In Vitro Evaluation of Anti-Inflammatory and Protective Potential of an Extract from Cornus mas L. Fruit against H2O2-Induced Oxidative Stress in Human Skin Keratinocytes and Fibroblasts
Abstract
1. Introduction
2. Results
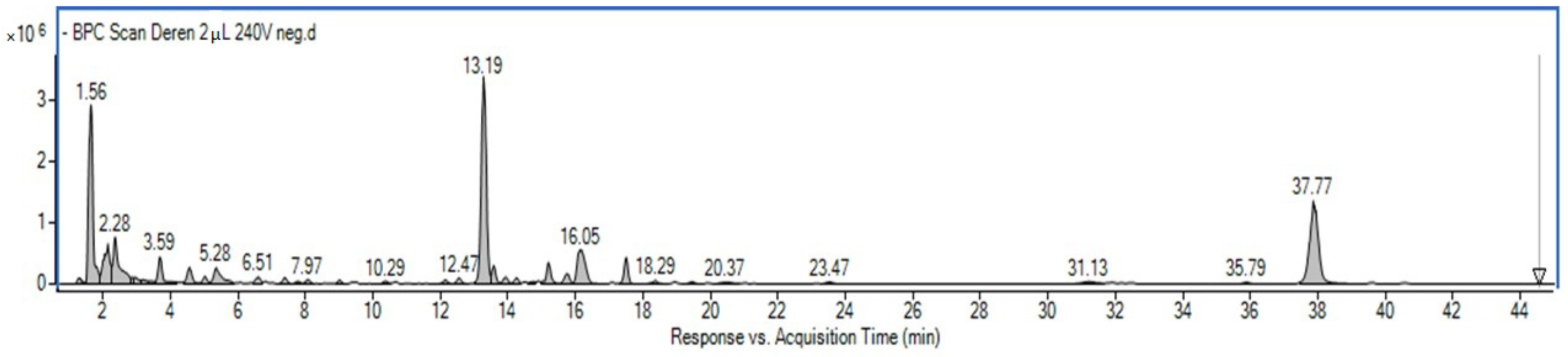
2.1. Phytochemical Profiling of the Cornus mas L. Fruit Extract and Quantitative Analysis of the Main Constituents
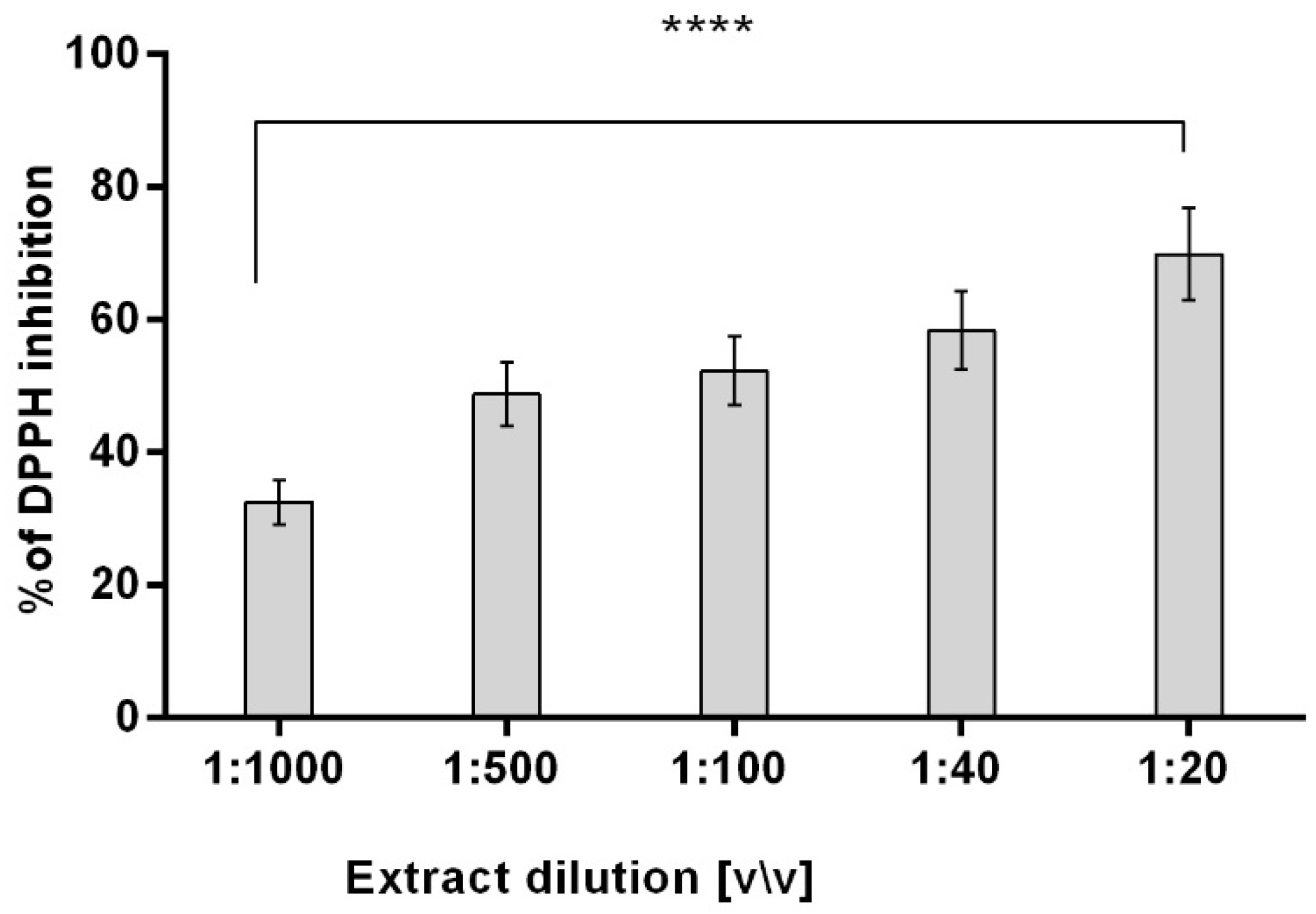
2.2. DPPH Radical Scavenging Assay
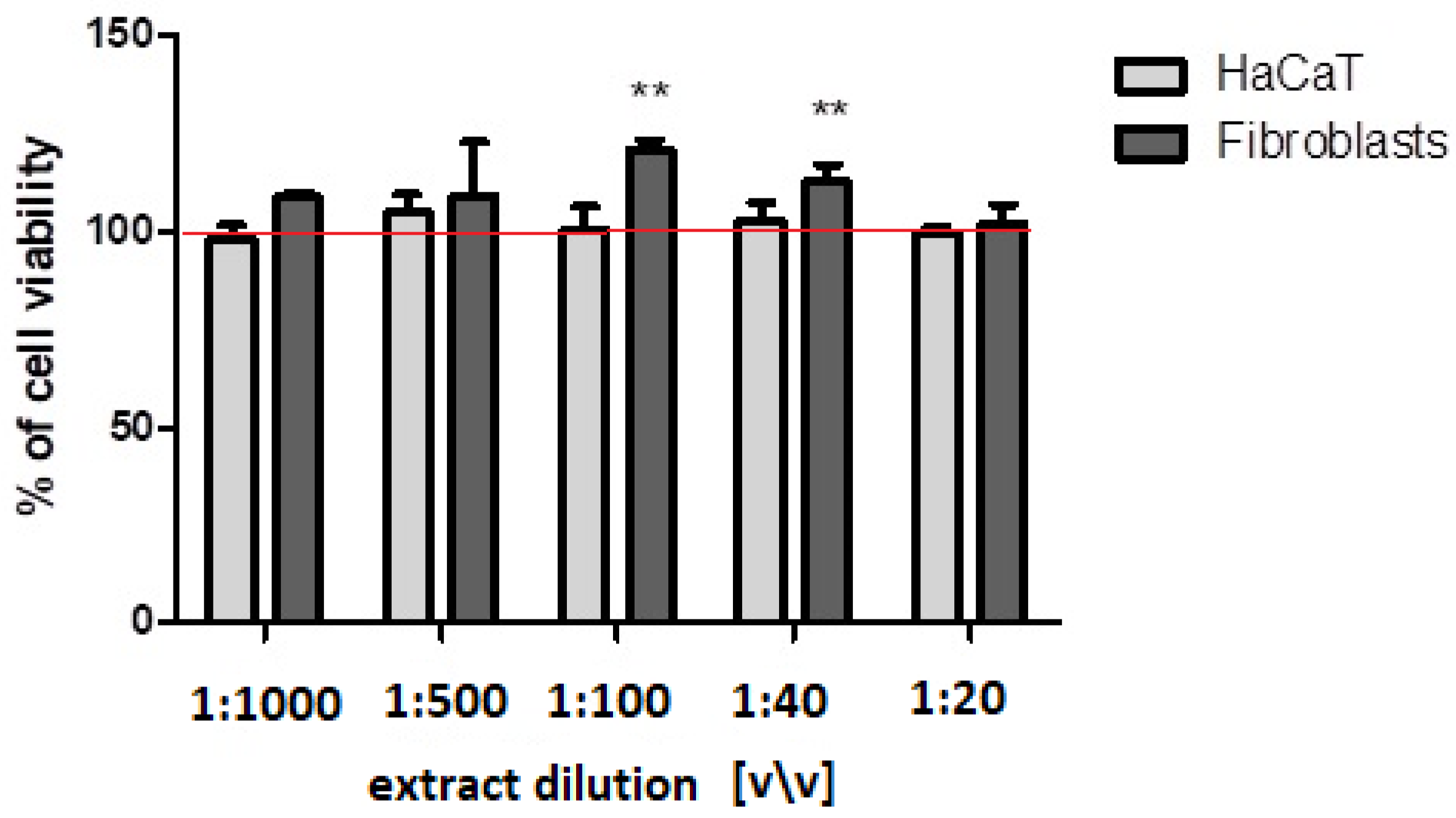
2.3. Cell Viability and Proliferation
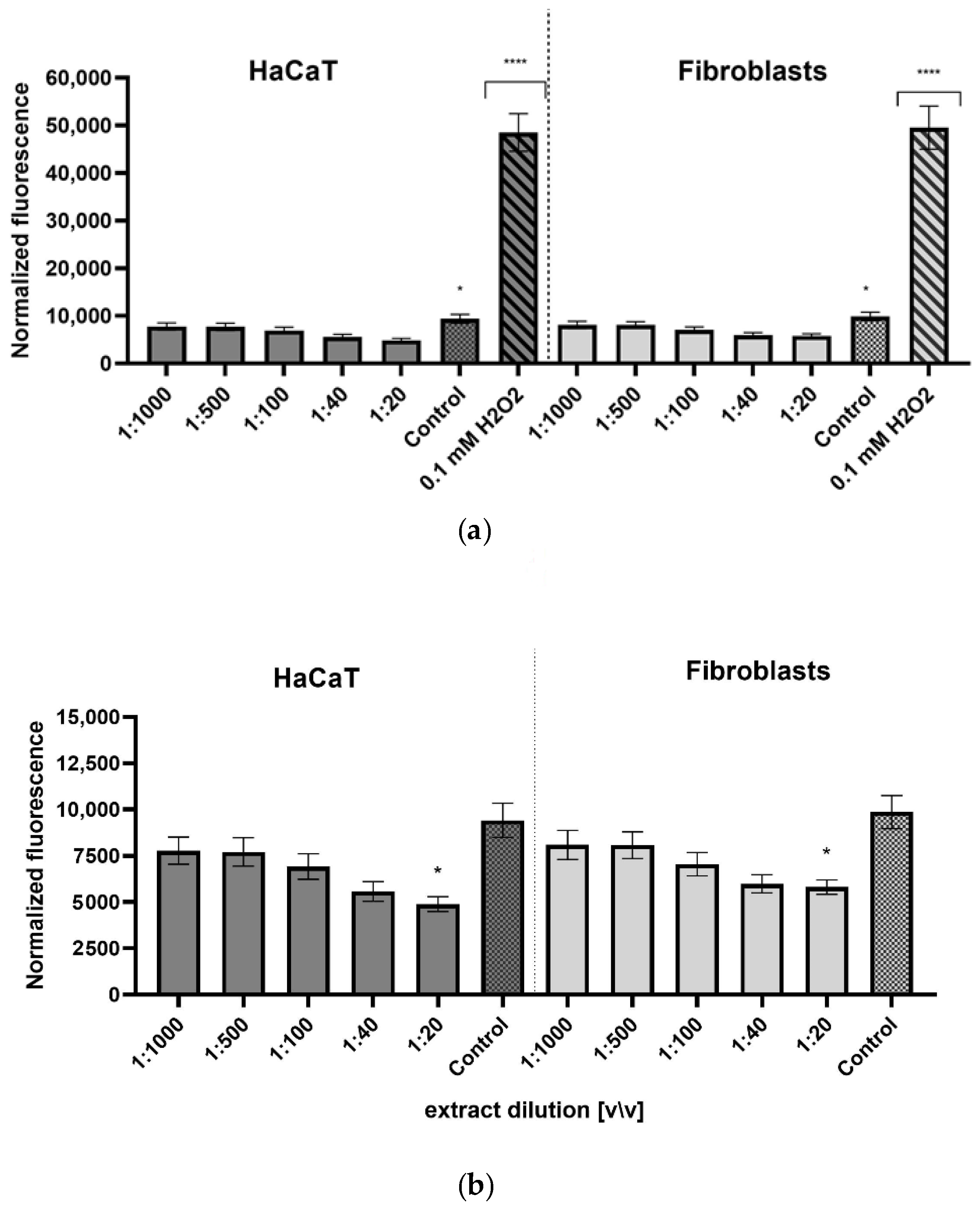
2.4. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
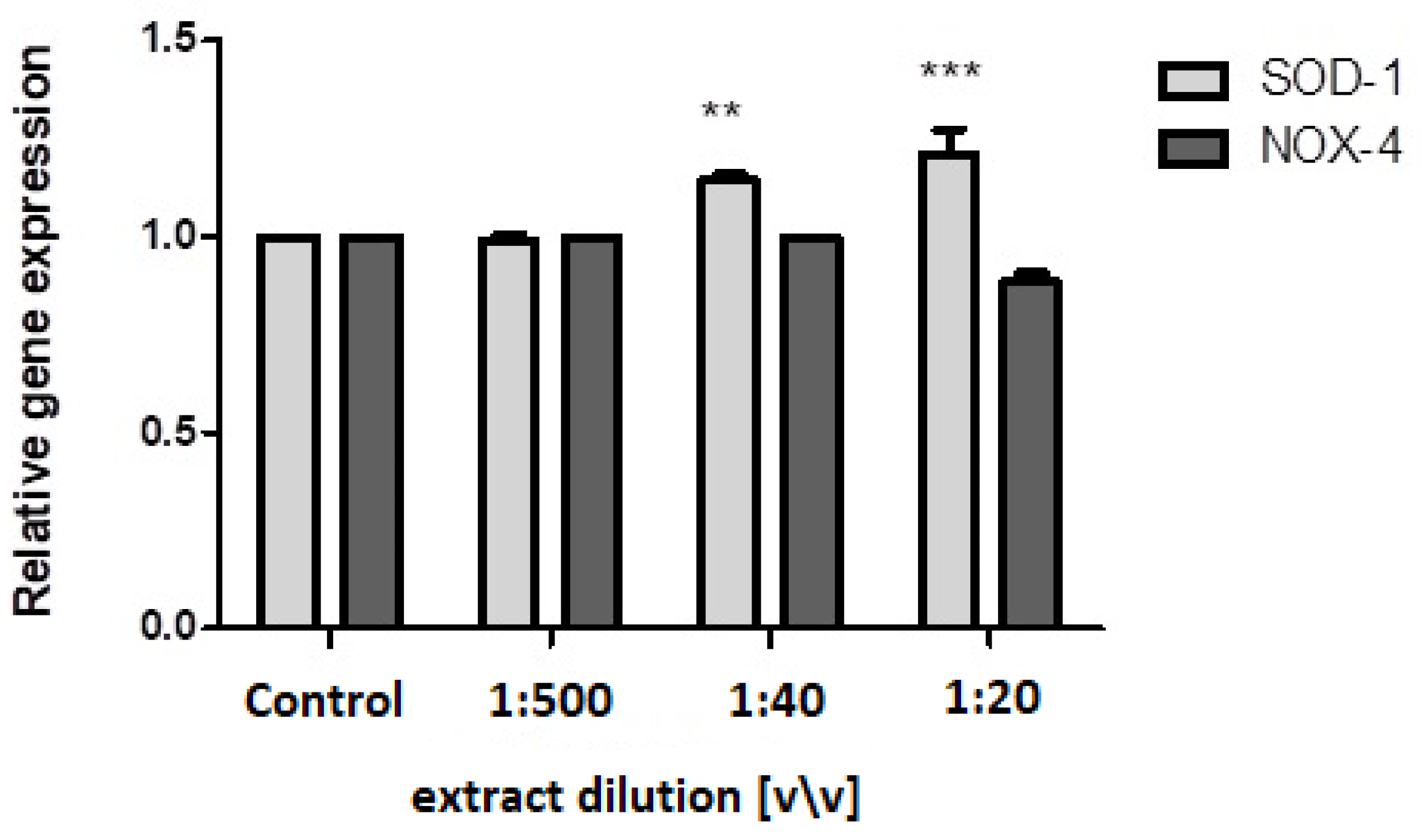
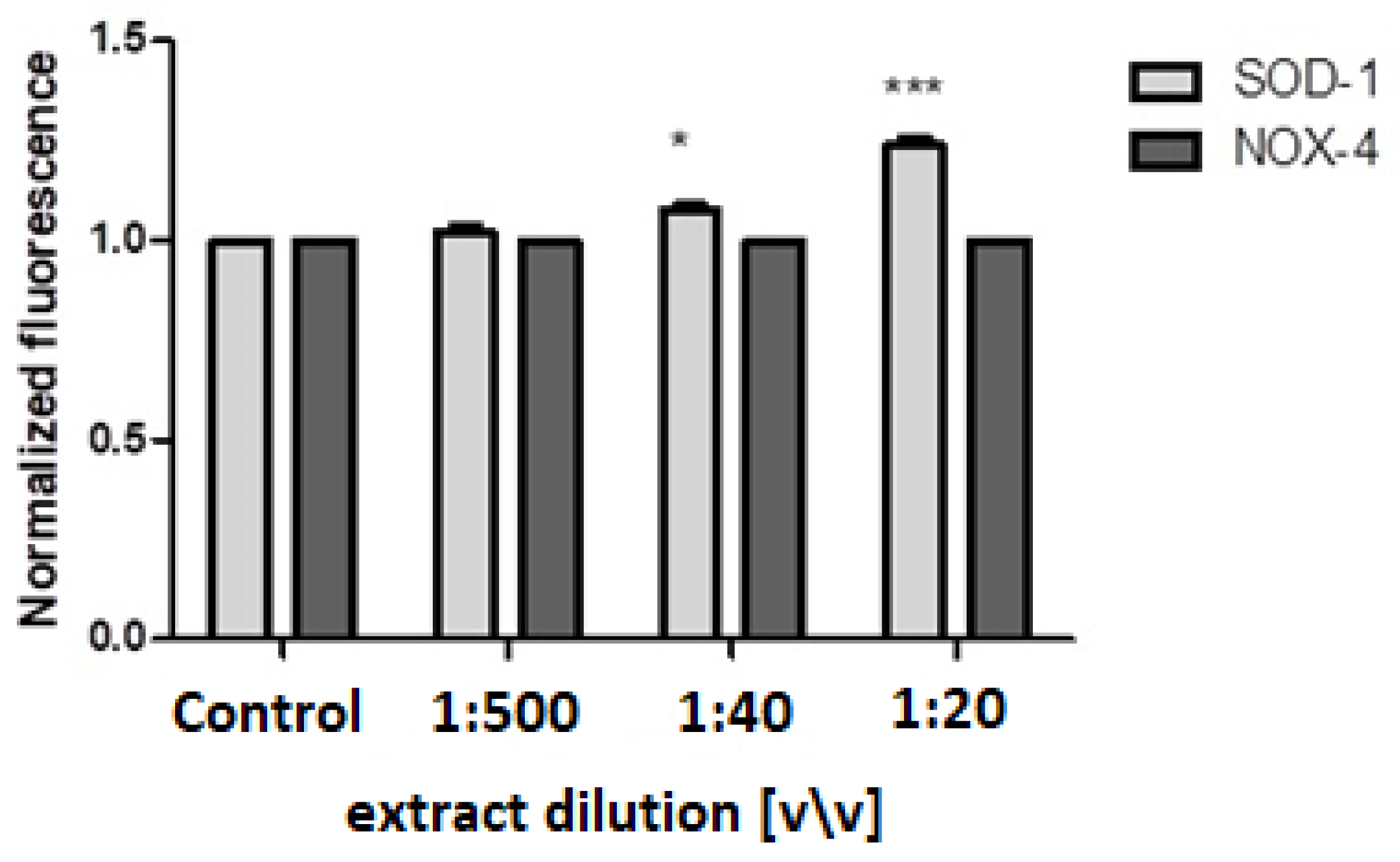
2.5. SOD-1 and Nox-4 Expression
2.6. Anti-Inflammatory Assay
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction Procedure
4.2. Total Phenolic Content
4.3. Total Flavonoid Content
4.4. Total Anthocyanin Content
4.5. UHPLC-MS Analysis
4.6. DPPH Radical Scavenging Assay
4.7. Cell Culture
4.8. Cell Viability Assay
4.9. Detection of Intracellular Reactive Oxygen Species and Interleukins Level
4.10. Real-Time PCR for mRNA Expression Analyses
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Gunduz, K.; Saracoglu, O.; Özgen, M.; Serce, S. Antioxidant, physical and chemical characteristics of cornelian cherry fruits (Cornus mas L.) at different stages of ripenees. Acta Sci. Pol. Hortorum Cultus 2013, 12, 59–66. [Google Scholar]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G.R. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Camangi, F.; Braca, A. Quali-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem. 2010, 119, 1257–1261. [Google Scholar] [CrossRef]
- Seeram, N.P.; Nair, M.G. Inhibition of lipid peroxidation and structure-activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J. Agric. Food Chem. 2002, 50, 5308–5312. [Google Scholar] [CrossRef]
- Seeram, N.; Schutzki, R.; Chandra, A.; Nair, M.G. Characterization, quantification and bioactivities of anthocyanins in Cornus species. J. Agric. Food Chem. 2002, 50, 2519–2523. [Google Scholar]
- Kruk, J.; Duchnik, E. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Yilmaz, K.U.; Ercisli, S.; Zengin, Y.; Sengul, M.; Kafkas, E.Y. Preliminary characterisation of cornelian cherry (Cornus mas L.) genotypes for their physico-chemical properties. Food Chem. 2008, 114, 408–412. [Google Scholar] [CrossRef]
- Vareed, S.K.; Reddy, M.K.; Schutzki, R.E.; Nair, M.G. Anthocyanins in Cornus alternifolia, Cornuscontroversa, Cornuskousa and Cornusflorida fruits with health benefits. Life Sci. 2006, 11, 777–784. [Google Scholar] [CrossRef]
- Kazimierski, M.; Regula, J.; Molska, M. Cornelian cherry (Cornus mas L.)—characteristics, nutritional and pro-health properties. Acta Sci. Pol. Technol. Aliment. 2019, 18, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef]
- Przybylska, D.; Kucharska, A.Z.; Cybulska, I.; Sozański, T.; Piórecki, N.; Fecka, I. Cornus mas L. Stones: A Valuable By-Product as an Ellagitannin Source with High Antioxidant Potential. Molecules 2020, 25, 4646. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, Z.L.; Chen, H.B. Corni Fructus: A review of chemical constituents and pharmacological activities. Chin Med. 2018, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A.; Kucharska, A.Z.; Fecka, I. Composition and Antibacterial Activity of Aronia melanocarpa (Michx.) Elliot, Cornus mas L. and Chaenomeles superba Lindl. Leaf Extracts. Molecules 2020, 25, 2011. [Google Scholar] [CrossRef]
- Kucharska, A.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Sardesai, V.M. Role of antioxidants in health maintenance. Nutr. Clin. Pract. 1995, 10, 19–25. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Moldovan, B.; Filip, A.; Clichici, S.; Suharoschi, R.; Bolfă, P.; David, L. Antioxidant activity of Cornelian cherry (Cornus mas L.) fruits extract and the in vivo evaluation of its anti-inflammatory effects. J. Funct. Foods 2016, 26, 77–87. [Google Scholar]
- Perova, I.B.; Zhogova, A.A.; Poliakova, A.V.; Éller, K.I.; Ramenskaia, G.V.; Samylina, I.A. Biologically active substances of cornelian cherry fruits (Cornus mas L.). Vopr. Pitan. 2014, 83, 86–94. [Google Scholar]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Yigit, D. Antimicrobial and antioxidant evaluation of fruit extract from Cornus mas L. Aksaray J. Sci. Eng. 2018, 2, 41–51. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Wasilewski, T.; Bujak, T.; Gaweł-Bęben, K.; Osika, P.; Czerwonka, D. Cornus mas L. extract as a multifunctional material for manufacturing cosmetic emulsions. Chin. J. Nat. Med. 2018, 16, 284–292. [Google Scholar] [CrossRef]
- Popović, B.M.; Stajner, D.; Slavko, K.; Sandra, B. Antioxidant capacity of cornelian cherry (Cornus mas L.)—comparison between permanganate reducing antioxidant capacity and other antioxidant methods. Food Chem. 2012, 134, 734–741. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Osika, P.; Wasilewski, T.; Bujak, T. Hydrophilic Dogwood Extracts as Materials for Reducing the Skin Irritation Potential of Body Wash Cosmetics. Molecules 2017, 22, 320. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Kefalas, P.; Kallithraka, S.; Parejo, I.; Makris, D.P. A comparative study on the in vitro antiradical activity and hydroxyl free radical scavenging activity in aged red wines. Food Sci. Technol. Int. 2003, 9, 383–387. [Google Scholar] [CrossRef]
- Pavelescu, L.A. On reactive oxygen species measurement in living systems. J. Med. Life 2015, 8, 38–42. [Google Scholar]
- Quan, N.V.; Xuan, T.D.; Tran, H.D.; Thuy, N.T.D.; Trang, L.T.; Huong, C.T.; Andriana, Y.; Tuyen, P.T. Antioxidant, α-Amylase and α-Glucosidase Inhibitory Activities and Potential Constituents of Canarium tramdenum Bark. Molecules 2019, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; St Clair, D.K. Regulation of Superoxide Dismutase Genes: Implications in Diseases. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Francik, R.; Krośniak, J.; Krosniak, M.; Berköz, M.; Sanocka, I.; Francik, S. The neuroprotective effect of Cornus mas on brain tissue of Wistar rats. Sci. World J. 2014, 2014, 847368. [Google Scholar] [CrossRef] [PubMed]
- Yarim, G.F.; Kazak, F.; Sozmen, M.; Koca, I.; Albayrak, H.; Yarim, M.; Cenesiz, S.; Ozan, E. Investigation of the effect of cornelian cherry (Cornus mas L.) fruit extract against cisplatin-induced renal cell injury in vitro. Turk. J. Biochem. 2017, 42, 435–443. [Google Scholar] [CrossRef]
- Eshaghi, M.; Zare, S.; Banihabib, N.; Nejati, V.; Farokhi, F.; Mikaili, P. Cardioprotective effect of Cornus mas fruit extract against car-bon tetrachloride induced-cardiotoxicity in Albino rats. J. Basic Appl. Sci. Res. 2012, 2, 11106–11114. [Google Scholar]
- Ko, M.J.; Cheigh, C.I.; Chung, M.S. Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chem. 2014, 143, 147–155. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, G.; Zhang, Y.; He, J.; Zheng, S.; Lin, J. Tormentic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NF-κB signaling pathway. Mol. Med. Rep. 2016, 14, 3559–3564. [Google Scholar] [CrossRef]
- Recio, M.C.; Giner, R.M.; Máñez, S.; Ríos, J.L. Structural considerations on the iridoids as anti-inflammatory agents. Planta Med. 1994, 60, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid–loganic acid versus anthocyanins from the Cornus mas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef]
- Wei, S.; Chi, H.; Kodama, H.; Chen, G. Anti-inflammatory effect of three iridoids in human neutrophils. Nat. Prod. Res. 2013, 27, 911–915. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jin, G.Y.; Li, G.Z.; Yan, G.H. Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-kappa B activation in RAW 264.7 macrophages. Biol. Pharm. Bull. 2011, 34, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.G.; Moon, M.K.; Lee, A.S.; Kwon, T.O.; Kim, J.S.; Lee, H.S. Cornuside suppresses cytokine-induced proinflammatory and adhesion molecules in the human umbilical vein endothelial cells. Biol. Pharm. Bull. 2007, 30, 1796–1799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sepulveda, L.; Ascacio, A.; Rodriguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. Ellagic acid: Biological properties and biotechnological development for production processes. Afr. J. Biotechnol. 2011, 10, 4518–4523. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 288, 152–178. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apis. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Akande, O.E.; Galam, D.C.A. Total Anthocyanin Content of Strawberry and the Profile Changes by Extraction Methods and Sample Processing. Foods 2022, 11, 1072. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Page, B.; Page, M.; Noel, C. A new fluorometric assay for cytotoxicity measurements in-vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta DeltaC(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Total Phenolic Content (mg GA/100 g ± SD) | Total Flavonoid Content (mg QE/100 g ± SD) | Total Anthocyanin Content (mg CG/100 g ± SD) |
|---|---|---|
| 626.0 ± 3.2 | 35.0 ± 1.7 | 47.3 ± 0.3 |
| TR (min.) | Observed Ion Mass [M-H]-/(Fragments) | Δ ppm | Formula | Identified | Ref |
|---|---|---|---|---|---|
| 1.56 | 191.05648 | 1.92 | C7H12O6 | Quinic acid | str |
| 3.59 | 331.06855/(125, 169) | 4.46 | C13H16O10 | O-galloyl-d-glucose 1 | [13] |
| 4.48 | 169.01506/(125) | 4.78 | C7H6O5 | Gallic acid | str [13] |
| 4.96 | 633.07521 | 2.95 | C27H22O18 | Gemin D (1) | [13] |
| 5.28 | 361.07912/(125, 169) | 4.10 | C14H18O11 | 7-O-Galloyl-d-sedoheptulose 1 | [14] |
| 6.03 | 299.07843/(137) | 3.96 | C13H16O8 | Hydroxybenzoic acid hexoside | [15] |
| 7.29 | 633.07421 | 1.38 | C27H22O18 | Gemin D (2) | [13] |
| 7.97 | 153.01971 | 2.45 | C7H6O4 | Protocatechuic acid | str, [15] |
| 7.97 | 331.06801/(125, 169) | 2.83 | C13H16O10 | O-galloyl-d-glucose 1 | [13] |
| 9.37 | 483.07821 | 0.37 | C20H20O14 | 2,3-Di-galloyl glucose 1 | [13] |
| 13.19 | 375.12902 | −1.37 | C16H24O10 | Loganic acid | str [16] |
| 13.84 | 311.04087 (179, 135, 149) | 0.05 | C13H12O9 | Caftaric acid | [15] |
| 15.65 | 353.08812 (191, 179) | 0.89 | C16H18O9 | Chlorogenic acid | str, [15] |
| 16.05 | 491.14096 (375) | 0.67 | C20H28O14 | Loganic acid derivative | [15] |
| 17.75 | 447.09322 (285) | 2.31 | C21H20O11 | Cyanidin 3-O-galactoside | [16] |
| 18.01 | 431.09891 (269) | 1.25 | C21H20O10 | Pelargonidin 3-O-galactoside | [16] |
| 18.11 | 403.12490 | 0.78 | C17H24O11 | Secoxyloganin | [15] |
| 19.37 | 337.09347 (173, 191) | 1.71 | C16H18O8 | p-Coumaroylquinic acid | [15] |
| 31.13 | 301.00042 | 4.73 | C14H6O8 | Ellagic acid | str, [13] |
| 31.29 | 609.14899 (300, 463) | 4.72 | C27H30O16 | Quercetin rutoside | [15] |
| 31.81 | 477.06846 | 2.08 | C21H18O13 | Quercetin-3-O-glucuronide 2 | [15] |
| 37.77 | 541.15662 | 0.63 | C24H30O14 | Cornuside | str, [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójciak, M.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Furman-Toczek, D.; Szczepanek, D.; Sowa, I. In Vitro Evaluation of Anti-Inflammatory and Protective Potential of an Extract from Cornus mas L. Fruit against H2O2-Induced Oxidative Stress in Human Skin Keratinocytes and Fibroblasts. Int. J. Mol. Sci. 2022, 23, 13755. https://doi.org/10.3390/ijms232213755
Wójciak M, Zagórska-Dziok M, Nizioł-Łukaszewska Z, Ziemlewska A, Furman-Toczek D, Szczepanek D, Sowa I. In Vitro Evaluation of Anti-Inflammatory and Protective Potential of an Extract from Cornus mas L. Fruit against H2O2-Induced Oxidative Stress in Human Skin Keratinocytes and Fibroblasts. International Journal of Molecular Sciences. 2022; 23(22):13755. https://doi.org/10.3390/ijms232213755
Chicago/Turabian StyleWójciak, Magdalena, Martyna Zagórska-Dziok, Zofia Nizioł-Łukaszewska, Aleksandra Ziemlewska, Dominika Furman-Toczek, Dariusz Szczepanek, and Ireneusz Sowa. 2022. "In Vitro Evaluation of Anti-Inflammatory and Protective Potential of an Extract from Cornus mas L. Fruit against H2O2-Induced Oxidative Stress in Human Skin Keratinocytes and Fibroblasts" International Journal of Molecular Sciences 23, no. 22: 13755. https://doi.org/10.3390/ijms232213755
APA StyleWójciak, M., Zagórska-Dziok, M., Nizioł-Łukaszewska, Z., Ziemlewska, A., Furman-Toczek, D., Szczepanek, D., & Sowa, I. (2022). In Vitro Evaluation of Anti-Inflammatory and Protective Potential of an Extract from Cornus mas L. Fruit against H2O2-Induced Oxidative Stress in Human Skin Keratinocytes and Fibroblasts. International Journal of Molecular Sciences, 23(22), 13755. https://doi.org/10.3390/ijms232213755






