Knockdown of Tet2 Inhibits the Myogenic Differentiation of Chicken Myoblasts Induced by Ascorbic Acid
Abstract
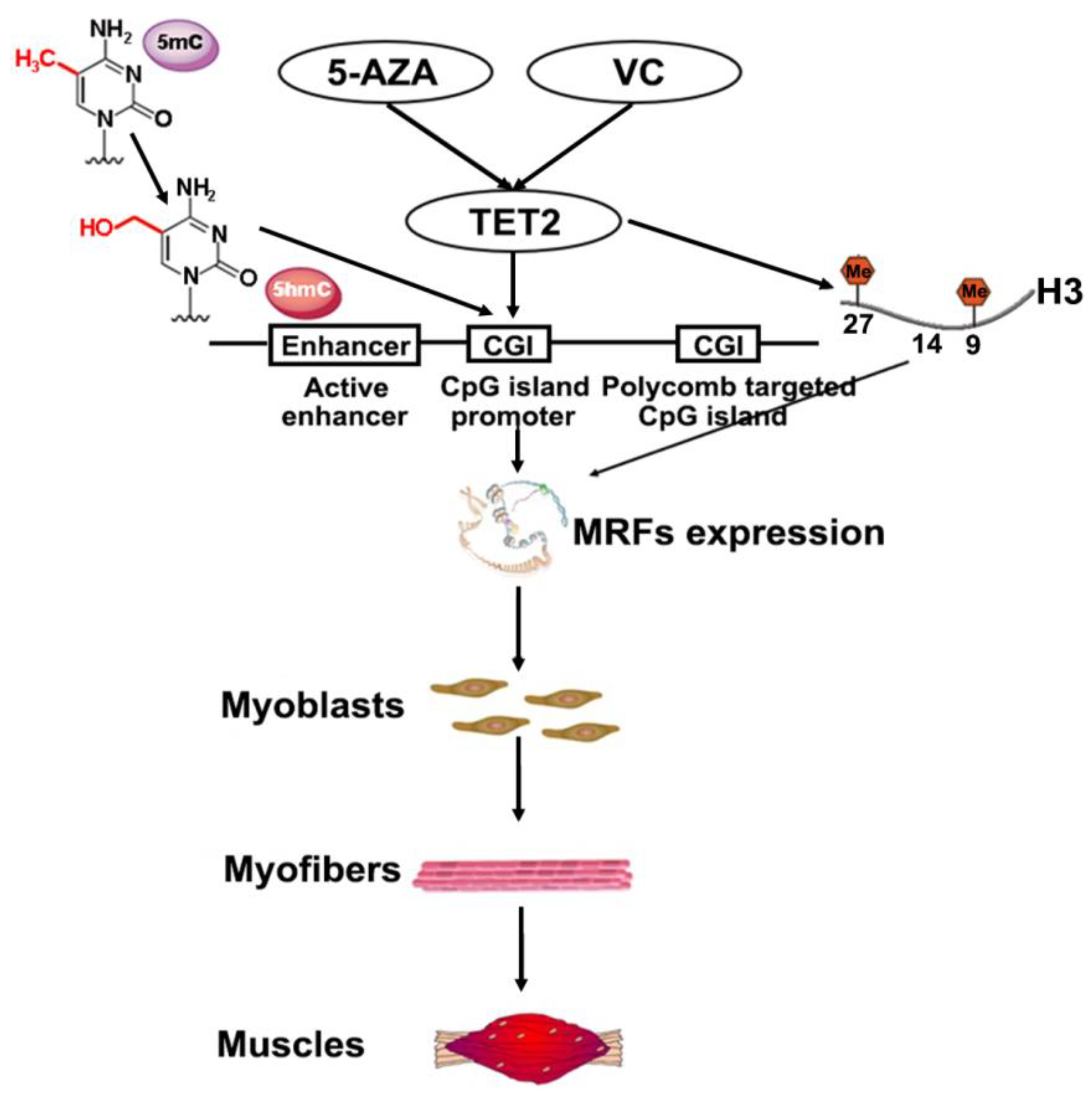
1. Introduction
2. Results
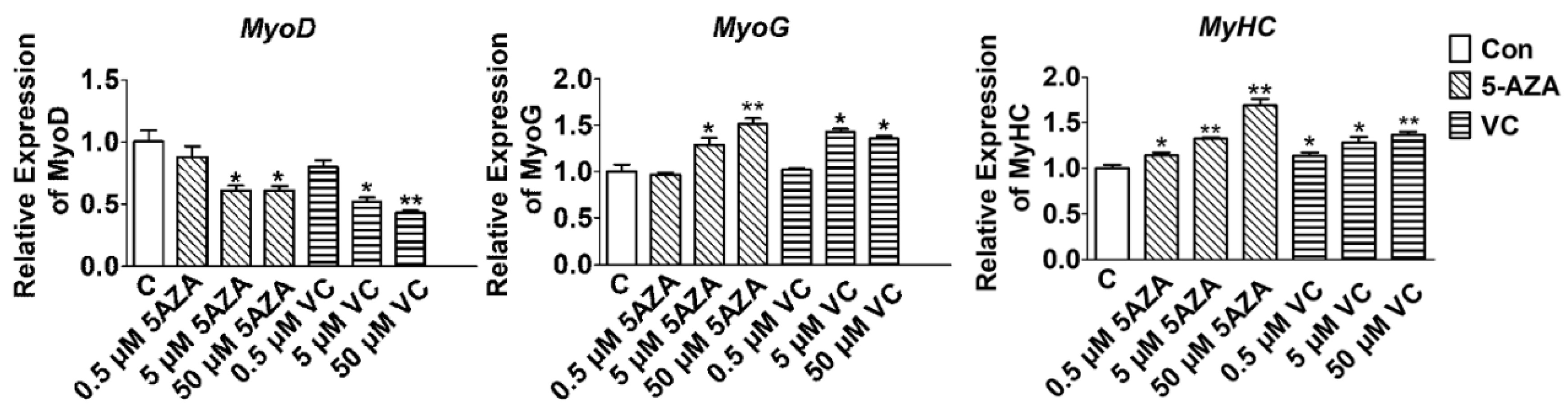
2.1. Effect of 5-AZA and VC on Myogenic Related Gene Expression
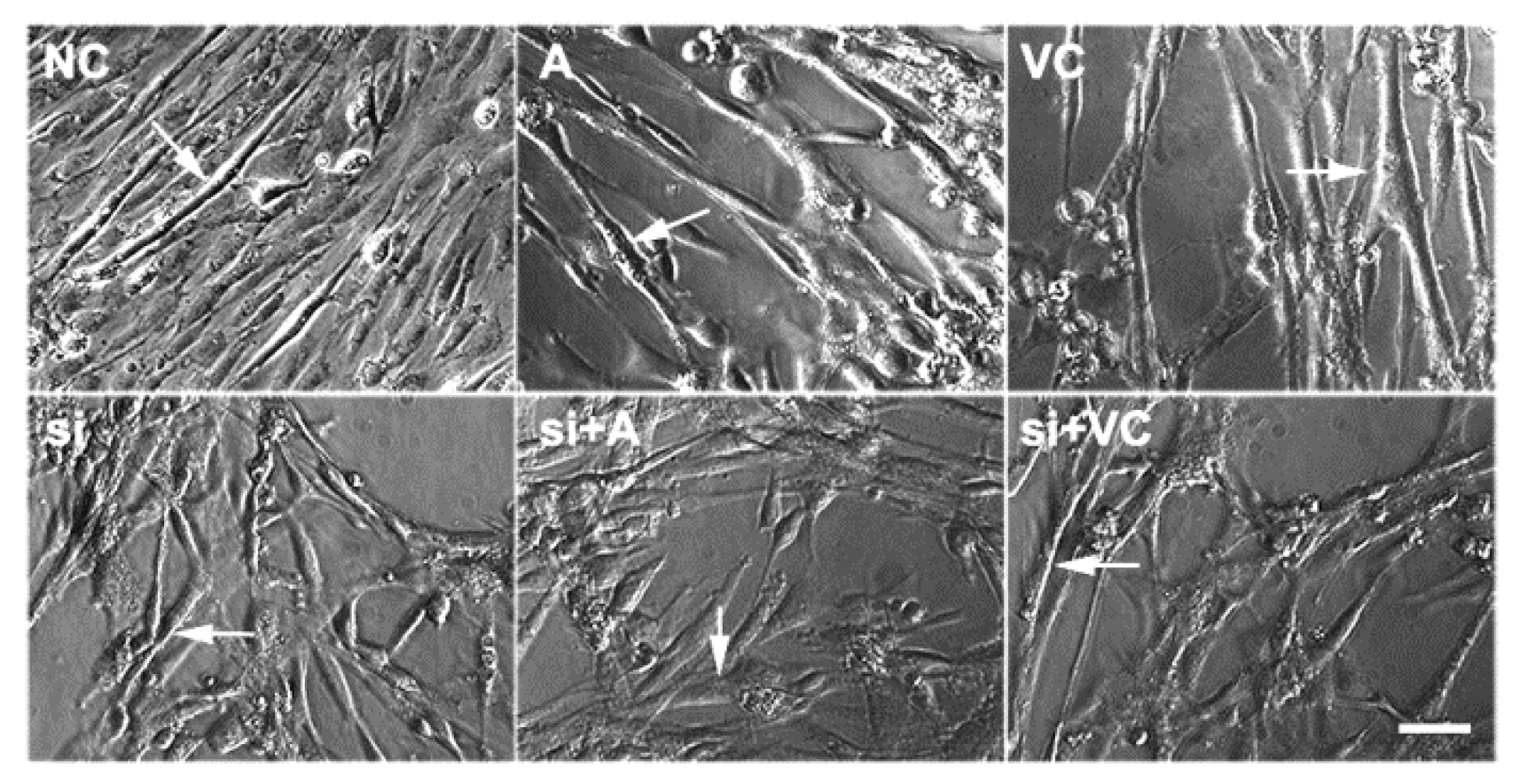
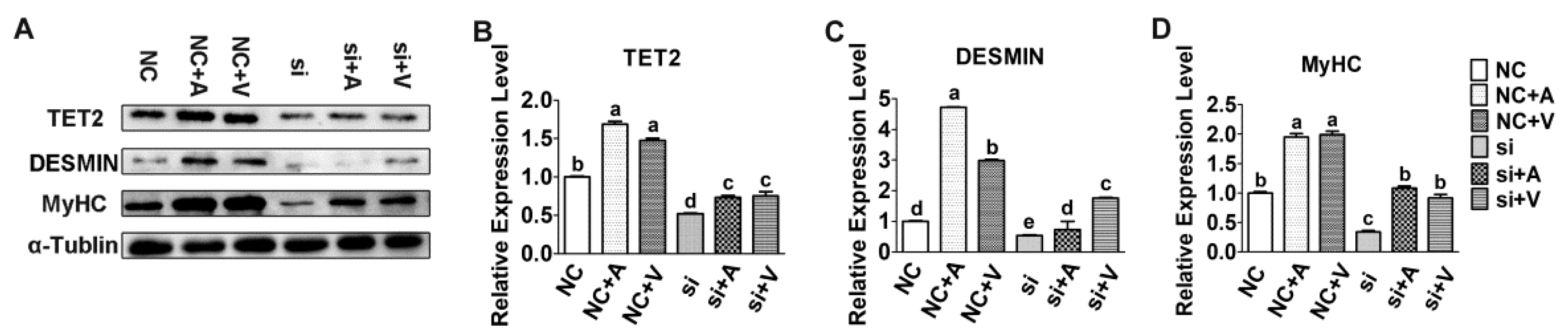
2.2. Effects of 5-AZA and VC on Myoblast Differentiation after Tet2 Knockdown
2.3. Effects of 5-AZA and VC on 5hmC Level after Tet2 Knockdown
2.4. Effects of 5-AZA and VC on H3K9me2 and H3K27me3 Level after Tet2 Knockdown
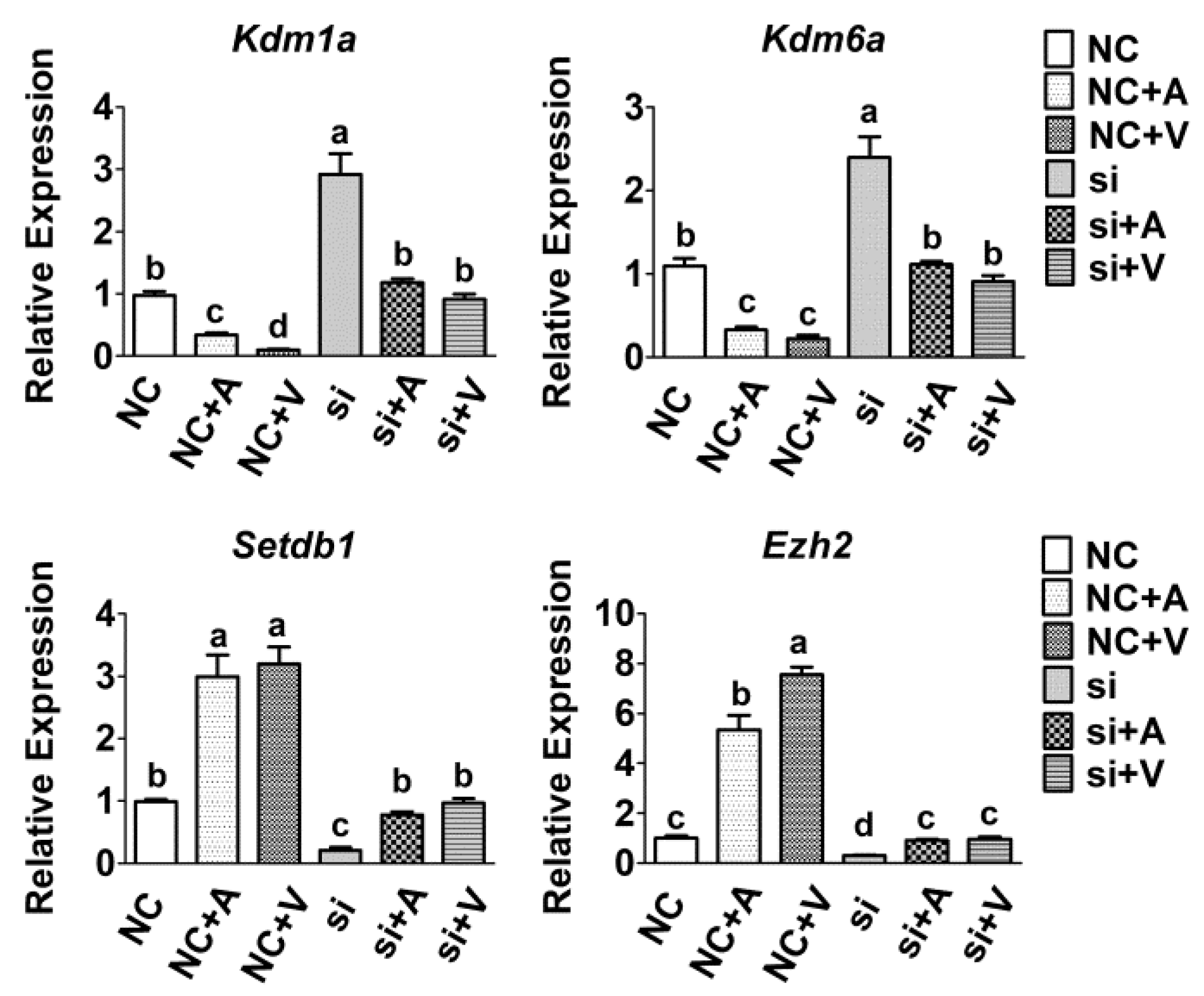
2.5. Effects of 5-AZA and VC on Histone Methyltransferases Expression after Tet2 Knockdown
3. Discussion
4. Materials and Methods
4.1. Myoblast Cell Culture
4.2. The RNA Interference Assays
4.3. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.4. Western Blotting
4.5. Immunocytochemistry
4.6. DNA Extraction and Dot Blot Assay
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Massenet, J.; Gardner, E.; Chazaud, B.; Dilworth, F.J. Epigenetic regulation of satellite cell fate during skeletal muscle regeneration. Skelet. Muscle 2021, 11, 4. [Google Scholar] [CrossRef]
- Asp, P.; Blum, R.; Vethantham, V.; Parisi, F.; Micsinai, M.; Cheng, J.; Bowman, C.; Kluger, Y.; Dynlacht, B.D. Genome-wide remodeling of the epigenetic landscape during myogenic differentiation. Proc. Natl. Acad. Sci. USA 2011, 108, E149–E158. [Google Scholar] [CrossRef]
- Tsumagari, K.; Baribault, C.; Terragni, J.; Varley, K.E.; Gertz, J.; Pradhan, S.; Badoo, M.; Crain, C.M.; Song, L.; Crawford, G.E.; et al. Early de novo DNA methylation and prolonged demethylation in the muscle lineage. Epigenetics 2013, 8, 317–332. [Google Scholar] [CrossRef]
- Perdiguero, E.; Sousa-Victor, P.; Ballestar, E.; Munoz-Canoves, P. Epigenetic regulation of myogenesis. Epigenetics 2009, 4, 541–550. [Google Scholar] [CrossRef]
- Cai, S.; Zhu, Q.; Guo, C.; Yuan, R.; Zhang, X.; Nie, Y.; Chen, L.; Fang, Y.; Chen, K.; Zhang, J.; et al. MLL1 promotes myogenesis by epigenetically regulating Myf5. Cell Prolif. 2020, 53, e12744. [Google Scholar] [CrossRef]
- Berkes, C.A.; Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell. Dev. Biol. 2005, 16, 585–595. [Google Scholar] [CrossRef]
- Chao, Z.; Zheng, X.L.; Sun, R.P.; Liu, H.L.; Huang, L.L.; Cao, Z.X.; Deng, C.Y.; Wang, F. Characterization of the Methylation Status of Pax7 and Myogenic Regulator Factors in Cell Myogenic Differentiation. Asian-Australas J. Anim. Sci. 2016, 29, 1037–1043. [Google Scholar] [CrossRef]
- Shi, K.; Lu, Y.; Chen, X.; Li, D.; Du, W.; Yu, M. Effects of Ten-Eleven Translocation-2 (Tet2) on myogenic differentiation of chicken myoblasts. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 252, 110540. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, K.; Zhu, Y.; Wang, T.; Shan, Y.; Long, B.; Li, Y.; Chen, Q.; Wang, P.; Zhao, S.; et al. Vitamin C-dependent lysine demethylase 6 (KDM6)-mediated demethylation promotes a chromatin state that supports the endothelial-to-hematopoietic transition. J. Biol. Chem. 2019, 294, 13657–13670. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, Q.Q.; Li, J.W.; Zhang, Y.M.; An, X.R.; Hou, J. Ten-Eleven Translocation-2 (Tet2) Is Involved in Myogenic Differentiation of Skeletal Myoblast Cells in Vitro. Sci. Rep. 2017, 7, 43539. [Google Scholar] [CrossRef]
- Palacios, D.; Puri, P.L. The epigenetic network regulating muscle development and regeneration. J. Cell. Physiol. 2006, 207, 1–11. [Google Scholar] [CrossRef]
- Lucarelli, M.; Fuso, A.; Strom, R.; Scarpa, S. The dynamics of myogenin site-specific demethylation is strongly correlated with its expression and with muscle differentiation. J. Biol. Chem. 2001, 276, 7500–7506. [Google Scholar] [CrossRef]
- Steffens, A.A.; Hong, G.M.; Bain, L.J. Sodium arsenite delays the differentiation of C2C12 mouse myoblast cells and alters methylation patterns on the transcription factor myogenin. Toxicol. Appl. Pharmacol. 2011, 250, 154–161. [Google Scholar] [CrossRef]
- Fuso, A.; Ferraguti, G.; Grandoni, F.; Ruggeri, R.; Scarpa, S.; Strom, R.; Lucarelli, M. Early demethylation of non-CpG, CpC-rich, elements in the myogenin 5′-flanking region: A priming effect on the spreading of active demethylation. Cell Cycle 2010, 9, 3965–3976. [Google Scholar] [CrossRef]
- Blum, R.; Dynlacht, B.D. The role of MyoD1 and histone modifications in the activation of muscle enhancers. Epigenetics 2013, 8, 778–784. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.; He, X.B.; Wulansari, N.; Yoon, B.H.; Bae, D.H.; Huh, N.; Kim, Y.S.; Lee, S.H.; Kim, S.Y. Vitamin C Promotes Astrocyte Differentiation Through DNA Hydroxymethylation. Stem. Cells 2018, 36, 1578–1588. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.; Xu, Y.; Yu, D.; Li, D.; Liu, X.; Du, W. Insulin-like growth factor-1 (IGF-1) promotes myoblast proliferation and skeletal muscle growth of embryonic chickens via the PI3K/Akt signalling pathway. Cell Biol. Int. 2015, 39, 910–922. [Google Scholar] [CrossRef]
- Montesano, A.; Luzi, L.; Senesi, P.; Terruzzi, I. Modulation of Cell Cycle Progression by 5-Azacytidine Is Associated with Early Myogenesis Induction in Murine Myoblasts. Int. J. Biol. Sci. 2013, 9, 391–402. [Google Scholar] [CrossRef]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martinez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef]
- Bhanu, N.V.; Sidoli, S.; Yuan, Z.F.; Molden, R.C.; Garcia, B.A. Regulation of proline-directed kinases and the trans-histone code H3K9me3/H4K20me3 during human myogenesis. J. Biol. Chem. 2019, 294, 8296–8308. [Google Scholar] [CrossRef]
- Faralli, H.; Wang, C.C.; Nakka, K.; Benyoucef, A.; Sebastian, S.; Zhuang, L.N.; Chu, A.; Palii, C.G.; Liu, C.Y.; Camellato, B.; et al. UTX demethylase activity is required for satellite cell-mediated muscle regeneration. J. Clin. Investig. 2016, 126, 1555–1565. [Google Scholar] [CrossRef]
- Tao, Y.; Neppl, R.L.; Huang, Z.P.; Chen, J.; Tang, R.H.; Cao, R.; Zhang, Y.; Jin, S.W.; Wang, D.Z. The histone methyltransferase Set7/9 promotes myoblast differentiation and myofibril assembly. J. Cell Biol. 2011, 194, 551–565. [Google Scholar] [CrossRef]
- Luo, D.; de Morree, A.; Boutet, S.; Quach, N.; Natu, V.; Rustagi, A.; Rando, T.A. Deltex2 represses MyoD expression and inhibits myogenic differentiation by acting as a negative regulator of Jmjd1c. Proc. Natl. Acad. Sci. USA 2017, 114, E3071–E3080. [Google Scholar] [CrossRef]
- Agarwal, N.; Hardt, T.; Brero, A.; Nowak, D.; Rothbauer, U.; Becker, A.; Leonhardt, H.; Cardoso, M.C. MeCP2 interacts with HP1 and modulates its heterochromatin association during myogenic differentiation. Nucleic Acids Res. 2007, 35, 5402–5408. [Google Scholar] [CrossRef]
- Shima, A.; Pham, J.; Blanco, E.; Barton, E.R.; Sweeney, H.L.; Matsuda, R. IGF-I and vitamin C promote myogenic differentiation of mouse and human skeletal muscle cells at low temperatures. Exp. Cell Res. 2011, 317, 356–366. [Google Scholar] [CrossRef]
- Ebata, K.T.; Mesh, K.; Liu, S.; Bilenky, M.; Fekete, A.; Acker, M.G.; Hirst, M.; Garcia, B.A.; Ramalho-Santos, M. Vitamin C induces specific demethylation of H3K9me2 in mouse embryonic stem cells via Kdm3a/b. Epigenet. Chromatin 2017, 10, 36. [Google Scholar] [CrossRef]
- Rocha, M.A.; Veronezi, G.M.B.; Felisbino, M.B.; Gatti, M.S.V.; Tamashiro, W.; Mello, M.L.S. Sodium valproate and 5-aza-2′-deoxycytidine differentially modulate DNA demethylation in G1 phase-arrested and proliferative HeLa cells. Sci. Rep. 2019, 9, 18236. [Google Scholar] [CrossRef]
- Sajadian, S.O.; Ehnert, S.; Vakilian, H.; Koutsouraki, E.; Damm, G.; Seehofer, D.; Thasler, W.; Dooley, S.; Baharvand, H.; Sipos, B.; et al. Induction of active demethylation and 5hmC formation by 5-azacytidine is TET2 dependent and suggests new treatment strategies against hepatocellular carcinoma. Clin. Epigenet. 2015, 7, 98. [Google Scholar] [CrossRef]


| Gene | Accession No. | Primer Sequence (5′-3′) | Size (bp) |
|---|---|---|---|
| Gapdh | NM_204305.1 | F: CTGTTGTTGACCTGACCTGC | 166 |
| R:TCAAAGGTGGAGGAAATGGCT | |||
| MyoD | NM_204214 | F: ATGTCCCATACTGCCTCCAG | 235 |
| R: GTCTTGGAGCTTGGCTGAAC | |||
| MyoG | NM_204184 | F: GGCTTTGGAGGAGAAGGACT | 184 |
| R: CAGAGTGCTGCGTTTCAGAG | |||
| MyHC | NM_001044683 | F: GCTTGAACACACTGCAGGAA | 236 |
| R: CTTCAGCCCCTCAGCATAAC | |||
| Setdb1 | XM_040690848 | F: ATCTGAAGGTTGGCATGAGG | 170 |
| R: GGGGTGGTAGTCGTAAGCAA | |||
| Ezh2 | XM_046912581 | F: AGCAAAAAGATCGGGAAGGT | 169 |
| R: GCTCCTGGAAGTTGCTGTTC | |||
| Kdm1a | XM_417719 | F: GCATTTTGCAAAACCTGGAAT | 219 |
| R: GCAATCACTTCACAGCCTGA | |||
| Kdm6a | XM_040662413 | F: ATGGAAACGTGCCTTACCTG | 151 |
| R: GGACCTGCCAAATGTGAACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Shi, K.; Wang, H.; Cao, H.; Li, F.; Zhou, J.; Yu, M.; Yu, D. Knockdown of Tet2 Inhibits the Myogenic Differentiation of Chicken Myoblasts Induced by Ascorbic Acid. Int. J. Mol. Sci. 2022, 23, 13758. https://doi.org/10.3390/ijms232213758
Lu Y, Shi K, Wang H, Cao H, Li F, Zhou J, Yu M, Yu D. Knockdown of Tet2 Inhibits the Myogenic Differentiation of Chicken Myoblasts Induced by Ascorbic Acid. International Journal of Molecular Sciences. 2022; 23(22):13758. https://doi.org/10.3390/ijms232213758
Chicago/Turabian StyleLu, Yinglin, Kai Shi, Haobin Wang, Heng Cao, Fan Li, Jing Zhou, Minli Yu, and Debing Yu. 2022. "Knockdown of Tet2 Inhibits the Myogenic Differentiation of Chicken Myoblasts Induced by Ascorbic Acid" International Journal of Molecular Sciences 23, no. 22: 13758. https://doi.org/10.3390/ijms232213758
APA StyleLu, Y., Shi, K., Wang, H., Cao, H., Li, F., Zhou, J., Yu, M., & Yu, D. (2022). Knockdown of Tet2 Inhibits the Myogenic Differentiation of Chicken Myoblasts Induced by Ascorbic Acid. International Journal of Molecular Sciences, 23(22), 13758. https://doi.org/10.3390/ijms232213758







