New Angucycline Glycosides from a Marine-Derived Bacterium Streptomyces ardesiacus
Abstract
:1. Introduction
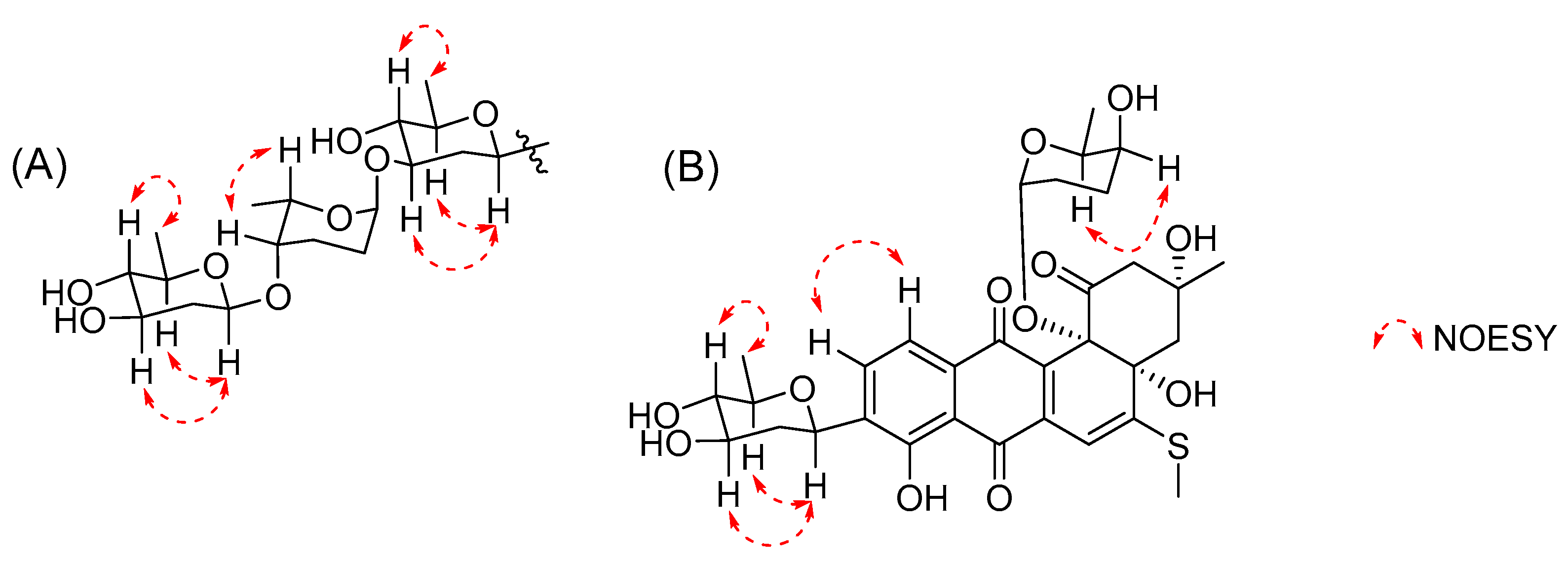
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Bacterial Strain, Fermentation, and Isolation of 1–10 from Streptomyces ardesiacus 156VN-095
3.3. Antibacterial Assay
3.4. Cytotoxicity Test by SRB Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quinn, G.A.; Banat, A.M.; Abdelhameed, A.M.; Banat, I.M. Streptomyces from traditional medicine: Sources of new innovations in antibiotic discovery. J. Med. Microbiol. 2020, 69, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Mast, Y.; Stegmann, E. Actinomycetes: The Antibiotics Producers. Antibiotics 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Xiaohanyao, Y.; Zhou, T.; Nakajima-Shimada, J.; Tashiro, E.; Triningsih, D.W.; Harunari, E.; Oku, N.; Igarashi, Y. Phytohabitols A–C, δ-Lactone-Terminated Polyketides from an Actinomycete of the Genus Phytohabitans. J. Nat. Prod. 2022, 85, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Nuzillard, J.-M.; Renault, J.-H. Dereplication strategies in natural product research: How many tools and methodologies behind the same concept? Phytochem. Rev. 2017, 16, 55–95. [Google Scholar] [CrossRef]
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How different are marine microbial natural products compared to their terrestrial counterparts? Nat. Prod. Rep. 2021, 39, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of Polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, H.J.; Rutledge, P.J. Recently Discovered Secondary Metabolites from Streptomyces Species. Molecules 2022, 27, 887. [Google Scholar] [CrossRef] [PubMed]
- Kharel, M.K.; Pahari, P.; Shepherd, M.D.; Tibrewal, N.; Nybo, S.E.; Shaaban, K.A.; Rohr, J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012, 29, 264–325. [Google Scholar] [CrossRef] [PubMed]
- Hulst, M.B.; Grocholski, T.; Neefjes, J.J.C.; van Wezel, G.P.; Metsä-Ketelä, M. Anthracyclines: Biosynthesis, engineering and clinical applications. Nat. Prod. Rep. 2022, 39, 814–841. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Nie, Q.-Y.; Pan, H.-X.; Tang, G.-L. 1.07-Bacterial Type II Polyketide Synthases. In Comprehensive Natural Products III; Liu, H.-W., Begley, T.P., Eds.; Elsevier: Oxford, UK, 2020; Volume 1, pp. 198–249. [Google Scholar] [CrossRef]
- Rohr, J.; Thiericke, R. Angucycline group antibiotics. Nat. Prod. Rep. 1992, 9, 103–137. [Google Scholar] [CrossRef] [PubMed]
- Dan, V.M.; Vinodh, J.S.; Sandesh, C.J.; Sanawar, R.; Lekshmi, A.; Kumar, R.A.; Santhosh Kumar, T.R.; Marelli, U.K.; Dastager, S.G.; Pillai, M.R. Molecular networking and whole-genome analysis aid discovery of an angucycline that inactivates mTORC1/C2 and induces programmed cell death. ACS Chem. Biol. 2020, 15, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Drautz, H.; Zähner, H.; Rohr, J.; Zeeck, A. Metabolic products of microorganisms. 234. Urdamycins, new angucycline antibiotics from Streptomyces fradiae. I. Isolation, characterization and biological properties. J. Antibiot. 1986, 39, 1657–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohr, J.; Zeeck, A. Metabolic products of microorganisms. 240 Urdamycins, new angucycline antibiotics from Streptomyces fradiae. II Structural studies of urdamycins B to F. J. Antibiot. 1987, 40, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hou, L.K.; Li, H.Y.; Li, W.L. Antibiotic angucycline derivatives from the deepsea-derived Streptomyces lusitanus. Nat. Prod. Res. 2020, 34, 3444–3450. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Ferris, Z.E.; Sweeney, D.; Chase, A.B.; Yuan, C.; Hui, Y.; Hou, L.; Older, E.A.; Xue, D.; Tang, X.; et al. Grincamycins P–T: Rearranged Angucyclines from the Marine Sediment-Derived Streptomyces sp. CNZ-748 Inhibit Cell Lines of the Rare Cancer Pseudomyxoma Peritonei. J. Nat. Prod. 2021, 84, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.; Schoenewolf, M.; Udvarnoki, G.; Eckardt, K.; Schumann, G.; Wagner, C.; Beale, J.M.; Sorey, S.D. Investigations on the biosynthesis of the angucycline group antibiotics aquayamycin and the urdamycins A and B. Results from the structural analysis of novel blocked mutant products. J. Org. Chem. 1993, 58, 2547–2551. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-K.; Trinh, P.T.H.; Lee, H.-S.; Choi, B.-W.; Kang, J.S.; Ngoc, N.T.D.; Van, T.T.T.; Shin, H.J. New Ophiobolin Derivatives from the Marine Fungus Aspergillus flocculosus and Their Cytotoxicities against Cancer Cells. Mar. Drugs 2019, 17, 346. [Google Scholar] [CrossRef] [PubMed]



| MIC (µg/mL) | |||
|---|---|---|---|
| B. subtilis KCTC 1021 | Micrococcus luteus KCTC 1915 | Staphylococcus aureus KCTC 1927 | |
| 1 | 8.0 | 64.0 | 32.0 |
| 2 | >128 | 64.0 | 64.0 |
| 9 | 32.0 | >128 | >128 |
| Kanamycin | 0.25 | 1.0 | 0.5 |
| Compounds | 1 | 2 | 3 | 9 | Adr. |
|---|---|---|---|---|---|
| ACHN | 0.104 ± 0.012 | 0.093 ± 0.004 | 0.060 ± 0.001 | 3.422 ± 0.357 | 0.140 ± 0.009 |
| HCT-15 | 0.075 ± 0.012 | 0.150 ± 0.015 | 0.095 ± 0.037 | 3.886 ± 0.351 | 0.162 ± 0.012 |
| MDA-MB-231 | 0.033 ± 0.008 | 0.077 ± 0.017 | 0.093 ± 0.005 | 3.500 ± 0.472 | 0.162 ± 0.000 |
| NCI-H23 | 0.031 ± 0.002 | 0.050 ± 0.004 | 0.036 ± 0.002 | 3.245 ± 0.179 | 0.145 ± 0.003 |
| NUGC-3 | 0.019 ± 0.003 | 0.028 ± 0.006 | 0.030 ± 0.006 | 3.037 ± 0.045 | 0.151 ± 0.014 |
| PC-3 | 0.022 ± 0.006 | 0.103 ± 0.002 | 0.062 ± 0.012 | 2.750 ± 0.344 | 0.148 ± 0.005 |
| 1 a | 2 a | 9 b | ||||||
|---|---|---|---|---|---|---|---|---|
| Pos. | δH, Mult (J in Hz) | δC | Pos. | δH, Mult (J in Hz) | δC | Pos. | δH, Mult (J in Hz) | δC |
| 1 | 206.6 | 1 | 204.7 | 1 | 175.7 | |||
| 2 | 2.85, d (13.0) 2.66, d (13.3) | 53.3 | 2 | 2.78, d (13.1) 2.56, dd (13.1, 2.6) | 55.0 | 2 | 3.00, m | 47.0 |
| 3 | 77.3 | 3 | 76.8 | 3 | 72.4 | |||
| 4 | 2.13, s | 46.6 | 4 | 2.21, d (14.9) 2.06, dd (15.0, 2.6) | 46.1 | 4 | 3.44, m | 41.4 |
| 4a | 84.2 | 4a | 84.7 | 4a | 136.6 | |||
| 5 | 165.8 | 5 | 165.8 | 5 | 8.03, d (7.6) | 140.6 | ||
| 6 | 6.42, s | 106.5 | 6 | 6.49, s | 106.0 | 6 | 7.88, d (7.6) | 119.3 |
| 6a | 139.6 | 6a | 138.7 | 6a | 132.3 | |||
| 7 | 190.1 | 7 | 190.3 | 7 | 188.9 | |||
| 7a | 115.3 | 7a | 115.5 | 7a | 116.3 | |||
| 8 | 158.7 | 8 | 158.7 | 8 | 159.5 | |||
| 9 | 138.5 | 9 | 138.6 | 9 | 139.4 | |||
| 10 | 7.73, d (7.6) | 134.4 | 10 | 7.84, d (7.8) | 134.4 | 10 | 8.09, d (7.8) | 134.1 |
| 11 | 7.42, d (6.8) | 119.7 | 11 | 7.57, d (7.8) | 120.1 | 11 | 7.98, d (7.8) | 119.7 |
| 11a | 132.3 | 11a | 132.6 | 11a | 132.7 | |||
| 12 | 183.1 | 12 | 183.9 | 12 | 188.8 | |||
| 12a | 134.7 | 12a | 135.4 | 12a | 116.3 | |||
| 12b | 79.5 | 12b | 83.5 | 12b | 162.3 | |||
| 13 | 1.24, s | 30.3 | 13 | 1.21, s | 30.0 | 13 | 1.69, s | 28.1 |
| 14 | 2.49, s | 14.6 | 14 | 2.48, s | 14.6 | 14 | ||
| 1′ | 4.75, d (11.1) | 72.3 | 1′ | 4.89, d (11.0) | 72.4 | 1′ | 5.11, d (11.2) | 72.1 |
| 2′ | 2.45, m 1.22, m | 37.6 | 2′ | 2.40, dd (12.7, 4.7) 1.38, m | 41.1 | 2′ | 2.81, dd (12.0, 3.1) 1.61, m | 37.7 |
| 3′ | 3.73, m | 77.8 | 3′ | 3.69, m | 73.6 | 3′ | 4.21, m | 78.2 |
| 4′ | 3.12, t (8.9) | 76.8 | 4′ | 3.03, t (8.9) | 78.8 | 4′ | 3.64, m | 76.4 |
| 5′ | 3.45, m | 77.7 | 5′ | 3.44, dq (12.2, 6.1) | 77.8 | 5′ | 3.81, m | 77.8 |
| 6′ | 1.38, d (5.9) | 18.9 | 6′ | 1.37, d (6.2) | 18.6 | 6′ | 1.69, d (6.3) | 19.4 |
| 1″ | 4.96, s | 95.2 | 1b | 5.28, s | 95.7 | 1″ | 5.27, s | 95.1 |
| 2″ | 2.05, m 1.44, m | 25.6 | 2b | 1.86, m | 24.2 | 2″ | 2.38, m 2.25, m | 25.7 |
| 3″ | 2.05, m 1.94, m | 25.4 | 3b | 2.01, m 1.58, dd (2.5, 13.2) | 26.5 | 3″ | 2.25, m 1.62, m | 25.7 |
| 4″ | 3.55, s | 77.7 | 4b | 3.35, s | 67.8 | 4″ | 3.67, s | 76.9 |
| 5″ | 4.24, q (6.4) | 67.8 | 5b | 3.64, q (6.5) | 68.3 | 5″ | 4.67, q (6.0) | 67.3 |
| 6″ | 1.17, d (6.4) | 17.4 | 6b | 0.55, d (6.6) | 17.0 | 6″ | 1.38, d (6.4) | 17.9 |
| 1‴ | 4.57, d (9.6) | 102.8 | 1‴ | 4.80, d (9.6) | 102.9 | |||
| 2‴ | 2.20, dd (12.3, 4.9) 1.55, m | 40.6 | 2‴ | 2.66, dd (12.1, 4.3) 2.17, m | 41.4 | |||
| 3‴ | 3.50, m | 72.3 | 3‴ | 4.08, m | 72.5 | |||
| 4‴ | 2.91, t (9.0) | 78.4 | 4‴ | 3.56, t (8.7) | 78.9 | |||
| 5‴ | 3.23, dq (12.5, 6.1) | 73.2 | 5‴ | 3.64, m | 73.4 | |||
| 6‴ | 1.26, d (6.0) | 18.4 | 6‴ | 1.61, d (6.1) | 19.2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anh, C.V.; Kwon, J.-H.; Kang, J.S.; Lee, H.-S.; Heo, C.-S.; Shin, H.J. New Angucycline Glycosides from a Marine-Derived Bacterium Streptomyces ardesiacus. Int. J. Mol. Sci. 2022, 23, 13779. https://doi.org/10.3390/ijms232213779
Anh CV, Kwon J-H, Kang JS, Lee H-S, Heo C-S, Shin HJ. New Angucycline Glycosides from a Marine-Derived Bacterium Streptomyces ardesiacus. International Journal of Molecular Sciences. 2022; 23(22):13779. https://doi.org/10.3390/ijms232213779
Chicago/Turabian StyleAnh, Cao Van, Joo-Hee Kwon, Jong Soon Kang, Hwa-Sun Lee, Chang-Su Heo, and Hee Jae Shin. 2022. "New Angucycline Glycosides from a Marine-Derived Bacterium Streptomyces ardesiacus" International Journal of Molecular Sciences 23, no. 22: 13779. https://doi.org/10.3390/ijms232213779






