Circulating Inflammatory Biomarkers in Early Prediction of Stroke-Associated Infections
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics and Rate of Infection
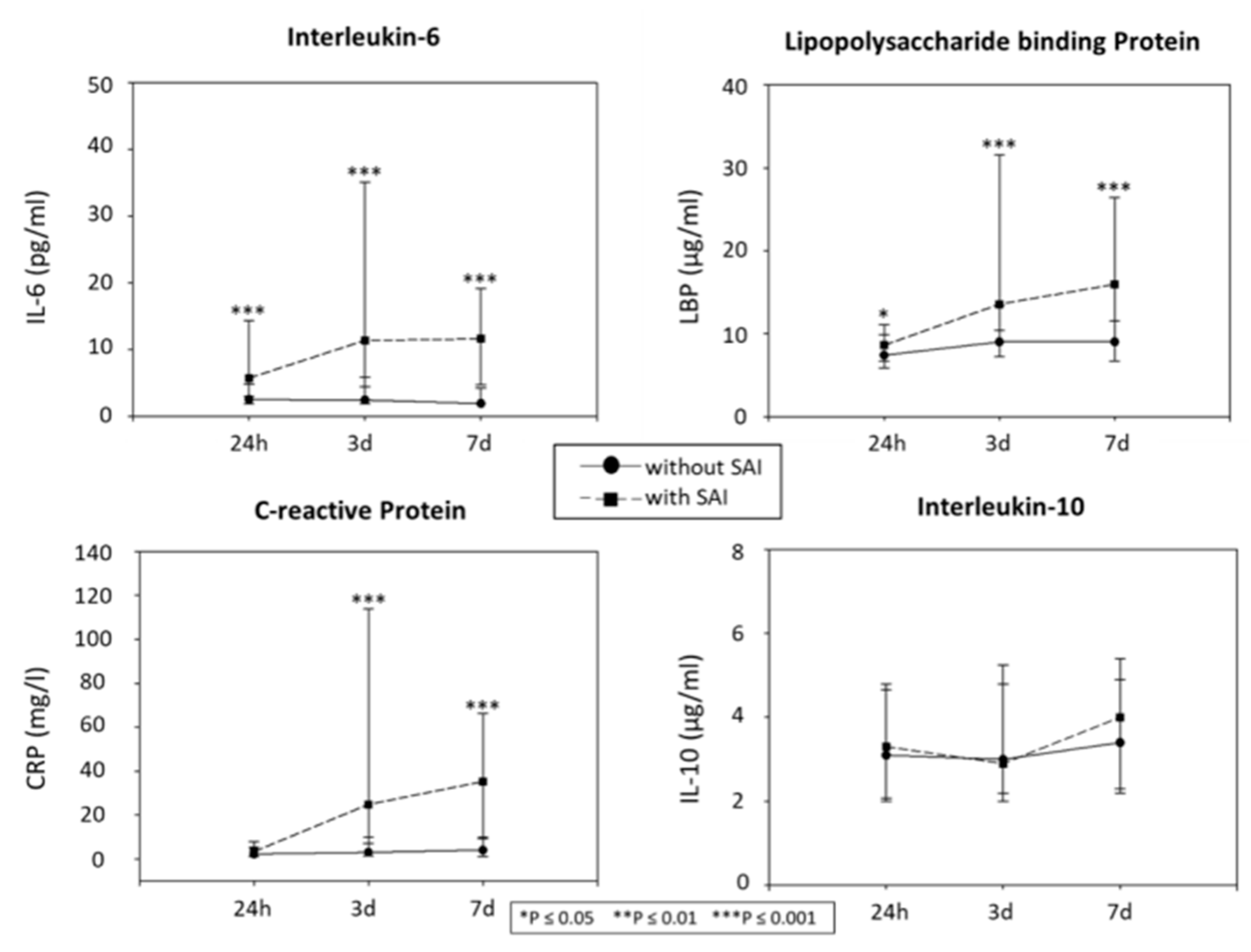
2.2. Biochemical Markers of the Inflammation and Status of SAIs
2.3. Association of the Biochemical Markers of Inflammation with the Outcome
2.4. Association of the Type of Infection with the Outcome
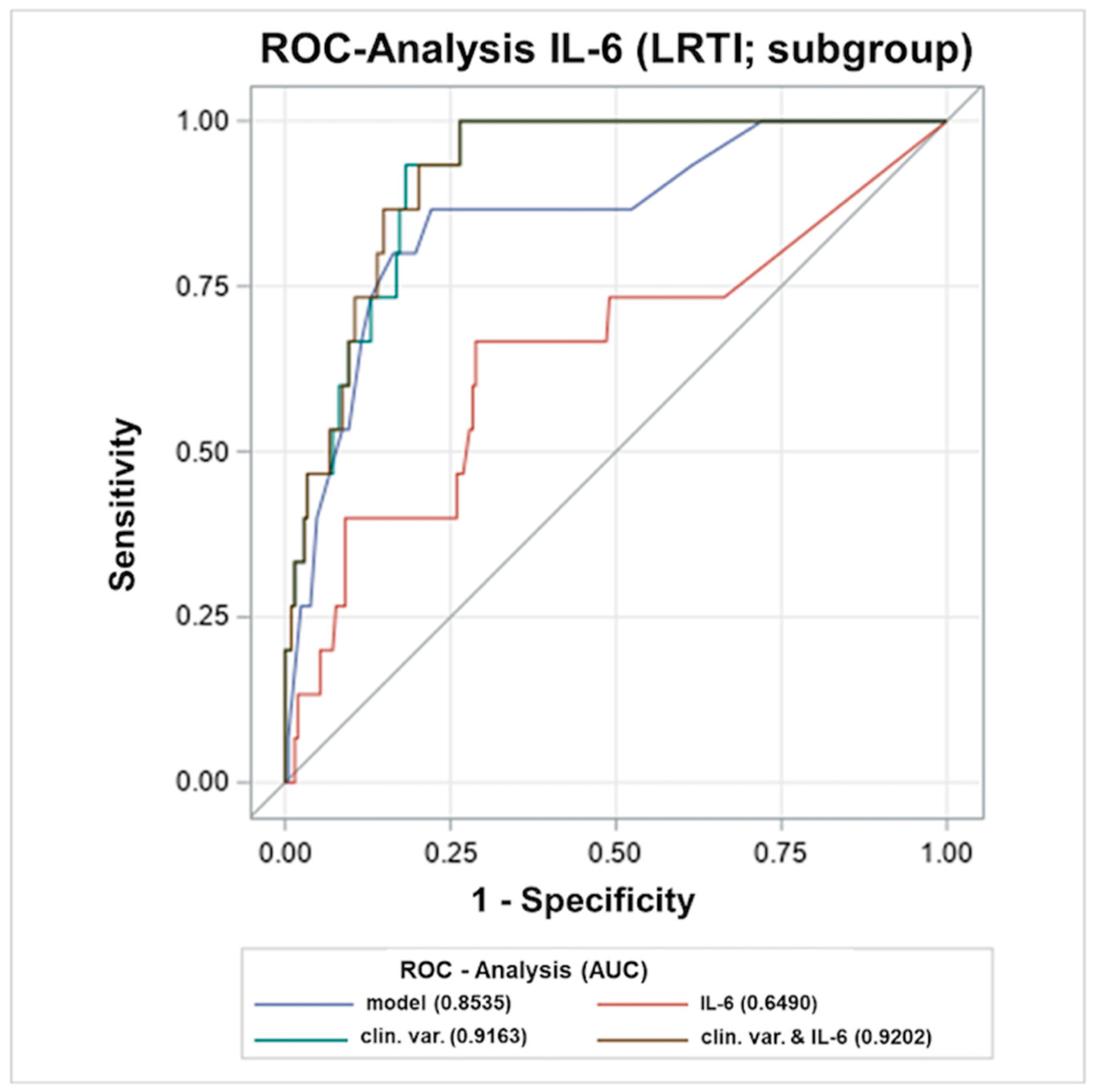
2.5. Early Levels of Inflammation Markers ≤12 h in Association to LRTIs
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Blood Samples and Marker Determination
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Suda, S.; Aoki, J.; Shimoyama, T.; Suzuki, K.; Sakamoto, Y.; Katano, T.; Okubo, S.; Nito, C.; Nishiyama, Y.; Mishina, M.; et al. Stroke-associated infection independently predicts 3-month poor functional outcome and mortality. J. Neurol. 2018, 265, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, W.F.; Nederkoorn, P.J.; Vermeij, J.D.; Dijkgraaf, M.G.; van de Beek, D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011, 11, 110. [Google Scholar] [CrossRef] [Green Version]
- Macrez, R.; Ali, C.; Toutirais, O.; Le Mauff, B.; Defer, G.; Dirnagl, U.; Vivien, D. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet. Neurol. 2011, 10, 471–480. [Google Scholar] [CrossRef]
- Haeusler, K.G.; Schmidt, W.U.H.; Föhring, F.; Meisel, C.; Helms, T.; Jungehulsing, G.J.; Nolte, C.H.; Schmolke, K.; Wegner, B.; Meisel, A.; et al. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc. Dis. 2008, 25, 50–58. [Google Scholar] [CrossRef]
- Westendorp, W.F.; Vermeij, J.D.; Zock, E.; Hooijenga, I.J.; Kruyt, N.D.; Bosboom, H.J.L.W.; Kwa, V.I.H.; Weisfelt, M.; Remmers, M.J.M.; ten Houten, R.; et al. The Preventive Antibiotics in Stroke Study (PASS): A pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015, 385, 1519–1526. [Google Scholar] [CrossRef]
- Kalra, L.; Irshad, S.; Hodsoll, J.; Simpson, M.; Gulliford, M.; Smithard, D.; Patel, A.; Rebollo-Mesa, I.; STROKE-INF Investigators. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): A prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 2015, 386, 1835–1844. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Huang, J.; Jia, X.; Peng, L.; Yan, K.; Zan, X.; Ma, L. Preventive Antibiotics for Poststroke Infection in Patients with Acute Stroke: A Systematic Review and Meta-analysis. Neurologist 2018, 23, 35–42. [Google Scholar] [CrossRef]
- Schwarz, S.; Al-Shajlawi, F.; Sick, C.; Meairs, S.; Hennerici, M.G. Effects of prophylactic antibiotic therapy with mezlocillin plus sulbactam on the incidence and height of fever after severe acute ischemic stroke: The Mannheim infection in stroke study (MISS). Stroke 2008, 39, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Wartenberg, K.E.; Stoll, A.; Funk, A.; Meyer, A.; Schmidt, J.M.; Berrouschot, J. Infection after acute ischemic stroke: Risk factors, biomarkers, and outcome. Stroke Res. Treat. 2011, 2011, 830614. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Biber, K.; Finsen, B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2012, 32, 1677–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worthmann, H.; Tryc, A.B.; Dirks, M.; Schuppner, R.; Brand, K.; Klawonn, F.; Lichtinghagen, R.; Weissenborn, K. Lipopolysaccharide binding protein, interleukin-10, interleukin-6 and C-reactive protein blood levels in acute ischemic stroke patients with post-stroke infection. J. Neuroinflamm. 2015, 12, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, H.C.; Smith, C.J.; Gavin, C.M.; Georgiou, R.F.; Vail, A.; Barberan, E.M.; Hallenbeck, J.M.; del Zoppo, G.J.; Rothwell, N.J.; Tyrrell, P.J.; et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: Relationships with infection and atherosclerosis. J. Neuroimmunol. 2003, 139, 93–101. [Google Scholar] [CrossRef]
- Schumann, R.R.; Leong, S.R.; Flaggs, G.W.; Gray, P.W.; Wright, S.D.; Mathison, J.C.; Tobias, P.S.; Ulevitch, R.J. Structure and function of lipopolysaccharide binding protein. Science 1990, 249, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Urra, X.; Cervera, A.; Obach, V.; Climent, N.; Planas, A.M.; Chamorro, A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke 2009, 40, 1262–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamorro, A.; Amaro, S.; Vargas, M.; Obach, V.; Cervera, Á.; Torres, F.; Planas, A.M. Interleukin 10, monocytes and increased risk of early infection in ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1279–1281. [Google Scholar] [CrossRef] [Green Version]
- Fluri, F.; Morgenthaler, N.G.; Mueller, B.; Christ-Crain, M.; Katan, M. Copeptin, procalcitonin and routine inflammatory markers-predictors of infection after stroke. PLoS ONE 2012, 7, e48309. [Google Scholar] [CrossRef]
- Hotter, B.; Hoffmann, S.; Ulm, L.; Montaner, J.; Bustamante, A.; Meisel, C.; Meisel, A. Inflammatory and stress markers predicting pneumonia, outcome, and etiology in patients with stroke: Biomarkers for predicting pneumonia, functional outcome, and death after stroke. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e692. [Google Scholar] [CrossRef] [Green Version]
- Salat, D.; Penalba, A.; García-Berrocoso, T.; Campos-Martorell, M.; Flores, A.; Pagola, J.; Bustamante, A.; Quintana, M.; Giralt, D.; Molina, C.; et al. Immunological biomarkers improve the accuracy of clinical risk models of infection in the acute phase of ischemic stroke. Cerebrovasc. Dis. 2013, 35, 220–227. [Google Scholar] [CrossRef]
- Meisel, C.; Schwab, J.M.; Prass, K.; Meisel, A.; Dirnagl, U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005, 6, 775–786. [Google Scholar] [CrossRef]
- Harms, H.; Prass, K.; Meisel, C.; Klehmet, J.; Rogge, W.; Drenckhahn, C.; Göhler, J.; Bereswill, S.; Göbel, U.; Wernecke, K.D.; et al. Preventive antibacterial therapy in acute ischemic stroke: A randomized controlled trial. PLoS ONE 2008, 3, e2158. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, A.; Giralt, D.; García-Berrocoso, T.; Rubiera, M.; Álvarez-Sabín, J.; Molina, C.; Serena, J.; Montaner, J. The impact of post-stroke complications on in-hospital mortality depends on stroke severity. Eur. Stroke J. 2017, 2, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faura, J.; Bustamante, A.; Reverté, S.; García-Berrocoso, T.; Millán, M.; Castellanos, M.; Lara-Rodríguez, B.; Zaragoza, J.; Ventura, O.; Hernández-Pérez, M.; et al. Blood Biomarker Panels for the Early Prediction of Stroke-Associated Complications. J. Am. Heart Assoc. 2021, 10, e018946. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Mason, L.J.; Mackin, K.E.; Srikhanta, Y.N.; Lyras, D.; Prakash, M.D.; Nurgali, K.; Venegas, A.; Hill, M.D.; Moore, R.J.; et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 2016, 22, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Eltringham, S.A.; Kilner, K.; Gee, M.; Sage, K.; Bray, B.D.; Smith, C.J.; Pownall, S. Factors Associated with Risk of Stroke-Associated Pneumonia in Patients with Dysphagia: A Systematic Review. Dysphagia 2020, 35, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Maier, I.L.; Becker, J.C.; Leyhe, J.R.; Schnieder, M.; Behme, D.; Psychogios, M.N.; Liman, J. Influence of beta-blocker therapy on the risk of infections and death in patients at high risk for stroke induced immunodepression. PLoS ONE 2018, 13, e0196174. [Google Scholar] [CrossRef] [Green Version]
- Rudilosso, S.; Rodríguez-Vázquez, A.; Urra, X.; Arboix, A. The Potential Impact of Neuroimaging and Translational Research on the Clinical Management of Lacunar Stroke. Int. J. Mol. Sci. 2022, 23, 1497. [Google Scholar] [CrossRef]
- Maiese, A.; Del Nonno, F.; Dell’Aquila, M.; Moauro, M.; Baiocchini, A.; Mastracchio, A.; Bolino, G. Postmortem diagnosis of sepsis: A preliminary immunohistochemical study with an anti-procalcitonin antibody. Leg. Med. 2017, 28, 1–5. [Google Scholar] [CrossRef]
- La Russa, R.; Maiese, A.; Viola, R.V.; De Matteis, A.; Pinchi, E.; Frati, P.; Fineschi, V. Searching for highly sensitive and specific biomarkers for sepsis: State-of-the-art in post-mortem diagnosis of sepsis through immunohistochemical analysis. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419855226. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.J.; Kishore, A.K.; Vail, A.; Chamorro, A.; Garau, J.; Hopkins, S.J.; Di Napoli, M.; Kalra, L.; Langhorne, P.; Montaner, J.; et al. Diagnosis of Stroke-Associated Pneumonia: Recommendations from the Pneumonia in Stroke Consensus Group. Stroke 2015, 46, 2335–2340. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]


| All Patients (n = 223) | SAI = Yes (n = 47) | SAI = No (n = 176) | p= | |
|---|---|---|---|---|
| Female [%] | 84 [37.67] | 23 [48.94] | 61 [34.66] | 0.073 |
| Age in years [IQR] | 74 [64.00–81.00] | 79 [68.00–84.00] | 73 [64.00–80.75] | 0.035 |
| BMI [IQR) | 26.0 [24.14–28.91] | 25.7 [23.80–29.00] | 26.1 [24.17–28.90] | 0.763 |
| Blood glucose upon admission [IQR] | 6.5 [5.7–7.9] | 6.8 [6.20–8.50] | 6.5 [5.70–7.80] | 0.178 |
| NIHSS upon admission [IQR] | 5 [2.00–10.00] | 13 [6.00–17.00] | 4 [2.00–8.00] | <0.001 |
| Reduced consciousness upon admission [%] | 26 [11.66] | 17 [36.17] | 9 [5.11] | <0.001 |
| Dysphagia [%] | 76 [34.08] | 33 [70.21] | 43 [24.43] | <0.001 |
| CHA2DS2-VASc [IQR] | 5 [4.00–6.00] | 6 [4.00–7.00] | 5 [4.00–6.00] | 0.089 |
| ESRS [IQR] | 4 [3.00–5.00] | 4 [3.00–6.00] | 4 [3.00–5.00] | 0.335 |
| Stroke cause (TOAST) | 0.019 | |||
| TOAST large-artery atherosclerosis [%] | 19 [8.52] | 2 [4.26] | 17 [9.66] | |
| TOAST cardioembolism [%] | 88 [39.46] | 25 [53.19] | 63 [35.80] | |
| TOAST small-vessel occlusion [%] | 16 [7.17] | 0 [0.00] | 16 [9.09] | |
| TOAST other determined etiology [%] | 4 [1.79] | 2 [4.26] | 2 [1.14] | |
| TOAST undetermined etiology [%] | 96 [43.05] | 18 [38.30] | 78 [44.32] | |
| Atrial fibrillation [%] | 66 [29.60] | 19 [40.43] | 47 [26.70] | 0.067 |
| Coronary heart disease [%] | 30 [13.45] | 8 [17.02] | 22 [12.50] | 0.420 |
| Renal dysfunction [%] | 50 [22.42] | 11 [23.40] | 39 [22.16] | 0.856 |
| History of stroke [%] | 48 [21.52] | 13 [27.66] | 35 [19.89] | 0.249 |
| Family history of stroke [%] | 66 [29.60] | 10 [21.28] | 56 [31.82] | 0.160 |
| Obesity (BMI ≥ 30 kg/m2) [%] | 47 [21.08] | 12 [25.53] | 35 [19.89] | 0.399 |
| Arterial hypertension [%] | 172 [77.13] | 33 [70.21] | 139 [78.98] | 0.204 |
| Hyperlipoproteinemia [%] | 54 [24.21] | 12 [25.53] | 42 [23.86] | 0.813 |
| Alcohol abuse [%] | 25 [11.21] | 6 [12.77] | 19 [10.80] | 0.704 |
| Nicotine abuse [%] | 119 [53.36] | 27 [57.45] | 92 [52.27] | 0.528 |
| Diabetes [%] | 42 [18.83] | 6 [12.77] | 36 [20.45] | 0.231 |
| IL-6 24 h (pg/mL) [IQR] | 3.1 [1.90–6.50] | 5.8 [3.10–14.90] | 2.6 [1.90–4.68] | <0.001 |
| LBP 24 h (ug/mL) [IQR] | 7.8 [6.00–10.40] | 8.6 [6.70–11.10] | 7.5 [5.93–9.90] | 0.05 |
| IL-10 24 h (ug/mL) [IQR] | 3.2 [2.00–5.00] | 3.5 [2.30–5.90] | 3.1 [1.90–4.98] | 0.301 |
| CRP 24 h (mg/L) [IQR] | 2.9 [1.15–6.63] | 3.35 [1.26–8.09] | 2.38 [1.15–6.16] | 0.148 |
| All Patients (n = 223) | Favourable Outcome (mRS 0–3) (n = 171) | Unfavourable Outcome (mRS 4–6) (n = 52) | p= | |
|---|---|---|---|---|
| Female [%] | 84 [37.67] | 57 [33.33] | 27 [51.92] | 0.013 |
| Age in years [IQR] | 74 [64–81] | 71 [62–80] | 81 [75–87] | <0.001 |
| BMI [IQR) | 26.0 [24.1–28.9] | 26.2 [24.1–29.1] | 25.7 [23.8–27.8] | 0.415 |
| Blood glucose on admission [IQR] | 6.5 [5.7–7.9] | 6.5 [5.6–7.7] | 7.5 [6.3–9.3] | 0.003 |
| Reduced consciousness on admission [%] | 26 [11.66] | 6 [3.51] | 20 [38.46] | <0.001 |
| Dysphagia [%] | 76 [34.08] | 34 [19.88] | 42 [80.77] | <0.001 |
| NIHSS on admission [IQR] | 5 [2–10] | 4 [2–7] | 13 [5–18] | <0.001 |
| SAI within 7d [%] | 47 [21.08] | 17 [9.94] | 30 [57.69] | <0.001 |
| UTI within 7d [%] | 19 [8.52] | 9 [5.26] | 10 [19.23] | 0.004 |
| LRTI within 7d [%] | 15 [6.73] | 2 [1.17] | 13 [25.00] | <0.001 |
| Alcohol abuse [%] | 25 [11.21] | 20 [11.70] | 5 [9.62] | 0.448 |
| Atrial fibrillation [%] | 66 [29.60] | 43 [25.15] | 23 [44.23] | 0.008 |
| Arterial Hypertension [%] | 172 [77.13] | 129 [75.44] | 43 [82.69] | 0.184 |
| IL-6 24 h (pg/mL) [IQR] | 3.1 [1.9–6.5] | 2.6 [1.9–4.7] | 5.6 [2.6–14.9] | <0.001 |
| LBP 24 h (ug/mL) [IQR] | 7.8 [6.0–10.4] | 7.5 [6.0–9.9] | 8.7 [6.3–10.9] | 0.039 |
| IL-10 24 h (ug/mL) [IQR] | 3.2 [2.0–5.0] | 3.2 [1.9–5.0] | 3.1 [2.3–5.0] | 0.610 |
| CRP 24 h (mg/L) [IQR] | 2.39 [1.15–6.63] | 2.27 [1.15–4.86] | 5.23 [1.15–8.28] | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasse, I.M.C.; Grosse, G.M.; Schuppner, R.; Van Gemmeren, T.; Gabriel, M.M.; Weissenborn, K.; Lichtinghagen, R.; Worthmann, H. Circulating Inflammatory Biomarkers in Early Prediction of Stroke-Associated Infections. Int. J. Mol. Sci. 2022, 23, 13747. https://doi.org/10.3390/ijms232213747
Hasse IMC, Grosse GM, Schuppner R, Van Gemmeren T, Gabriel MM, Weissenborn K, Lichtinghagen R, Worthmann H. Circulating Inflammatory Biomarkers in Early Prediction of Stroke-Associated Infections. International Journal of Molecular Sciences. 2022; 23(22):13747. https://doi.org/10.3390/ijms232213747
Chicago/Turabian StyleHasse, Isabel M. C., Gerrit M. Grosse, Ramona Schuppner, Till Van Gemmeren, Maria M. Gabriel, Karin Weissenborn, Ralf Lichtinghagen, and Hans Worthmann. 2022. "Circulating Inflammatory Biomarkers in Early Prediction of Stroke-Associated Infections" International Journal of Molecular Sciences 23, no. 22: 13747. https://doi.org/10.3390/ijms232213747
APA StyleHasse, I. M. C., Grosse, G. M., Schuppner, R., Van Gemmeren, T., Gabriel, M. M., Weissenborn, K., Lichtinghagen, R., & Worthmann, H. (2022). Circulating Inflammatory Biomarkers in Early Prediction of Stroke-Associated Infections. International Journal of Molecular Sciences, 23(22), 13747. https://doi.org/10.3390/ijms232213747






