Fabrication of Multilayered Biofunctional Material with an Enamel-like Structure
Abstract
1. Introduction
2. Results and Discussion
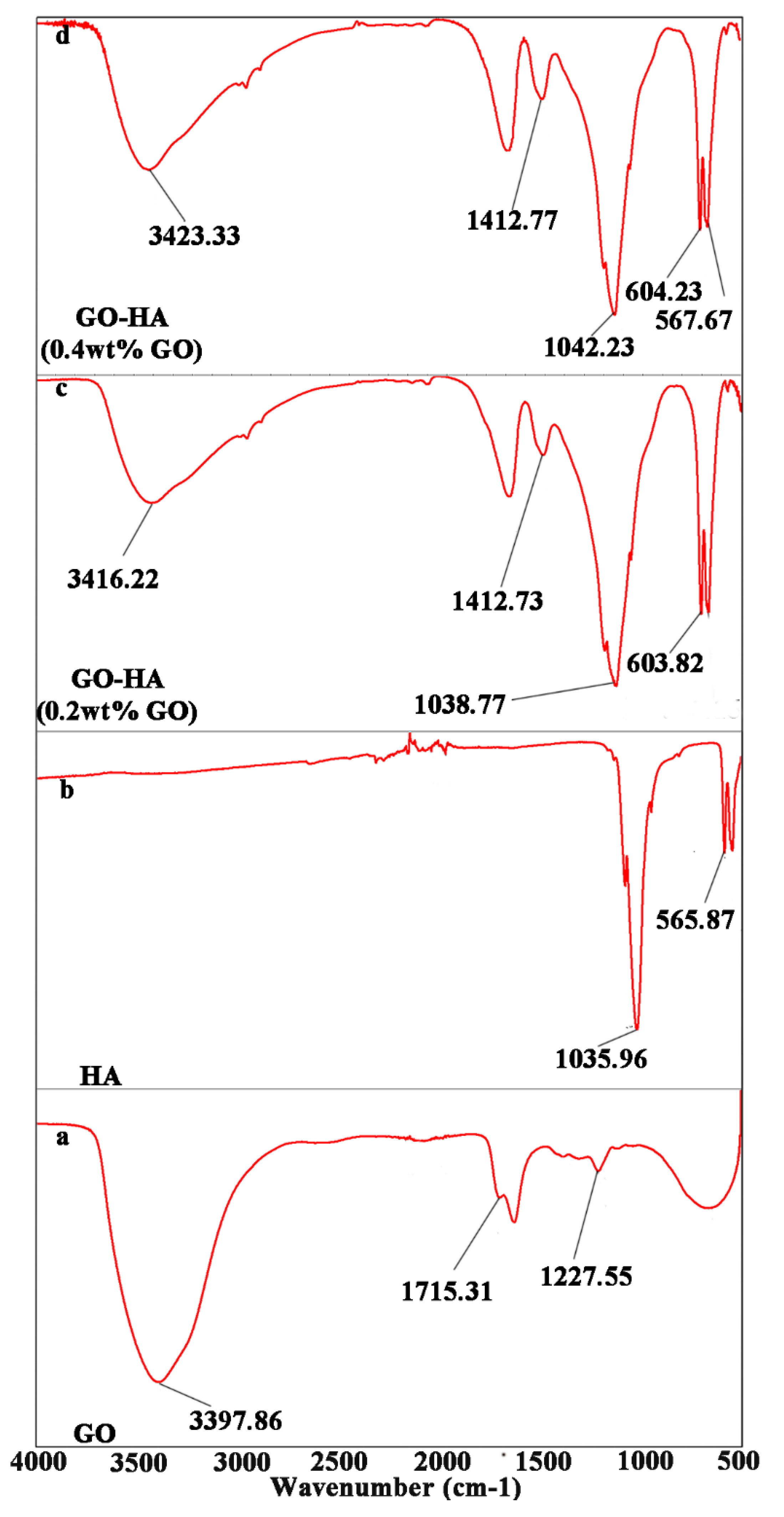
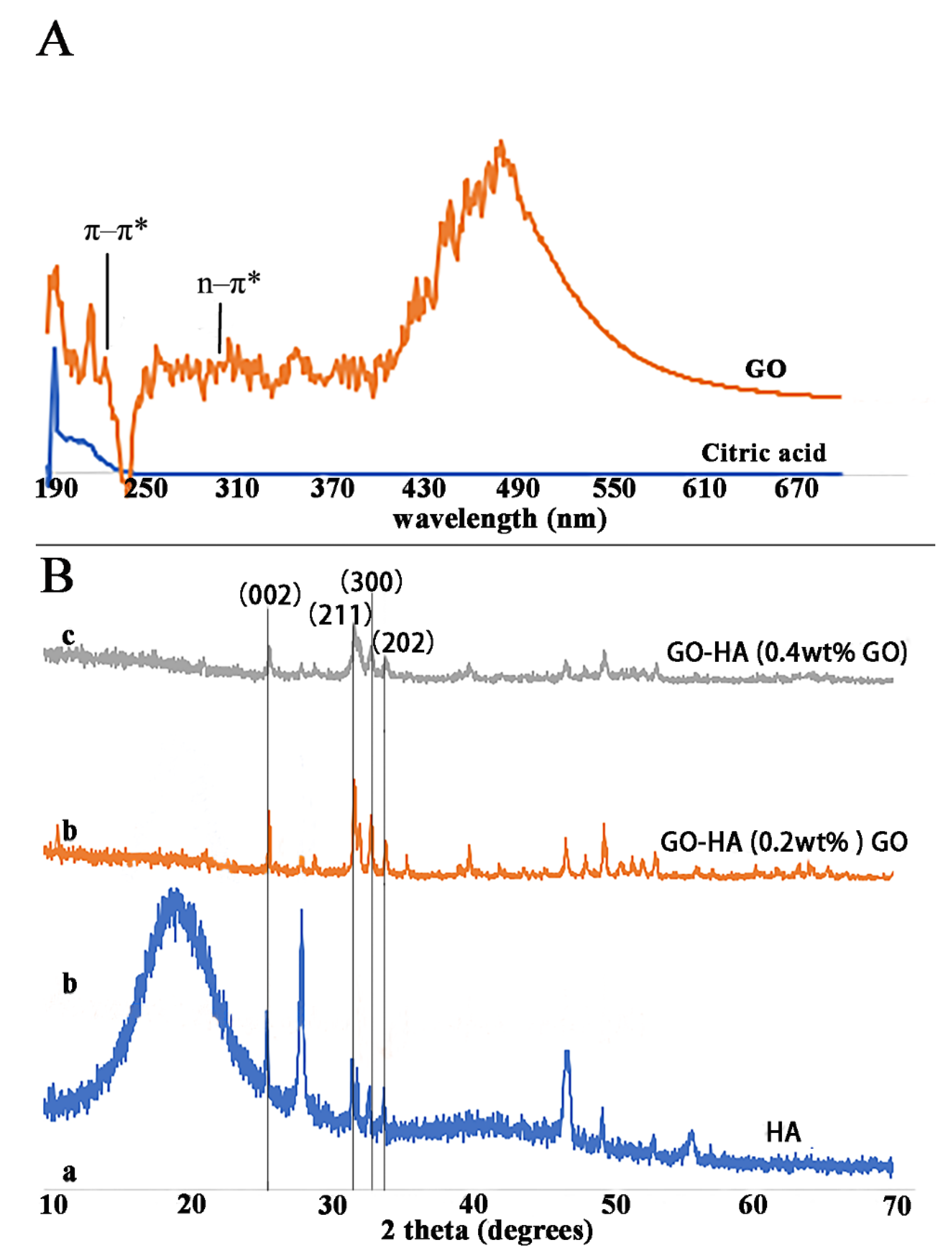
2.1. Synthesis of GO
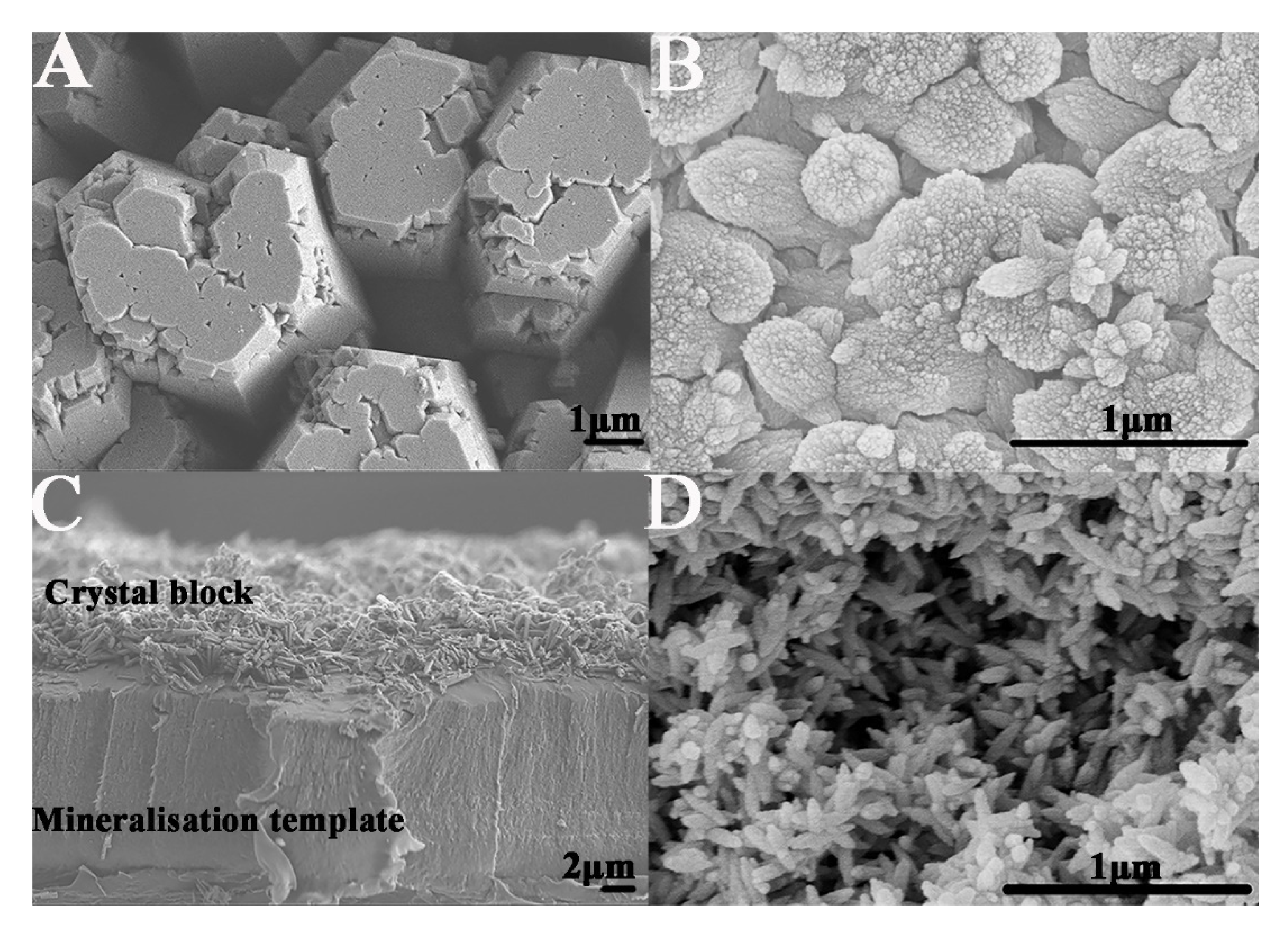
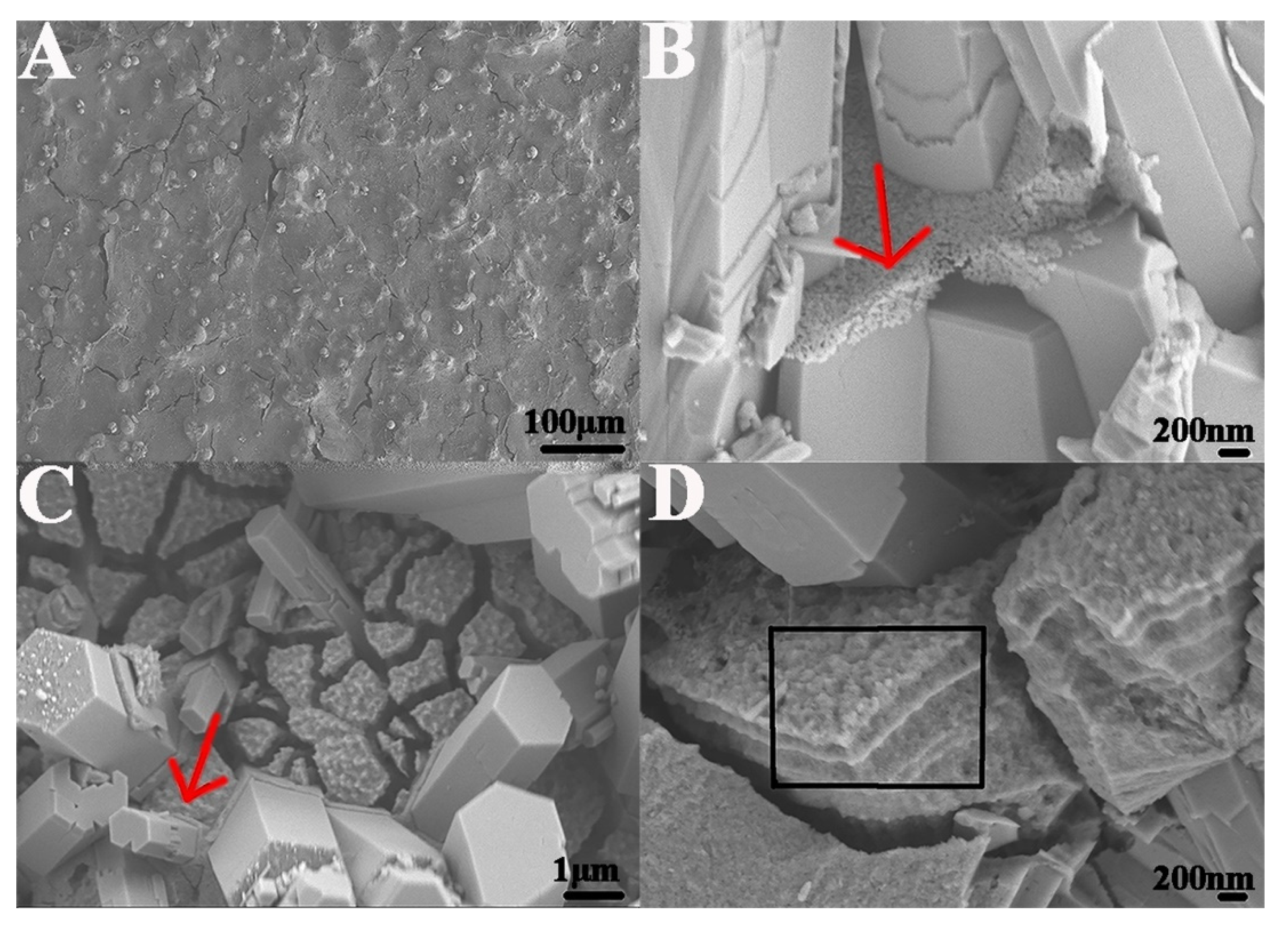
2.2. Formation of Enamel-like GO–HA Crystals
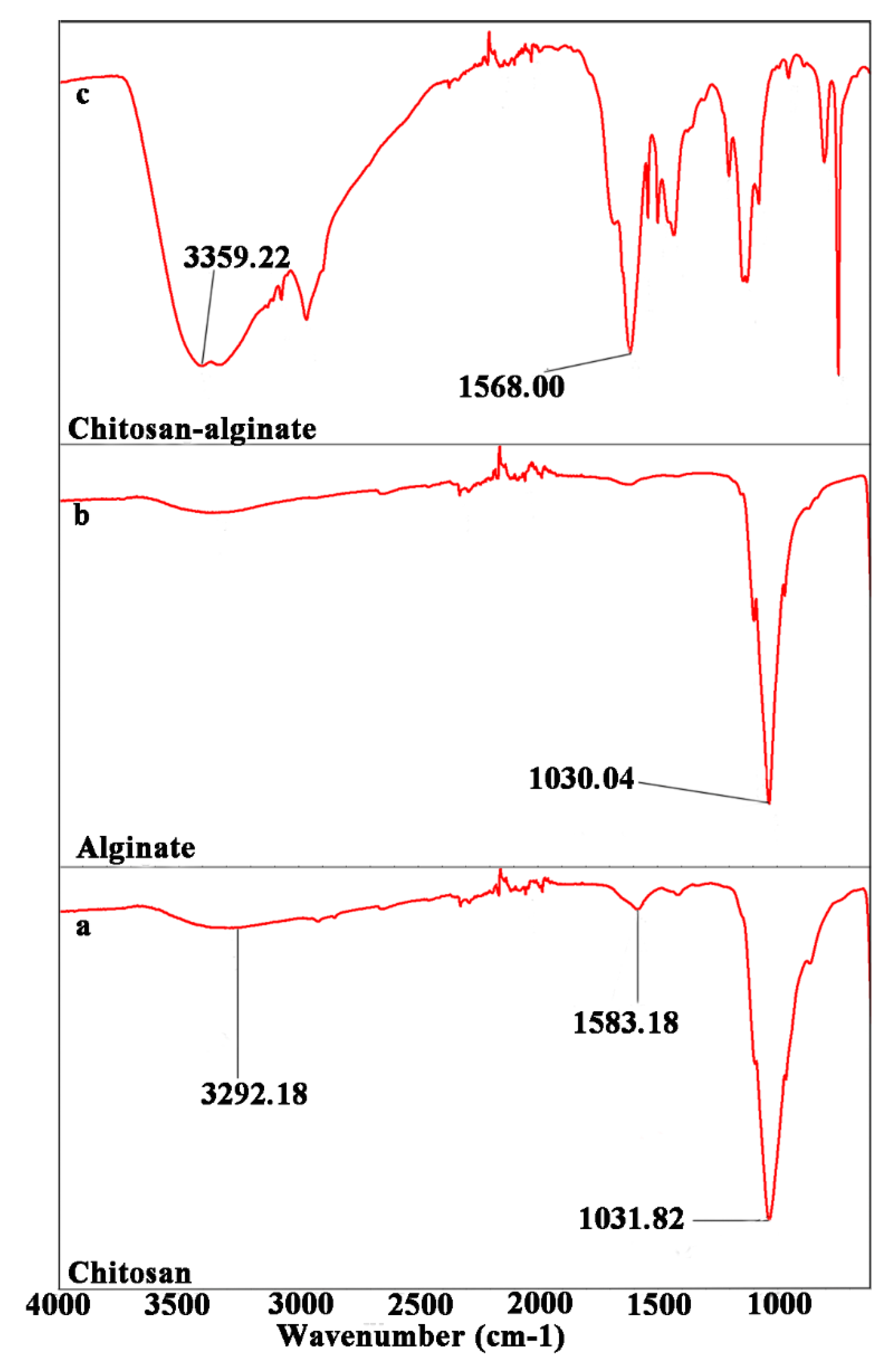
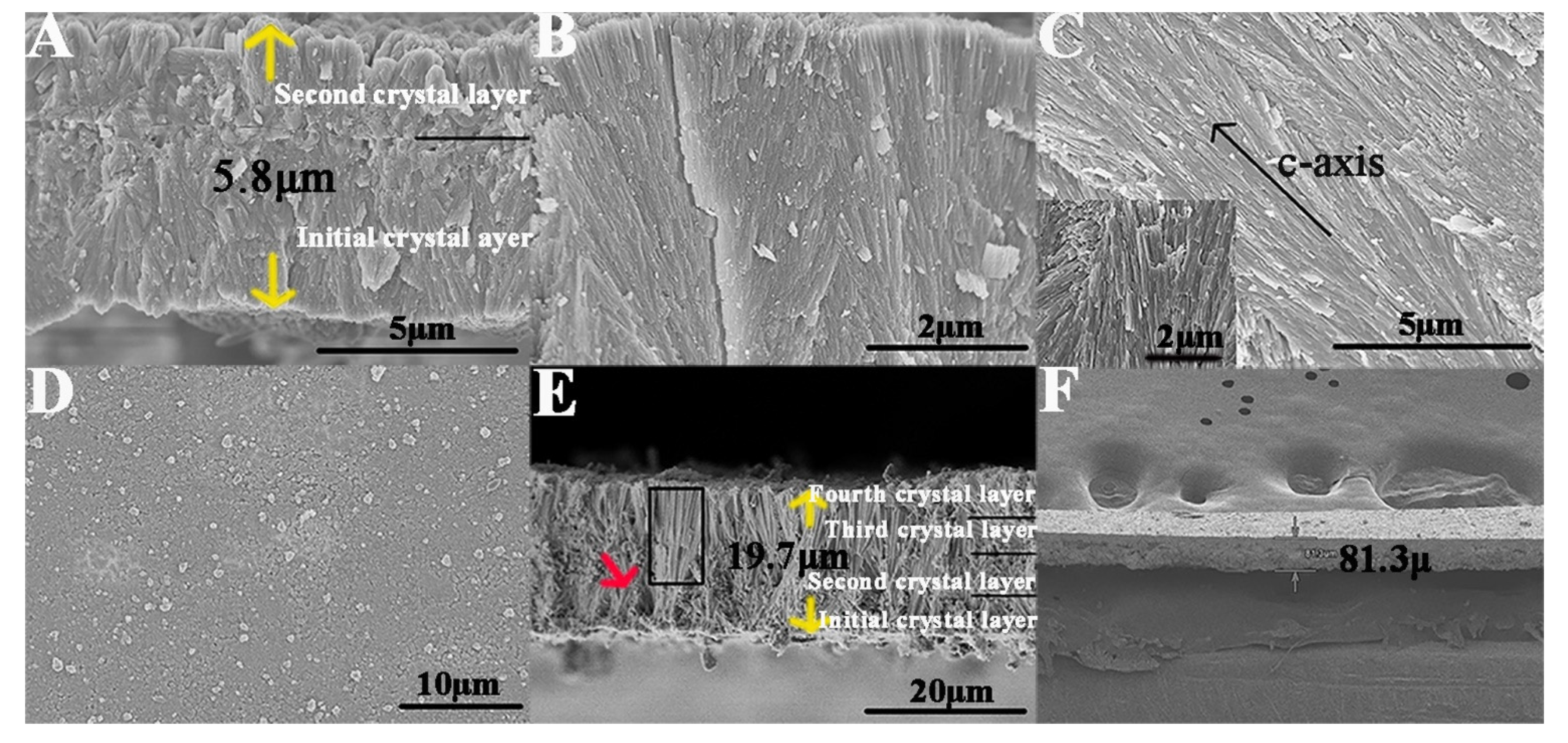
2.3. Fabrication of an Enamel-like, Multilayered Microstructure
2.4. Mechanical Evaluation of Synthesized Materials
2.5. Antibacterial and Biocompatibility of the Synthesized Materials
3. Materials and Methods
3.1. Synthesis of GO
3.2. Preparation of a Metastable Mineralization Solution Containing GO
3.3. Preparation of Chitosan and Alginate Solutions
3.4. Preparation of Dopamine-Coated Polyethylene Membranes
3.5. Fabrication of Bulk Multilayered GO−HA Crystals
3.5.1. Mineralization Process
3.5.2. LBL Deposition
3.6. Isolation of Synthesized Material
3.7. Characterization and Assessments
- Scanning electron microscopy (SEM)
- Energy dispersive spectroscopy (EDS)
- Fourier transform infrared spectroscopy (FTIR)
- X-ray diffraction (XRD)
- Ultraviolet−visible (UV−Vis) absorption
3.8. Mechanical Evaluation
3.9. Antibacterial and Cytocompatibility Evaluation
3.9.1. Colony-Forming Unit (CFU) Assay
3.9.2. Cytocompatibility Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bhushan, B. Hierarchical structure and mechanical properties of nacre: A review. RSC Adv. 2012, 2, 7617–7632. [Google Scholar] [CrossRef]
- Yao, H.B.; Ge, J.; Mao, L.B.; Yan, Y.X.; Yu, S.H. 25th Anniversary Article: Artificial Carbonate Nanocrystals and Layered Structural Nanocomposites Inspired by Nacre: Synthesis, Fabrication and Applications. Adv. Mater. 2014, 26, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, Z.; Guo, L. Nacre-inspired composites with different macroscopic dimensions: Strategies for improved mechanical performance and applications. NPG Asia Mater. 2018, 10, 1–22. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Lin, K. Facile synthesis of dental enamel-like hydroxyapatite nanorod arrays via hydrothermal transformation of hillebrandite nanobelts. Mater. Chem. B 2015, 3, 7334–7339. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, X.; Chen, L.; Lin, K.; Chang, J. Dental enamel-like hydroxyapatite transformed directly from monetite. J. Mater. Chem. 2012, 22, 22637–22641. [Google Scholar] [CrossRef]
- Bakry, A.S.; Takahashi, H.; Otsuki, M.; Tagami, J. Evaluation of new treatment for incipient enamel demineralization using 45S5 bioglass. Dent. Mater. 2014, 30, 314–320. [Google Scholar] [CrossRef]
- Yang, S.; He, H.; Wang, L.; Jia, X.; Feng, H. Oriented crystallization of hydroxyapatite by the biomimetic amelogenin nanospheres from self-assemblies of amphiphilic dendrons. Chem. Commun. 2011, 47, 10100–10102. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural Polymers for the Maintenance of Oral Health: Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 10337. [Google Scholar] [CrossRef]
- Cox, D.; Brennan, M.; Moran, N. Integrins as therapeutic targets: Lessons and opportunities. Nat. Rev. Drug Discov. 2010, 9, 804–820. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mei, M.L.; Li, Q.L.; Lo, E.C.M.; Chu, C.H. Agarose hydrogel biomimetic mineralization model for the regeneration of enamel prismlike tissue. ACS Appl. Mater. Interfaces 2014, 6, 410. [Google Scholar] [CrossRef] [PubMed]
- Istikharoh, F.; Sujuti, H.; Mustamsir, E.; Swastirani, A. Preparation and biodegradable properties of hydroxyapatite nanoparticle composite coated with poly lactic-co-glycolic acid/polyvinyl alcohol for bone regeneration. Dent. Med. Probl. 2020, 57, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, Z.; Moradian-Oldak, J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials 2009, 30, 478. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Jiménez, A.Y.; Rodríguez-Vilchis, L.E.; Contreras-Bulnes, R.; Alatorre, J.Á.A.; Velazquez-Enriquez, U.; García-Fabila, M.M. Acid resistance of dental enamel treated with remineralizing agents, Er: YAG laser and combined treatments. Dent. Med. Probl. 2018, 55, 255–259. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef]
- Mukherjee, K.; Ruan, Q.; Liberman, D.; White, S.N.; Moradian-Oldak, J. Repairing human tooth enamel with leucine-rich amelogenin peptide–chitosan hydrogel. J. Mater. Res. 2016, 31, 556–563. [Google Scholar] [CrossRef]
- Ruan, Q.; Zhang, Y.; Yang, X.; Nutt, S.; Moradian-Oldak, J. An amelogenin–chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013, 9, 7289–7297. [Google Scholar] [CrossRef]
- Elsharkawy, S.; Al-Jawad, M.; Pantano, M.F.; Tejeda-Montes, E.; Mehta, K.; Jamal, H.; Agarwal, S.; Shuturminska, K.; Rice, A.; Tarakina, N.V.; et al. Protein disorder-order interplay to guide the growth of hierarchical mineralized structures. Nat. Commun. 2018, 9, 2145. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Wu, C.; Chang, J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014, 10, 4071–4102. [Google Scholar] [CrossRef]
- Xu, J.; Liu, L.; Munroe, P.; Xie, Z. Promoting bone-like apatite formation on titanium alloys through nanocrystalline tantalum nitride coatings. J. Mater. Chem. B 2015, 3, 4082–4094. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Tian, B.; Tang, S.; Ke, Q.; Zhang, C.; Zhu, Z.; Guo, Y. Hydroxyapatite coatings with oriented nanoplate arrays: Synthesis, formation mechanism and cytocompatibility. J. Mater. Chem. B 2015, 3, 1655–1666. [Google Scholar] [CrossRef]
- Zhao, C.; Xia, L.; Zhai, D.; Zhang, N.; Liu, J.; Fang, B.; Chang, J.; Lin, K. Designing ordered micropatterned hydroxyapatite bioceramics to promote the growth and osteogenic differentiation of bone marrow stromal cells. J. Mater. Chem. B 2015, 3, 968–976. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Poly. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control Rel. 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Liu, H.; Xi, P.; Xie, G.; Shi, Y.; Hou, F.; Huang, L.; Chen, F.; Zeng, Z.; Shao, C.; Wang, J. Simultaneous Reduction and Surface Functionalization of Graphene Oxide for Hydroxyapatite Mineralization. J. Phys. Chem. C 2012, 116, 3334–3341. [Google Scholar] [CrossRef]
- Zhu, J.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Spark plasma sintered hydroxyapatite/graphite nanosheet and hydroxyapatite/multiwalled carbon nanotube composites: Mechanical and in vitro cellular properties. Adv. Eng. Mater. 2011, 13, 336–341. [Google Scholar] [CrossRef]
- Menge, H.G.; Huynh, N.D.; Cho, C.; Choi, D.; Park, Y.T. Designable functional polymer nanocomposites via layer-by-layer assembly for highly deformable power-boosted triboelectric nanogenerators. Compos. B Eng. 2022, 230, 109513. [Google Scholar] [CrossRef]
- Mohammadi, E.; Zhao, C.; Meng, Y.; Qu, G.; Zhang, F.; Zhao, X.; Mei, J.; Zuo, J.M.; Shukla, D.; Diao, Y. Dynamic-template-directed multiscale assembly for large-area coating of highly-aligned conjugated polymer thin films. Nat. Commun. 2017, 8, 16070. [Google Scholar] [CrossRef]
- Mostafavi, E.; Soltantabar, P.; Webster, T.J. Nanotechnology and Picotechnology: A New Arena for Translational Medicine; Biomaterials in Translational Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 191–212. [Google Scholar]
- Ozin, G.A.; Arsenault, A. Nanochemistry: A Chemical Approach to Nanomaterials; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.; Sommerdijk, N.A.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef]
- Okada, M.; Furuzono, T. Hydroxylapatite nanoparticles: Fabrication methods and medical applications. Sci. Technol. Adv. Mater. 2012, 13, 064103. [Google Scholar] [CrossRef]
- Nielsen, M.H.; Aloni, S.; De Yoreo, J.J. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 2014, 345, 1158–1162. [Google Scholar] [CrossRef]
- Seker, U.O.S.; Demir, H.V. Material binding peptides for nanotechnology. Molecules 2011, 16, 1426–1451. [Google Scholar] [CrossRef]
- Fahlman, M.; Fabiano, S.; Gueskine, V.; Simon, D.; Berggren, M.; Crispin, X. Interfaces in organic electronics. Nat. Rev. Mater. 2019, 4, 627–650. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Cao, Y.; Liu, W.; Chu, C.H.; Li, Q.L. Polydopamine-induced tooth remineralization. ACS Appl. Mater. Interfaces 2012, 4, 6901–6910. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, S.; Moghaddam, E.; Basirun, W.J.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Moghaddam, M.N.; Alias, Y. Characterization of nickel-doped biphasic calcium phosphate/graphene nanoplatelet composites for biomedical application. Carbon 2014, 69, 32–45. [Google Scholar] [CrossRef]
- Li, D.; Sun, H.; Jiang, L.; Zhang, K.; Liu, W.; Zhu, Y.; Fangteng, J.; Shi, C.; Zhao, L.; Sun, H.; et al. Enhanced biocompatibility of PLGA nanofibers with gelatin/nano-hydroxyapatite bone biomimetics incorporation. ACS Appl. Mater. Interfaces 2014, 6, 9402–9410. [Google Scholar] [CrossRef] [PubMed]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and electronic structure of graphene-oxide. Microsc. Microanal 2010, 16, 1704–1705. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015. [Google Scholar] [CrossRef]
- Eda, G.; Lin, Y.Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.A.; Chen, I.S.; Chen, C.W.; Chhowalla, M. Blue photoluminescence from chemically derived graphene oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef]
- Lee, W.C.; Lim, C.H.Y.; Shi, H.; Tang, L.A.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Zeng, Y.; Pei, X.; Yang, S.; Qin, H.; Cai, H.; Hu, S.L.; Sui, Q.; Wang, J. Hydroxyapatite: Preparation, Properties and Its Biomedical Applications. Surf. Coat. Technol. 2016, 286, 72–79. [Google Scholar] [CrossRef]
- Mishra, D.; Bhunia, B.; Banerjee, I.; Datta, P.; Dhara, S.; Maiti, T.K. Enzymatically crosslinked carboxymethyl–chitosan/gelatin/nano-hydroxyapatite injectable gels for in situ bone tissue engineering application. Mater. Sci. Eng. C 2011, 31, 1295–1304. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Li, H. Synthesis of hydroxyapatite–reduced graphite oxide nanocomposites for biomedical applications: Oriented nucleation and epitaxial growth of hydroxyapatite. J. Mater. Chem. B 2013, 1, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Marine Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.B.; Gao, H.L.; Yao, H.B.; Liu, L.; Cölfen, H.; Liu, G.; Chen, S.M.; Li, S.K.; Yan, Y.X.; Liu, Y.Y.; et al. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Liu, Q.; Li, Q.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Mater. Chem. B 2013, 1, 475. [Google Scholar] [CrossRef]
- Fu, Y.C.; Chen, C.H.; Wang, C.Z.; Wang, Y.H.; Chang, J.K.; Wang, G.J.; Ho, M.L.; Wang, C.K. Preparation of porous bioceramics using reverse thermo-responsive hydrogels in combination with rhBMP-2 carriers: In vitro and in vivo evaluation. J. Mech. Behav. Biomed. Mater. 2013, 27, 64–76. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Rydzek, G.; Ji, Q.; Yonamine, Y.; Wu, K.C.W.; Hill, J.P. Layer-by-layer nanoarchitectonics: Invention, innovation, and evolution. Chem. Lett. 2014, 43, 36–68. [Google Scholar] [CrossRef]
- Wei, J.; Ping, H.; Xie, J.; Zou, Z.; Wang, K.; Xie, H.; Wang, W.; Lei, L.; Fu, Z. Bioprocess-inspired microscale additive manufacturing of multilayered TiO2/polymer composites with enamel-like structures and high mechanical properties. Adv. Funct. Mater. 2020, 30, 1904880. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.L.; Wong, H.M. Evaporation strategy generated antibacterial enamel-like fluorapatite-polyacrylic acid sheet for functional dental restoration. Compos. B Eng. 2022, 233, 109651. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]


| Carbon (wt%) | Oxygen (wt%) | |
|---|---|---|
| Citric acid | 35.34 | 64.66 |
| GO | 49.37 | 50.63 |
| Carbon (wt%) | Oxygen (wt%) | Fluorine (wt%) | Phosphorous (wt%) | Calcium (wt%) | |
|---|---|---|---|---|---|
| Pure HA crystal | 1.83 | 24.51 | 3.19 | 22.08 | 44.39 |
| GO−HA crystal fabricated with addition of 0.2 wt% GO | 7.86 | 26.08 | 7.97 | 18.69 | 39.40 |
| GO−HA crystal fabricated with addition of 0.4 wt% GO | 9.25 | 26.03 | 7.84 | 18.78 | 38.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.Y.; Li, Q.L.; Wong, H.M. Fabrication of Multilayered Biofunctional Material with an Enamel-like Structure. Int. J. Mol. Sci. 2022, 23, 13810. https://doi.org/10.3390/ijms232213810
Zhang YY, Li QL, Wong HM. Fabrication of Multilayered Biofunctional Material with an Enamel-like Structure. International Journal of Molecular Sciences. 2022; 23(22):13810. https://doi.org/10.3390/ijms232213810
Chicago/Turabian StyleZhang, Yu Yuan, Quan Li Li, and Hai Ming Wong. 2022. "Fabrication of Multilayered Biofunctional Material with an Enamel-like Structure" International Journal of Molecular Sciences 23, no. 22: 13810. https://doi.org/10.3390/ijms232213810
APA StyleZhang, Y. Y., Li, Q. L., & Wong, H. M. (2022). Fabrication of Multilayered Biofunctional Material with an Enamel-like Structure. International Journal of Molecular Sciences, 23(22), 13810. https://doi.org/10.3390/ijms232213810







