Pluripotent Stem Cells in Clinical Cell Transplantation: Focusing on Induced Pluripotent Stem Cell-Derived RPE Cell Therapy in Age-Related Macular Degeneration
Abstract
1. Introduction
2. Updates on Cell Therapy and PSC-Based Clinical Trials
3. From ESC to iPSC: Past and Ongoing iPSC- and ESC-Based Clinical Trials
4. RPE Physiology and Therapeutic Options for AMD
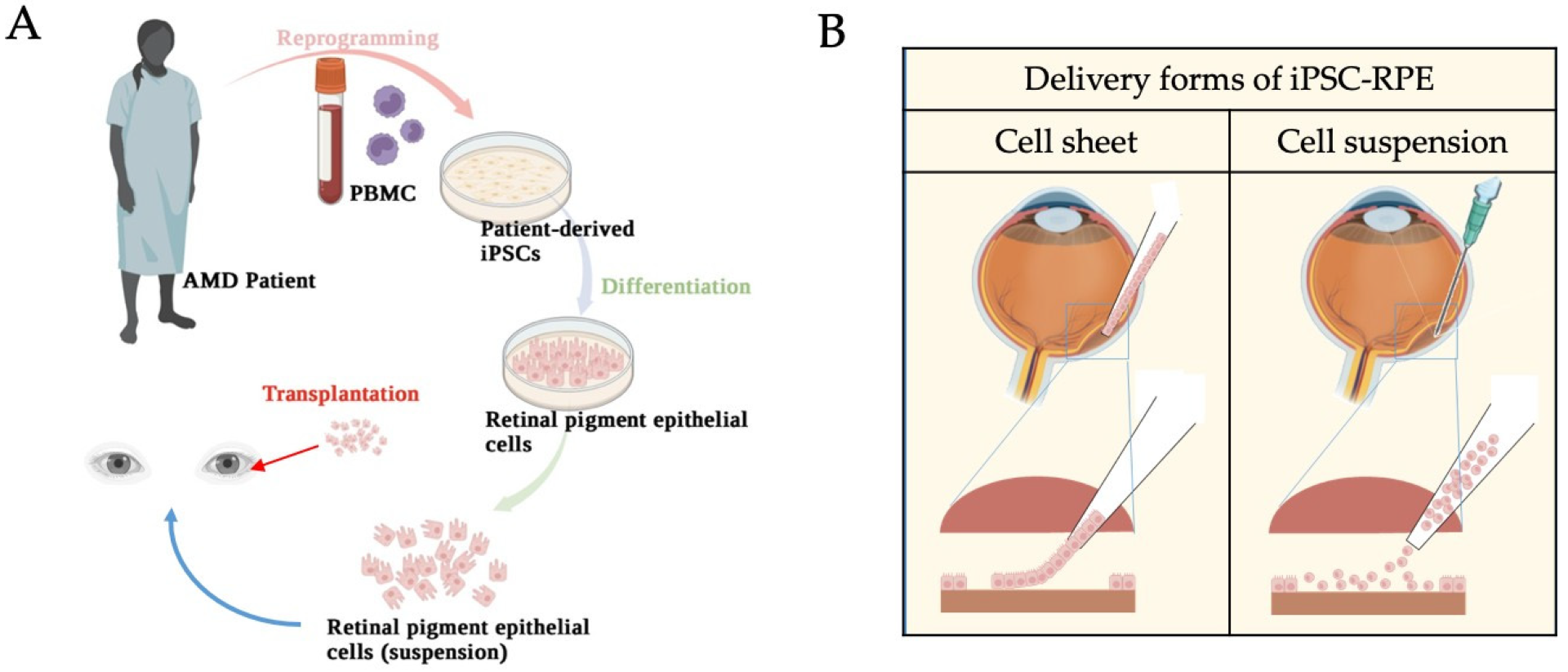
5. Application of iPSC-Derived RPEs in Disease Modeling and Cell Therapy
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Venugopalan, P.; Wang, Y.; Nguyen, T.; Huang, A.; Muller, K.J.; Goldberg, J.L. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 2016, 7, 10472. [Google Scholar] [CrossRef] [PubMed]
- Jindal, N.; Banik, A.; Prabhakar, S.; Vaiphie, K.; Anand, A. Alteration of Neurotrophic Factors After Transplantation of Bone Marrow Derived Lin-ve Stem Cell in NMDA-Induced Mouse Model of Retinal Degeneration. J. Cell. Biochem. 2017, 118, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Mesentier-Louro, L.A.; Teixeira-Pinheiro, L.C.; Gubert, F.; Vasques, J.F.; Silva-Junior, A.J.; Chimeli-Ormonde, L.; Nascimento-Dos-Santos, G.; Mendez-Otero, R.; Santiago, M.F. Long-term neuronal survival, regeneration, and transient target reconnection after optic nerve crush and mesenchymal stem cell transplantation. Stem Cell Res. Ther. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.-P.; Ng, T.K.; Liang, J.-J.; Zhuang, X.; Yao, X.; Yam, G.H.-F.; Chen, H.; Cheung, H.S.; Zhang, M.; Pang, C.P. Human Periodontal Ligament-Derived Stem Cells Promote Retinal Ganglion Cell Survival and Axon Regeneration After Optic Nerve Injury. Stem Cells 2018, 36, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Divya, M.S.; Rasheed, V.A.; Schmidt, T.; Lalitha, S.; Hattar, S.; James, J. Intraocular Injection of ES Cell-Derived Neural Progenitors Improve Visual Function in Retinal Ganglion Cell-Depleted Mouse Models. Front. Cell. Neurosci. 2017, 11, 295. [Google Scholar] [CrossRef]
- Wu, Y.-R.; Hashiguchi, T.; Sho, J.; Chiou, S.-H.; Takahashi, M.; Mandai, M. Transplanted Mouse Embryonic Stem Cell–Derived Retinal Ganglion Cells Integrate and Form Synapses in a Retinal Ganglion Cell-Depleted Mouse Model. Investig. Opthalmology Vis. Sci. 2021, 62, 26. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Esposito, M.T. Hematopoietic stem cells meet induced pluripotent stem cells technology. Haematologica 2016, 101, 999–1001. [Google Scholar] [CrossRef]
- Miyagawa, S.; Kainuma, S.; Kawamura, T.; Suzuki, K.; Ito, Y.; Iseoka, H.; Ito, E.; Takeda, M.; Sasai, M.; Mochizuki-Oda, N.; et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 950829. [Google Scholar] [CrossRef]
- Takahashi, J. iPS cell-based therapy for Parkinson’s disease: A Kyoto trial. Regen. Ther. 2020, 13, 18–22. [Google Scholar] [CrossRef]
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 2021, 18, 321–333. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Soma, Y.; Nakajima, K.; Kanazawa, H.; Tohyama, S.; Tabei, R.; Hirano, A.; Handa, N.; Yamada, Y.; Okuda, S.; et al. Intramyocardial Transplantation of Human iPS Cell–Derived Cardiac Spheroids Improves Cardiac Function in Heart Failure Animals. JACC Basic Transl. Sci. 2021, 6, 239–254. [Google Scholar] [CrossRef]
- Silver, S.E.; Barrs, R.W.; Mei, Y. Transplantation of Human Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Regenerative Therapy. Front. Cardiovasc. Med. 2021, 8, 707890. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Lu, B.; Malcuit, C.; Wang, S.; Girman, S.; Francis, P.; Lemieux, L.; Lanza, R.; Lund, R. Long-Term Safety and Function of RPE from Human Embryonic Stem Cells in Preclinical Models of Macular Degeneration. Stem Cells 2009, 27, 2126–2135. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Hubschman, J.-P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; Ingram, A.; Dang, W.; et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic RPE cell bioengineered implant for advanced dry age-related macular degeneration. Transl. Vis. Sci. Technol. 2021, 10, 13. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Takagi, S.; Mandai, M.; Gocho, K.; Hirami, Y.; Yamamoto, M.; Fujihara, M.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol. Retin. 2019, 3, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.J.; Hazen, J.L.; Nazor, K.L.; Rodriguez, A.R.; Gifford, W.; Martin, G.; Kupriyanov, S.; Baldwin, K.K. Adult mice generated from induced pluripotent stem cells. Nature 2009, 461, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Wang, J.; Zhang, Y.; Kou, Z.; Gao, S. iPS Cells Can Support Full-Term Development of Tetraploid Blastocyst-Complemented Embryos. Cell Stem Cell 2009, 5, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Li, X.; Fan, X.; Yang, D.; Li, L.; Chen, S.; Gu, Q.; Le, W. Retinal pigment epithelial cells secrete neurotrophic factors and synthesize dopamine: Possible contribution to therapeutic effects of RPE cell transplantation in Parkinson’s disease. J. Transl. Med. 2009, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Becerra, S.P.; Fariss, R.N.; Wu, Y.Q.; Montuenga, L.M.; Wong, P.; Pfeffer, B.A. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: Apical secretion and distribution. Exp. Eye Res. 2004, 78, 223–234. [Google Scholar] [CrossRef]
- Otani, A.; Dorrell, M.I.; Kinder, K.; Moreno, S.K.; Nusinowitz, S.; Banin, E.; Heckenlively, J.; Friedlander, M. Rescue of retinal degeneration by intravitreally injected adult bone marrow–derived lineage-negative hematopoietic stem cells. J. Clin. Investig. 2004, 114, 765–774. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.-F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- CATT Research Group; Martin, D.F.; Maguire, M.G.; Ying, G.-S.; E Grunwald, J.; Fine, S.L.; Jaffe, G.J. Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef]
- Hobbs, S.D.; Pierce, K. Wet Age-related Macular Degeneration (Wet AMD). In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tarallo, V.; Hirano, Y.; Gelfand, B.D.; Dridi, S.; Kerur, N.; Kim, Y.; Gil Cho, W.; Kaneko, H.; Fowler, B.J.; Bogdanovich, S.; et al. DICER1 Loss and Alu RNA Induce Age-Related Macular Degeneration via the NLRP3 Inflammasome and MyD88. Cell 2012, 149, 847–859. [Google Scholar] [CrossRef]
- Ufret-Vincenty, R.L.; Aredo, B.; Liu, X.; McMahon, A.; Chen, P.W.; Sun, H.; Niederkorn, J.Y.; Kedzierski, W. Transgenic Mice Expressing Variants of Complement Factor H Develop AMD-like Retinal Findings. Investig. Opthalmol. Vis. Sci. 2010, 51, 5878–5887. [Google Scholar] [CrossRef]
- Kozhevnikova, O.S.; Korbolina, E.E.; Stefanova, N.A.; Muraleva, N.A.; Orlov, Y.L.; Kolosova, N.G. Association of AMD-like retinopathy development with an Alzheimer’s disease metabolic pathway in OXYS rats. Biogerontology 2013, 14, 753–762. [Google Scholar] [CrossRef]
- Zahn, G.; Vossmeyer, D.; Stragies, R.; Wills, M.; Wong, C.G.; Löffler, K.U.; Adamis, A.P.; Knolle, J. Preclinical evaluation of the novel small-molecule integrin alpha5beta1 inhibitor JSM6427 in monkey and rabbit models of choroidal neovascularization. Arch. Ophthalmol. 2009, 127, 1329–1335. [Google Scholar] [CrossRef]
- Schreiter, S.; Vafia, K.; Barsacchi, R.; Tsang, S.H.; Bickle, M.; Ader, M.; Karl, M.O.; Tanaka, E.M.; Almedawar, S. A Human Retinal Pigment Epithelium-Based Screening Platform Reveals Inducers of Photoreceptor Outer Segments Phagocytosis. Stem Cell Rep. 2020, 15, 1347–1361. [Google Scholar] [CrossRef]
- Guo, F.; Ding, Y.; Caberoy, N.; Alvarado, G.; Wang, F.; Chen, R.; Li, W. ABCF1 extrinsically regulates retinal pigment epithelial cell phagocytosis. Mol. Biol. Cell 2015, 26, 2311–2320. [Google Scholar] [CrossRef]
- Wang, Z.; Dillon, J.; Gaillard, E.R. Antioxidant Properties of Melanin in Retinal Pigment Epithelial Cells. Photochem. Photobiol. 2006, 82, 474–479. [Google Scholar] [CrossRef]
- Machida, S.; Chaudhry, P.; Shinohara, T.; Singh, D.P.; Reddy, V.N.; Chylack, L.T.; A Sieving, P.; A Bush, R. Lens epithelium-derived growth factor promotes photoreceptor survival in light-damaged and RCS rats. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1087–1095. [Google Scholar]
- Mahendra, C.K.; Tan, L.T.H.; Pusparajah, P.; Htar, T.T.; Chuah, L.-H.; Lee, V.S.; Low, L.E.; Tang, S.Y.; Chan, K.-G.; Goh, B.H. Detrimental Effects of UVB on Retinal Pigment Epithelial Cells and Its Role in Age-Related Macular Degeneration. Oxidative Med. Cell. Longev. 2020, 2020, 1904178. [Google Scholar] [CrossRef]
- Carr, A.J.; Vugler, A.A.; Hikita, S.T.; Lawrence, J.M.; Gias, C.; Chen, L.L.; Buchholz, D.E.; Ahmado, A.; Semo, M.; Smart, M.J.K.; et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS ONE 2009, 4, e8152. [Google Scholar] [CrossRef]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kamao, H.; Mandai, M.; Shiina, T.; Ogasawara, K.; Hirami, Y.; Kurimoto, Y.; Takahashi, M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Rep. 2016, 7, 635–648. [Google Scholar] [CrossRef]
- Fisher, C.R.; Ebeling, M.C.; Geng, Z.; Kapphahn, R.J.; Roehrich, H.; Montezuma, S.R.; Dutton, J.R.; Ferrington, D.A. Human iPSC- and Primary-Retinal Pigment Epithelial Cells for Modeling Age-Related Macular Degeneration. Antioxidants 2022, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, N.; Chu, Y.; Cheng, S.K.; Cao, H.; Poliakov, E.; Berinstein, D.M. Repressed SIRT1/PGC-1alpha pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. J. Transl. Med. 2016, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Walsh, P.J.; Truong, V.; Hill, C.; Ebeling, M.; Kapphahn, R.J.; Montezuma, S.R.; Yuan, C.; Roehrich, H.; Ferrington, D.A.; et al. Generation of retinal pigmented epithelium from iPSCs derived from the conjunctiva of donors with and without age related macular degeneration. PLoS ONE 2017, 12, e0173575. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, M.C.; Geng, Z.; Stahl, M.R.; Kapphahn, R.J.; Roehrich, H.; Montezuma, S.R.; Ferrington, D.A.; Dutton, J.R. Testing Mitochondrial-Targeted Drugs in iPSC-RPE from Patients with Age-Related Macular Degeneration. Pharmaceuticals 2022, 15, 62. [Google Scholar] [CrossRef]
- Galloway, C.A.; Dalvi, S.; Hung, S.S.C.; MacDonald, L.A.; Latchney, L.R.; Wong, R.C.B.; Guymer, R.H.; Mackey, D.A.; Williams, D.S.; Chung, M.M.; et al. Drusen in patient-derived hiPSC-RPE models of macular dystrophies. Proc. Natl. Acad. Sci. USA 2017, 114, e8214–e8223. [Google Scholar] [CrossRef]
- Saini, J.S.; Corneo, B.; Miller, J.D.; Kiehl, T.R.; Wang, Q.; Boles, N.C.; Blenkinsop, T.A.; Stern, J.H.; Temple, S. Nicotinamide Ameliorates Disease Phenotypes in a Human iPSC Model of Age-Related Macular Degeneration. Cell Stem Cell 2017, 20, 635.e7–647.e7. [Google Scholar] [CrossRef]
- Ebeling, M.C.; Geng, Z.; Kapphahn, R.J.; Roehrich, H.; Montezuma, S.R.; Dutton, J.R.; Ferrington, D.A. Impaired Mitochondrial Function in iPSC-Retinal Pigment Epithelium with the Complement Factor H Polymorphism for Age-Related Macular Degeneration. Cells 2021, 10, 789. [Google Scholar] [CrossRef]
- Shi, P.; Tan, Y.S.E.; Yeong, W.Y.; Li, H.Y.; Laude, A. A bilayer photoreceptor-retinal tissue model with gradient cell density design: A study of microvalve-based bioprinting. J. Tissue Eng. Regen. Med. 2018, 12, 1297–1306. [Google Scholar] [CrossRef]
- Kim, S.; Cho, A.-N.; Min, S.; Kim, S.; Cho, S.-W. Organoids for Advanced Therapeutics and Disease Models. Adv. Ther. 2019, 2, 1800087. [Google Scholar] [CrossRef]
- Kratochvil, M.J.; Seymour, A.J.; Li, T.L.; Paşca, S.P.; Kuo, C.J.; Heilshorn, S.C. Engineered materials for organoid systems. Nat. Rev. Mater. 2019, 4, 606–622. [Google Scholar] [CrossRef]
- Garreta, E.; Kamm, R.D.; Lopes, S.M.C.D.S.; Lancaster, M.A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking organoid technology through bioengineering. Nat. Mater. 2020, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Chen, C.-Y.; Yu, Z.-Y.; Chung, J.H.; Liu, X.; Wu, C.-Y.; Chen, G.-Y. An electroactive hybrid biointerface for enhancing neuronal differentiation and axonal outgrowth on bio-subretinal chip. Mater. Today Bio 2022, 14, 100253. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, J.; Zhu, D.; Hinton, D.R. Polarized human embryonic stem cell-derived retinal pigment epithelial cell monolayers have higher resistance to oxidative stress-induced cell death than nonpolarized cultures. Stem Cells Transl. Med. 2015, 4, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Hirami, Y.; Osakada, F.; Takahashi, K.; Okita, K.; Yamanaka, S.; Ikeda, H.; Yoshimura, N.; Takahashi, M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci. Lett. 2009, 458, 126–131. [Google Scholar] [CrossRef]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cell Sheets Aiming for Clinical Application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef]
- Sugita, S.; Mandai, M.; Kamao, H.; Takahashi, M. Immunological aspects of RPE cell transplantation. Prog. Retin. Eye Res. 2021, 84, 100950. [Google Scholar] [CrossRef]
- Maeda, T.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Trends of Stem Cell Therapies in Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 1785. [Google Scholar] [CrossRef]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef]





| Treatments | Formulation | Procedure | Administration | Frequency | Main Function |
|---|---|---|---|---|---|
| Anti-VEGF therapy | Antiangiogenic drugs | Intravitreal injection, infusion | Monthly/weekly | More than 1 | Blocking or neutralizing VEGF expression |
| Laser photocoagulation | Laser light with special contact lens | Laser surgery | Monthly | More than 1 | Utilizing heat from a laser to shrink or destroy abnormal blood vessels |
| Photodynamic combined therapy | Laser- and light-activated drugs | Intravenous injection and shining a laser into the eye | Monthly | More than 1 | Creating blood clots to seal the abnormal blood vessels |
| Cell therapy | Pluripotent stem cell-derived RPE cells | Subretinal injection or transplantation | Once | 1 | Reversal of the degenerative loss of RPE cells |
| Cell | Trial ID | Trial Title | Condition | Status | Region | Age | Sample | Sponsors | Trial | Transplant | Phase | Delivery | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ESC-RPE | NCT03167203 | A Safety Surveillance Study in Subjects with Macular Degenerative Disease Treated with Human Embryonic Stem Cell-derived Retinal Pigment Epithelial Cell Therapy | MD | Enrolling via invitation | UK, US | 18 years and older | 36 | Astellas Institute for Regenerative Medicine | Allogenic | Suspension | I/II | Subretinal injection |
| 2 | ESC-RPE | NCT03963154 | Interventional Study of Implantation of hESC-derived RPEs in Patients with RP due to Monogenic Mutation | RP | Recruiting | France | 18 to 65 years | 12 | Centre d’Etude des Cellules Souches | Allogenic | Monolayer | I/II | Monolayer implantation |
| 3 | ESC-RPE | NCT02903576 | Stem Cell Therapy for Outer Retinal Degenerations | AMD; SMD; wet AMD | Completed | Brazil | 18 to 90 years | 15 | Federal University of São Paulo | Allogenic | Suspension | I/II | Subretinal injection |
| 4 | ESC-RPE | NCT01469832 | Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell-derived Retinal Pigmented Epithelial (hESC-RPE) in Patients with Stargardt’s Macular Dystrophy (SMD) | SMD | Completed | UK | 18 years and older | 12 | Astellas Institute for Regenerative Medicine | Allogenic | Suspension | I/II | Subretinal injection |
| 5 | ESC-RPE | NCT01344993 | Safety and Tolerability of Sub-retinal Transplantation of hESC-derived RPE (MA09-hRPE) in Patients with Advanced Dry AMD | Dry AMD | Completed | US | 55 years and older | 13 | Astellas Institute for Regenerative Medicine | Allogenic | Suspension | I/II | Subretinal injection |
| 6 | ESC-RPE | NCT01345006 | Sub-retinal Transplantation of hESC-derived RPEs (MA09-hRPE) in Patients with Stargardt’s Macular Dystrophy | SMD | Completed | US | 18 years and older | 13 | Astellas Institute for Regenerative Medicine | Allogenic | Suspension | I/II | Subretinal injection |
| 7 | iPSC-CEC | jRCTa031210199 | iPSC -derived corneal endothelial cell substitutes for bullous keratopathy | Bullous keratopathy | Recruiting | Japan | 45 to 85 years | 3 | Hirayama Masatoshi | Allogenic | Suspension | I | Subconjunctival injection |
| 8 | iPSC-RPE | jRCTa050210178 | Clinical Research of allogeneic iPSC-RPE strip transplantation for RPE impaired disease | RPE-impaired disease | Recruiting | Japan | 20 years and older | 50 | Kurimoto Yasuo | Allogenic | Cell strip | I/II | Subretinal transplantation |
| 9 | iPSC-RPE | UMIN000026003 | A Study of transplantation of allogenic iPSC-derived RPE suspension in subjects with neovascular AMD | Wet AMD | Completed | Japan | 50 to 85 years | 5 | Kobe City Eye Hospital | Allogenic | Suspension | I/II | Subretinal injection |
| 10 | iPSC-RPE | UMIN000011929 | A Study of transplantation of autologous iPSC-derived RPE cell sheet in subjects with exudative AMD | Wet AMD | Completed | Japan | 50 years and older | 2 | RIKEN | Autologous | Cell sheet | I | Subretinal transplantation |
| 11 | iPSC-RPE | NCT04339764 | A Phase I/IIa Trial for Autologous Transplantation of iPSC-derived RPEs for Geographic Atrophy Associated with AMD | Dry AMD | Recruiting | US | 55 years and older | 20 | National Eye Institute (NEI) | Autologous | RPE-plus-scaffold delivery | I/II | Subretinal transplantation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-P.; Hsiao, Y.-J.; Chang, K.-J.; Foustine, S.; Ko, Y.-L.; Tsai, Y.-C.; Tai, H.-Y.; Ko, Y.-C.; Chiou, S.-H.; Lin, T.-C.; et al. Pluripotent Stem Cells in Clinical Cell Transplantation: Focusing on Induced Pluripotent Stem Cell-Derived RPE Cell Therapy in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2022, 23, 13794. https://doi.org/10.3390/ijms232213794
Yang Y-P, Hsiao Y-J, Chang K-J, Foustine S, Ko Y-L, Tsai Y-C, Tai H-Y, Ko Y-C, Chiou S-H, Lin T-C, et al. Pluripotent Stem Cells in Clinical Cell Transplantation: Focusing on Induced Pluripotent Stem Cell-Derived RPE Cell Therapy in Age-Related Macular Degeneration. International Journal of Molecular Sciences. 2022; 23(22):13794. https://doi.org/10.3390/ijms232213794
Chicago/Turabian StyleYang, Yi-Ping, Yu-Jer Hsiao, Kao-Jung Chang, Shania Foustine, Yu-Ling Ko, Yi-Ching Tsai, Hsiao-Yun Tai, Yu-Chieh Ko, Shih-Hwa Chiou, Tai-Chi Lin, and et al. 2022. "Pluripotent Stem Cells in Clinical Cell Transplantation: Focusing on Induced Pluripotent Stem Cell-Derived RPE Cell Therapy in Age-Related Macular Degeneration" International Journal of Molecular Sciences 23, no. 22: 13794. https://doi.org/10.3390/ijms232213794
APA StyleYang, Y.-P., Hsiao, Y.-J., Chang, K.-J., Foustine, S., Ko, Y.-L., Tsai, Y.-C., Tai, H.-Y., Ko, Y.-C., Chiou, S.-H., Lin, T.-C., Chen, S.-J., Chien, Y., & Hwang, D.-K. (2022). Pluripotent Stem Cells in Clinical Cell Transplantation: Focusing on Induced Pluripotent Stem Cell-Derived RPE Cell Therapy in Age-Related Macular Degeneration. International Journal of Molecular Sciences, 23(22), 13794. https://doi.org/10.3390/ijms232213794







