillemette, C.; Hazarika, S.; Duan, S.X.; Greenblatt, D.J.; Lee, W.M. The UDP-glucuronosyltransferase (UGT) 1A polymorphism c.2042C>G (rs8330) is associated with increased human liver acetaminophen glucuronidation, increased UGT1A exon 5a/5b splice variant mRNA ratio, and decreased risk of unintentional acetaminophen-induced acute liver failure. J. Pharmacol. Exp. Ther. 2013, 345, 297–307. [Google Scholar] [CrossRef]
- Guan, S.; Chen, X.; Xin, S.; Liu, S.; Yang, Y.; Fang, W.; Huang, Y.; Zhao, H.; Zhu, X.; Zhuang, W.; et al. Establishment and Application of a Predictive Model for Gefitinib-Induced Severe Rash Based on Pharmacometabolomic Profiling and Polymorphisms of Transporters in Non-Small Cell Lung Cancer. Transl. Oncol. 2021, 14, 100951. [Google Scholar] [CrossRef]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Ween, M.P.; Lokman, N.A.; Tan, I.A.; Pyragius, C.E.; Oehler, M.K. Chemotherapy-Induced Hyaluronan Production: A Novel Chemoresistance Mechanism in Ovarian Cancer. BMC Cancer 2013, 13, 476. [Google Scholar] [CrossRef]
- Litviakov, N.V.; Cherdyntseva, N.V.; Tsyganov, M.M.; Slonimskaya, E.M.; Ibragimova, M.K.; Kazantseva, P.V.; Kzhyshkowska, J.; Choinzonov, E.L. Deletions of Multidrug Resistance Gene Loci in Breast Cancer Leads to the Down-Regulation of Its Expression and Predict Tumor Response to Neoadjuvant Chemotherapy. Oncotarget 2016, 7, 7829–7841. [Google Scholar] [CrossRef]
- Aleksunes, L.M.; Klaassen, C.D. Coordinated Regulation of Hepatic Phase I and II Drug-Metabolizing Genes and Transporters Using AhR-, CAR-, PXR-, PPARα-, and Nrf2-Null Mice. Drug Metab. Dispos. 2012, 40, 1366–1379. [Google Scholar] [CrossRef]
- De Mattia, E.; Polesel, J.; Roncato, R.; Labriet, A.; Bignucolo, A.; Dreussi, E.; Romanato, L.; Guardascione, M.; Buonadonna, A.; D’Andrea, M.; et al. Germline Polymorphisms in the Nuclear Receptors PXR and VDR as Novel Prognostic Markers in Metastatic Colorectal Cancer Patients Treated With FOLFIRI. Front. Oncol. 2019, 9, 1312. [Google Scholar] [CrossRef]
- Hakkola, J.; Bernasconi, C.; Coecke, S.; Richert, L.; Andersson, T.B.; Pelkonen, O. Cytochrome P450 Induction and Xeno-Sensing Receptors Pregnane X Receptor, Constitutive Androstane Receptor, Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor α at the Crossroads of Toxicokinetics and Toxicodynamics. Basic Clin. Pharmacol. Toxicol. 2018, 123, 42–50. [Google Scholar] [CrossRef]
- Kandel, B.A.; Thomas, M.; Winter, S.; Damm, G.; Seehofer, D.; Burk, O.; Schwab, M.; Zanger, U.M. Genomewide Comparison of the Inducible Transcriptomes of Nuclear Receptors CAR, PXR and PPARα in Primary Human Hepatocytes. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2016, 1859, 1218–1227. [Google Scholar] [CrossRef]
- Smutny, T.; Dusek, J.; Hyrsova, L.; Nekvindova, J.; Horvatova, A.; Micuda, S.; Gerbal-Chaloin, S.; Pavek, P. The 3′-Untranslated Region Contributes to the Pregnane X Receptor (PXR) Expression down-Regulation by PXR Ligands and up-Regulation by Glucocorticoids. Acta Pharm. Sin. B 2020, 10, 136–152. [Google Scholar] [CrossRef]
- Niu, X.; Wu, T.; Li, G.; Gu, X.; Tian, Y.; Cui, H. Insights into the Critical Role of the PXR in Preventing Carcinogenesis and Chemotherapeutic Drug Resistance. Int. J. Biol. Sci. 2022, 18, 742–759. [Google Scholar] [CrossRef]
- Skandalaki, A.; Sarantis, P.; Theocharis, S. Pregnane X Receptor (PXR) Polymorphisms and Cancer Treatment. Biomolecules 2021, 11, 1142. [Google Scholar] [CrossRef]
- Theocharis, S.; Giaginis, C.; Gourzi, S.; Alexandrou, P.; Tsourouflis, G.; Sarantis, P.; Danas, E.; Michail, A.; Tsoukalas, N.; Pergaris, A.; et al. High Pregnane X Receptor (PXR) Expression Is Correlated with Poor Prognosis in Invasive Breast Carcinoma. Diagnostics 2021, 11, 1946. [Google Scholar] [CrossRef]
- Revathidevi, S.; Sudesh, R.; Vaishnavi, V.; Kaliyanasundaram, M.; MaryHelen, K.G.; Sukanya, G.; Munirajan, A.K. Screening for the 3’UTR Polymorphism of the PXR Gene in South Indian Breast Cancer Patients and Its Potential Role in Pharmacogenomics. Asian Pac. J. Cancer Prev. 2016, 17, 3971–3977. [Google Scholar] [PubMed]
- Oleson, L.; von Moltke, L.L.; Greenblatt, D.J.; Court, M.H. Identification of Polymorphisms in the 3′-Untranslated Region of the Human Pregnane X Receptor (PXR) Gene Associated with Variability in Cytochrome P450 3A (CYP3A) Metabolism. Xenobiotica 2010, 40, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Pamuła-Piłat, J.; Tęcza, K.; Kalinowska-Herok, M.; Grzybowska, E. Genetic 3′UTR Variations and Clinical Factors Significantly Contribute to Survival Prediction and Clinical Response in Breast Cancer Patients. Sci. Rep. 2020, 10, 5736. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, G.; Li, J.; Luo, J.; Li, H.; Zhang, Z. Association of Polymorphism of CYP3A4, ABCB1, ABCC2, ABCG2, NFKB1, POR, and PXR with the Concentration of Cyclosporin A in Allogeneic Haematopoietic Stem Cell Transplantation Recipients. Xenobiotica 2021, 51, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Lee, K.E.; Jeong, H.; Chang, B.C.; Gwak, H.S. Impact of GATA4 Variants on Stable Warfarin Doses in Patients with Prosthetic Heart Valves. Pharmacogenom. J. 2015, 15, 33–37. [Google Scholar] [CrossRef]
- Hosseindokht, M.; Boroumand, M.; Salehi, R.; Mandegary, A.; Hajhosseini Talasaz, A.; Pourgholi, L.; Zare, H.; Ziaee, S.; Sharifi, M. Association between Four MicroRNA Binding Site-Related Polymorphisms and the Risk of Warfarin-Induced Bleeding Complications. EXCLI J. 2019, 18, 287–299. [Google Scholar] [CrossRef]
- Chen, J.; Cui, X.; Li, A.; Li, G.; Sun, F. Association of a GATA Binding Protein 4 Polymorphism with the Risk of Hypospadias in the Chinese Children. Urol. Int. 2021, 105, 1018–1023. [Google Scholar] [CrossRef]
- Li, Z.; Qing, Y.; Guan, W.; Li, M.; Peng, Y.; Zhang, S.; Xiong, Y.; Wang, D. Predictive Value of APE1, BRCA1, ERCC1 and TUBB3 Expression in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC) Receiving First-Line Platinum–Paclitaxel Chemotherapy. Cancer Chemother. Pharmacol. 2014, 74, 777–786. [Google Scholar] [CrossRef]
- McNeil, E.M.; Melton, D.W. DNA Repair Endonuclease ERCC1-XPF as a Novel Therapeutic Target to Overcome Chemoresistance in Cancer Therapy. Nucleic Acids Res. 2012, 40, 9990–10004. [Google Scholar] [CrossRef]
- Fritzell, K.; Xu, L.-D.; Lagergren, J.; Öhman, M. ADARs and Editing: The Role of A-to-I RNA Modification in Cancer Progression. Semin. Cell Dev. Biol. 2018, 79, 123–130. [Google Scholar] [CrossRef]
- Polakis, P. The Many Ways of Wnt in Cancer. Curr. Opin. Genet. Dev. 2007, 17, 45–51. [Google Scholar] [CrossRef]
- Sekiya, T.; Nakamura, T.; Kazuki, Y.; Oshimura, M.; Kohu, K.; Tago, K.-I.; Ohwada, S.; Akiyama, T. Overexpression of Icat Induces G(2) Arrest and Cell Death in Tumor Cell Mutants for Adenomatous Polyposis Coli, Beta-Catenin, or Axin. Cancer Res. 2002, 62, 3322–3326. [Google Scholar]
- Jiang, Y.; Ren, W.; Wang, W.; Xia, J.; Gou, L.; Liu, M.; Wan, Q.; Zhou, L.; Weng, Y.; He, T.; et al. Inhibitor of β-Catenin and TCF (ICAT) Promotes Cervical Cancer Growth and Metastasis by Disrupting E-Cadherin/β-Catenin Complex. Oncol. Rep. 2017, 38, 2597–2606. [Google Scholar] [CrossRef]
- Kosari-Monfared, M.; Nikbakhsh, N.; Fattahi, S.; Ghadami, E.; Ranaei, M.; Taheri, H.; Amjadi-Moheb, F.; Godazandeh, G.A.; Shafaei, S.; Pilehchian-Langroudi, M.; et al. CTNNBIP1 Downregulation Is Associated with Tumor Grade and Viral Infections in Gastric Adenocarcinoma. J. Cell. Physiol. 2019, 234, 2895–2904. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Tong, X.; Dai, H.; Shi, T.; Cheng, X.; Sun, M.; Chen, K.; Wei, Q.; Wang, M. Functional Genetic Variants of CTNNBIP1 Predict Platinum Treatment Response of Chinese Epithelial Ovarian Cancer Patients. J. Cancer 2020, 11, 6850–6860. [Google Scholar] [CrossRef]
- Sawamura, R.; Sakurai, H.; Wada, N.; Nishiya, Y.; Honda, T.; Kazui, M.; Kurihara, A.; Shinagawa, A.; Izumi, T. Bioactivation of Loxoprofen to a Pharmacologically Active Metabolite and Its Disposition Kinetics in Human Skin. Biopharm. Drug Dispos. 2015, 36, 352–363. [Google Scholar] [CrossRef]
- Quiñones-Lombraña, A.; Li, N.; del Solar, V.; Atilla-Gokcumen, G.E.; Blanco, J.G. CBR1 Rs9024 Genotype Status Impacts the Bioactivation of Loxoprofen in Human Liver. Biopharm. Drug Dispos. 2018, 39, 315–318. [Google Scholar] [CrossRef]
- Lacombe, J.; Ferron, M. VKORC1L1, An Enzyme Mediating the Effect of Vitamin K in Liver and Extrahepatic Tissues. Nutrients 2018, 10, 970. [Google Scholar] [CrossRef]
- Furie, K.L.; Goldstein, L.B.; Albers, G.W.; Khatri, P.; Neyens, R.; Turakhia, M.P.; Turan, T.N.; Wood, K.A. Oral Antithrombotic Agents for the Prevention of Stroke in Nonvalvular Atrial Fibrillation. Stroke 2012, 43, 3442–3453. [Google Scholar] [CrossRef]
- Koshy, L.; Harikrishnan, S.; Sudhakaran, P. Prioritizing Rs7294 as a MirSNP Contributing to Warfarin Dosing Variability. Pharmacogenomics 2020, 21, 257–267. [Google Scholar] [CrossRef]
- Moyerbrailean, G.A.; Richards, A.L.; Kurtz, D.; Kalita, C.A.; Davis, G.O.; Harvey, C.T.; Alazizi, A.; Watza, D.; Sorokin, Y.; Hauff, N.; et al. High-Throughput Allele-Specific Expression across 250 Environmental Conditions. Genome Res. 2016, 26, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Fairfax, B.P.; Humburg, P.; Makino, S.; Naranbhai, V.; Wong, D.; Lau, E.; Jostins, L.; Plant, K.; Andrews, R.; McGee, C.; et al. Innate Immune Activity Conditions the Effect of Regulatory Variants upon Monocyte Gene Expression. Science 2014, 343, 1246949. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Ziebarth, J.D.; Cui, Y. PolymiRTS Database 3.0: Linking Polymorphisms in MicroRNAs and Their Target Sites with Human Diseases and Biological Pathways. Nucleic Acids Res. 2014, 42, D86–D91. [Google Scholar] [CrossRef] [PubMed]
- Madamsetty, V.S.; Mohammadinejad, R.; Uzieliene, I.; Nabavi, N.; Dehshahri, A.; García-Couce, J.; Tavakol, S.; Moghassemi, S.; Dadashzadeh, A.; Makvandi, P.; et al. Dexamethasone: Insights into Pharmacological Aspects, Therapeutic Mechanisms, and Delivery Systems. ACS Biomater. Sci. Eng. 2022, 8, 1763–1790. [Google Scholar] [CrossRef] [PubMed]
- Melton, D.W.; McManus, L.M.; Gelfond, J.A.L.; Shireman, P.K. Temporal Phenotypic Features Distinguish Polarized Macrophages in Vitro. Autoimmunity 2015, 48, 161–176. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and Its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 Positivity Rates Associated with Circulating 25-Hydroxyvitamin D Levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Deng, J.; Wang, B.; Yang, X.; Yang, R.; Cheng, M.; Fang, W.; Qiu, F.; Zhang, X.; et al. Genetic Variant in the 3’-Untranslated Region of VEGFR1 Gene Influences Chronic Obstructive Pulmonary Disease and Lung Cancer Development in Chinese Population. Mutagenesis 2014, 29, 311–317. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Selenium and Redox Signaling. Arch. Biochem. Biophys. 2017, 617, 48–59. [Google Scholar] [CrossRef]
- Wu, B.-K.; Chen, Q.-H.; Pan, D.; Chang, B.; Sang, L.-X. A Novel Therapeutic Strategy for Hepatocellular Carcinoma: Immunomodulatory Mechanisms of Selenium and/or Selenoproteins on a Shift towards Anti-Cancer. Int. Immunopharmacol. 2021, 96, 107790. [Google Scholar] [CrossRef]
- Domínguez-Álvarez, E.; Rácz, B.; Marć, M.A.; Nasim, M.J.; Szemerédi, N.; Viktorová, J.; Jacob, C.; Spengler, G. Selenium and Tellurium in the Development of Novel Small Molecules and Nanoparticles as Cancer Multidrug Resistance Reversal Agents. Drug Resist. Updates 2022, 63, 100844. [Google Scholar] [CrossRef]
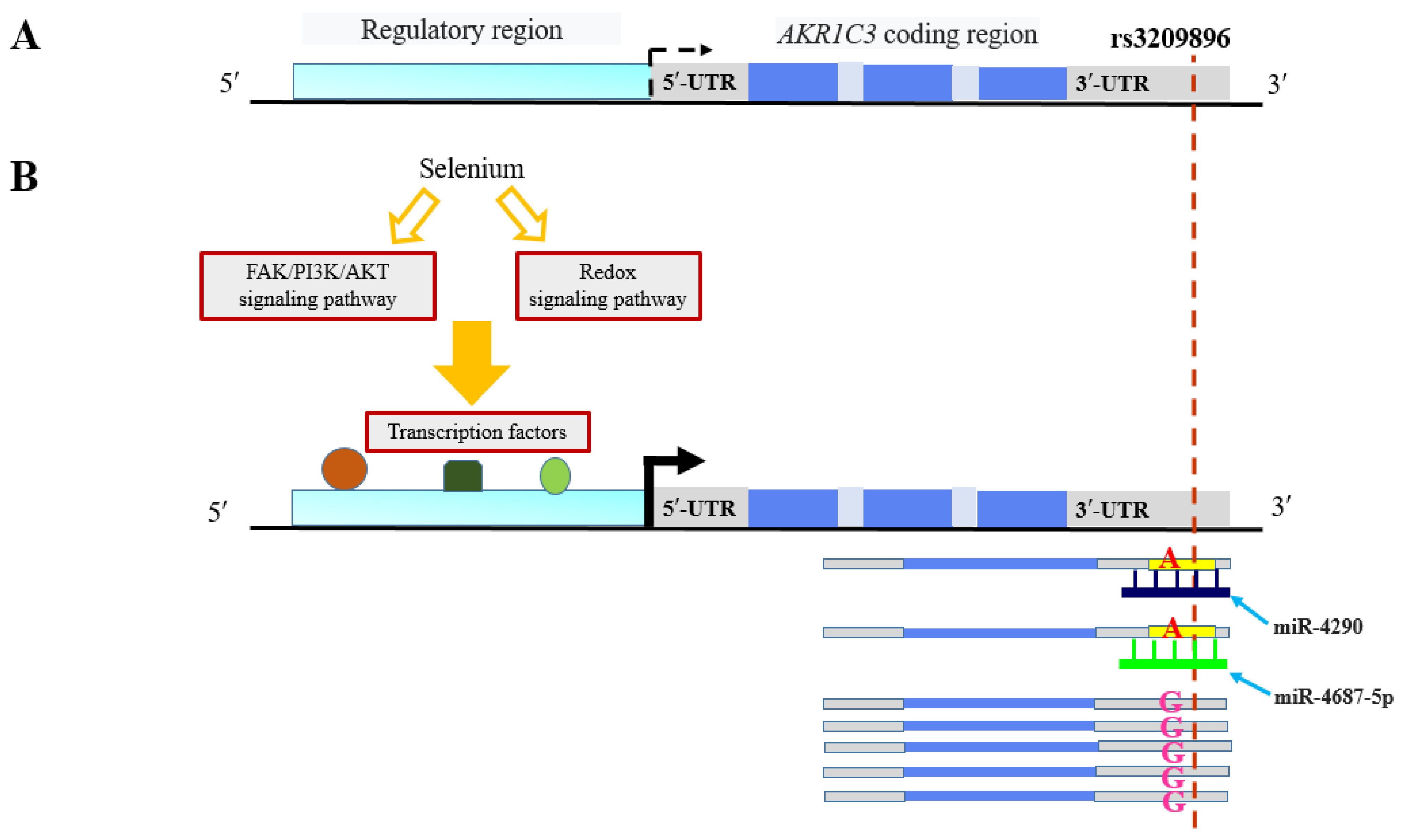
- Zhou, Q.; Tian, W.; Jiang, Z.; Huang, T.; Ge, C.; Liu, T.; Zhao, F.; Chen, T.; Cui, Y.; Li, H.; et al. A Positive Feedback Loop of AKR1C3-Mediated Activation of NF-ΚB and STAT3 Facilitates Proliferation and Metastasis in Hepatocellular Carcinoma. Cancer Res. 2021, 81, 1361–1374. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, X.; Zheng, Y.; Cao, R.; Zhang, M.; Sun, Y.; Wu, J. Construction and Analysis of the LncRNA-miRNA-mRNA Network Based on Competitive Endogenous RNA Reveals Functional Genes in Heart Failure. Mol. Med. Rep. 2018, 19, 994–1003. [Google Scholar] [CrossRef]
- Harvey, C.T.; Moyerbrailean, G.A.; Davis, G.O.; Wen, X.; Luca, F.; Pique-Regi, R. QuASAR: Quantitative Allele-Specific Analysis of Reads. Bioinformatics 2015, 31, 1235–1242. [Google Scholar] [CrossRef]
- Siegel, D.A.; le Tonqueze, O.; Biton, A.; Zaitlen, N.; Erle, D.J. Massively Parallel Analysis of Human 3′ UTRs Reveals That AU-Rich Element Length and Registration Predict MRNA Destabilization. G3 Genes Genomes Genet. 2022, 12, jkab404. [Google Scholar] [CrossRef]
- Abell, N.S.; DeGorter, M.K.; Gloudemans, M.J.; Greenwald, E.; Smith, K.S.; He, Z.; Montgomery, S.B. Multiple Causal Variants Underlie Genetic Associations in Humans. Science 2022, 375, 1247–1254. [Google Scholar] [CrossRef]
| mirSNPs/Gene/miRNA | In Silico Approaches | In Vitro Gene-by-Gene Approaches | PASSPORT-Seq | Drug Metabolisms and Drug Resistance | References |
|---|---|---|---|---|---|
| Neighboring rs2480256 and rs2480257/CYP2E1/miR-570 | MicroSNiPer computational service | Transient and stable luciferase reporter assay with miRNA cotransfection | Support | Associated with the trabectedin-induced hepatotoxicity in patients with advanced soft tissue sarcoma | [36,50] |
| rs15524/CYP3A5/hsa-miR-500a | MirSNP database search, SNPinfo | Transient luciferase reporter assay with miRNA cotransfection | Support | Associated with the tacrolimus serum concentration in myasthenia gravis therapy and sirolimus serum concentration of kidney-transplanted patients | [51,52,53] |
| rs70950385 = combination of rs70950385 and rs12979898/CYP2B6/miR-1275 | Analysis using different algorithms and databases | Luciferase reporter assays | Support | Associated with antiretroviral drug efavirenz hydroxylation activity in human liver microsomes and efavirenz pharmacokinetics | [54] |
| rs3181842/CYP2B6/miR-1581/4537 | TargetScan, SomamiR database | Luciferase transfection into HepG2 and LO2 cells | Support | Influence on the propofol target plasma concentration in patients under total intravenous anesthesia | [30,55] |
| rs6839 and rs1042157/SULT1A1/miR-631 | MicroInspector and RNAhybrid programs MFE calculation | Luciferase reporter assay with miRNA cotransfection, decrease in miR-631 level with inhibitor | Support | Associated with transformation of active metabolite 4-hydroxy-tamoxifen into inactive 4-hydroxy-tamoxifen sulfate in cancer treatment | [28,63] |
| rs8330/UGT1A/miR-1286 rs10929303/UGT1A/miR-21-3p, miR-141-3p, and miR-200a-3p | miRanda and RNAhybrid programs | Luciferase reporter assays with cotransfection of 2048 mimic miRNA library | Support | Influence on analgesic drug acetaminophen glucuronidation status in healthy subjects, gefitinib plasma concentration, and gefitinib-induced rash in cancer patients | [35,67,69] |
| rs241456/ABCB3/hsa-miR-1270 | PolymiRTS database, MFE calculation | RNA EMSA, luciferase reporter assay with miRNA cotransfection | ND | Associated with response to neoadjuvant chemotherapy in cancer patients | [27,72] |
| rs12458/GATA4/miR-556-5p, miR-4279, miR-500b, miR-502-5p, miR-526b, and miR-362-5p | In silico prediction (not detailed) | Luciferase reporter assay | ND | Associated with bleeding complication of warfarin anticoagulation therapy | [86,87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rykova, E.; Ershov, N.; Damarov, I.; Merkulova, T. SNPs in 3′UTR miRNA Target Sequences Associated with Individual Drug Susceptibility. Int. J. Mol. Sci. 2022, 23, 13725. https://doi.org/10.3390/ijms232213725
Rykova E, Ershov N, Damarov I, Merkulova T. SNPs in 3′UTR miRNA Target Sequences Associated with Individual Drug Susceptibility. International Journal of Molecular Sciences. 2022; 23(22):13725. https://doi.org/10.3390/ijms232213725
Chicago/Turabian StyleRykova, Elena, Nikita Ershov, Igor Damarov, and Tatiana Merkulova. 2022. "SNPs in 3′UTR miRNA Target Sequences Associated with Individual Drug Susceptibility" International Journal of Molecular Sciences 23, no. 22: 13725. https://doi.org/10.3390/ijms232213725
APA StyleRykova, E., Ershov, N., Damarov, I., & Merkulova, T. (2022). SNPs in 3′UTR miRNA Target Sequences Associated with Individual Drug Susceptibility. International Journal of Molecular Sciences, 23(22), 13725. https://doi.org/10.3390/ijms232213725





