Alcohol Exposure Induces Depressive and Anxiety-like Behaviors via Activating Ferroptosis in Mice
Abstract
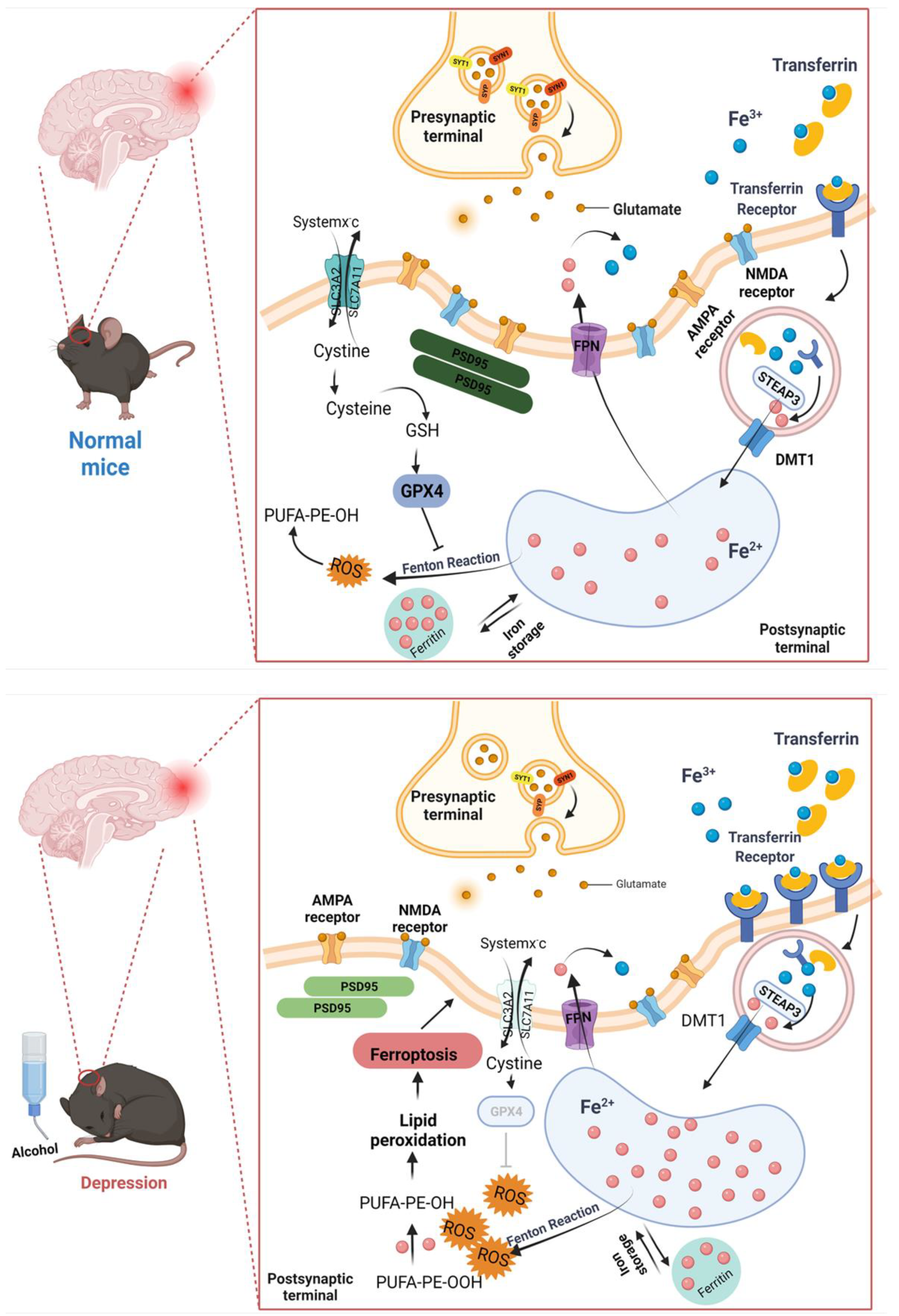
1. Introduction
2. Results
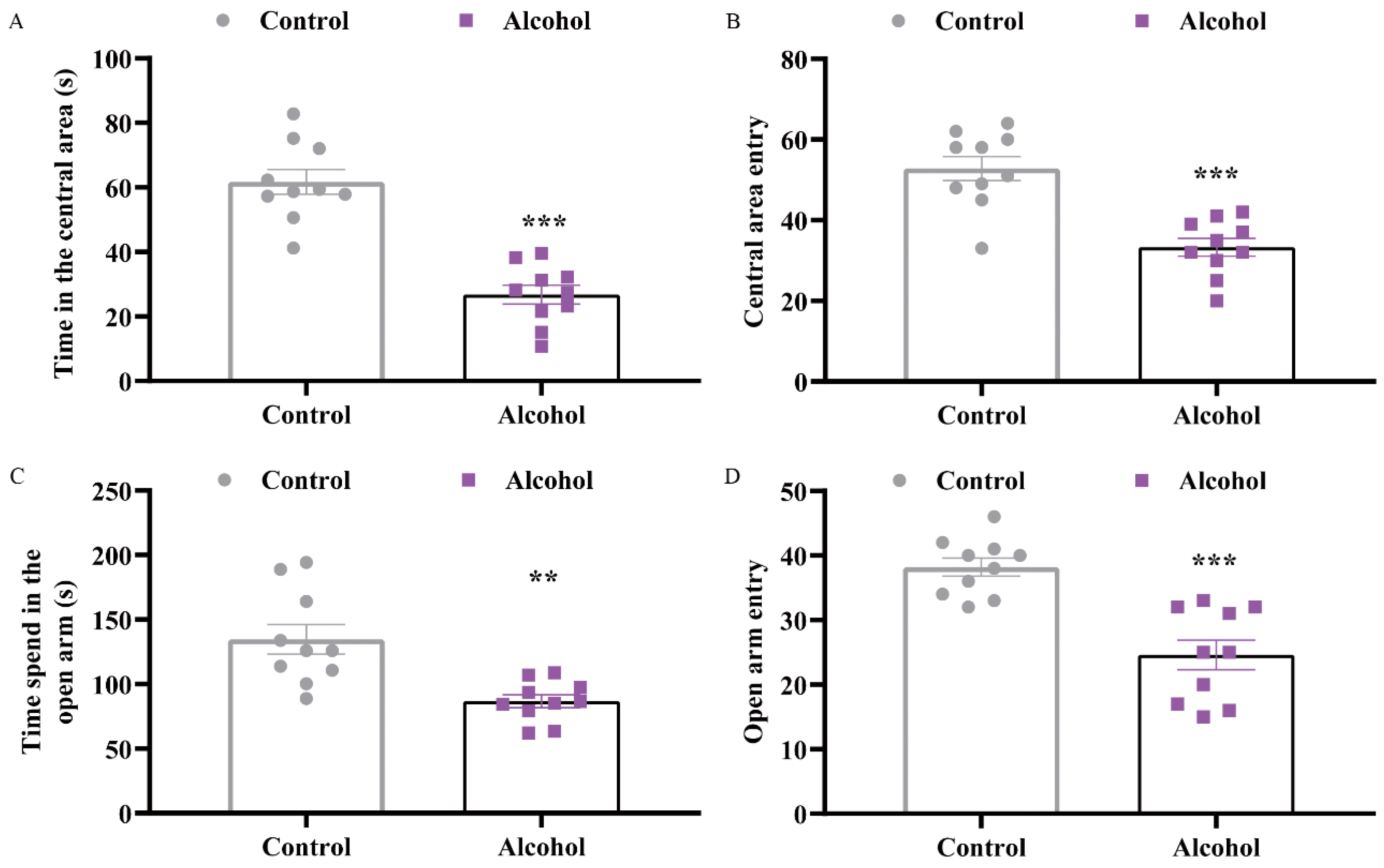
2.1. Alcohol Exposure Induced Depressive and Anxiety-Like Behaviors in Mice
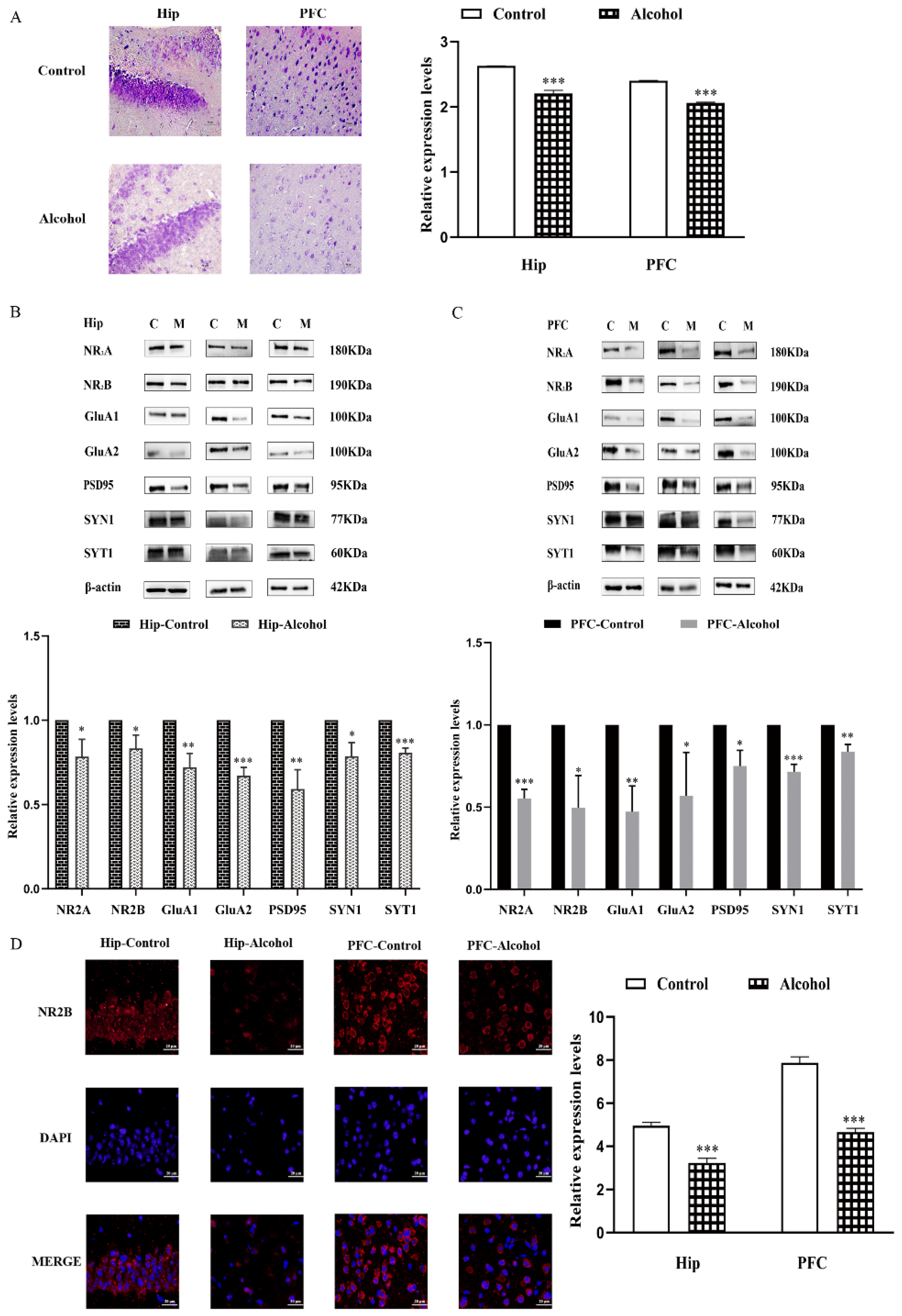
2.2. Alcohol Exposure Downregulated the Expression of Synapse-Related Proteins in the Hip and PFC
2.3. Ferroptosis Pathway Was Involved in Alcohol-Induced Mental Disorder
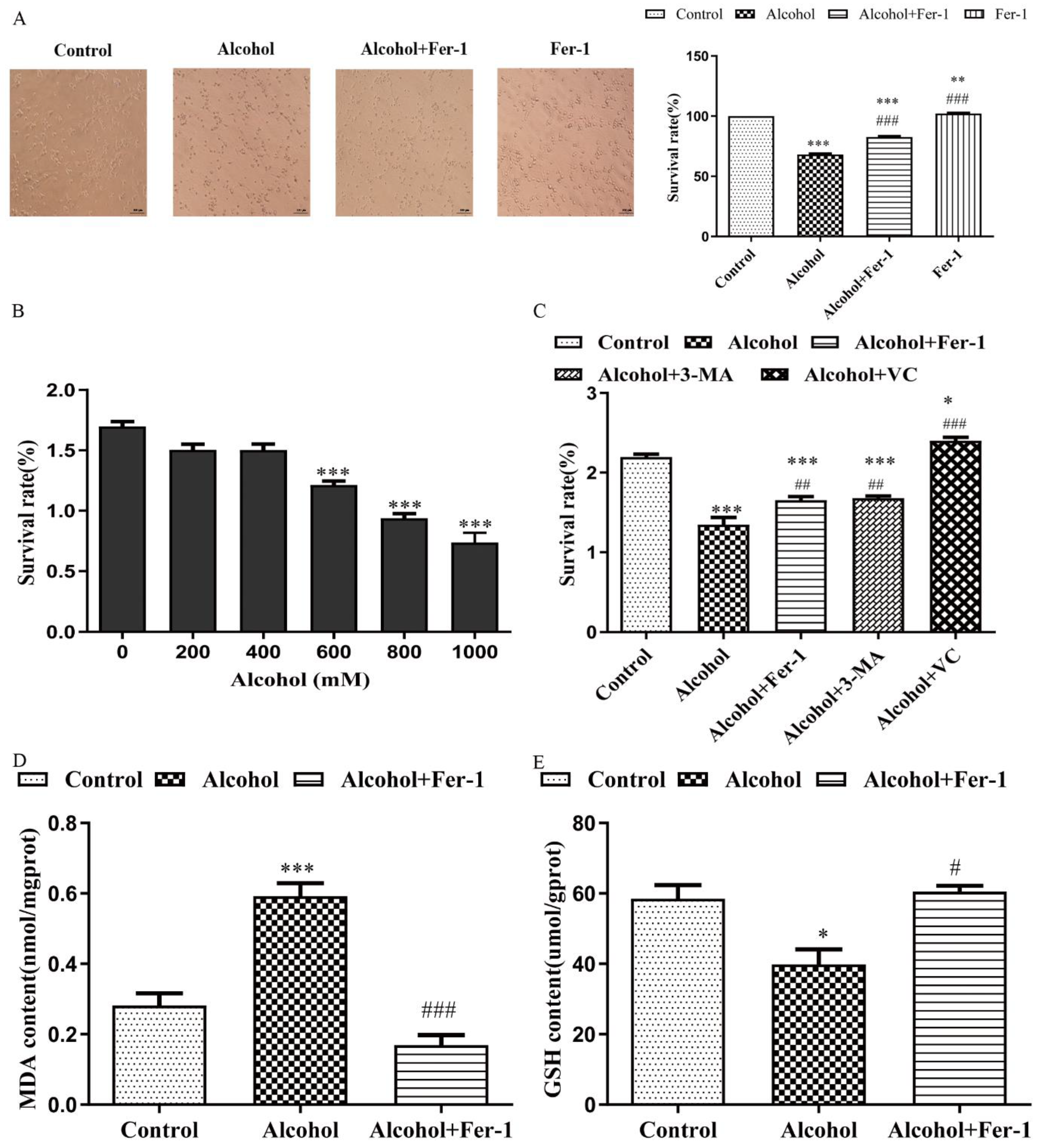
2.4. Fer-1 Improved Alcohol-Induced Cell Death In Vitro
2.5. Fer-1 Prevents Alcohol-Induced Ferroptosis in Cells
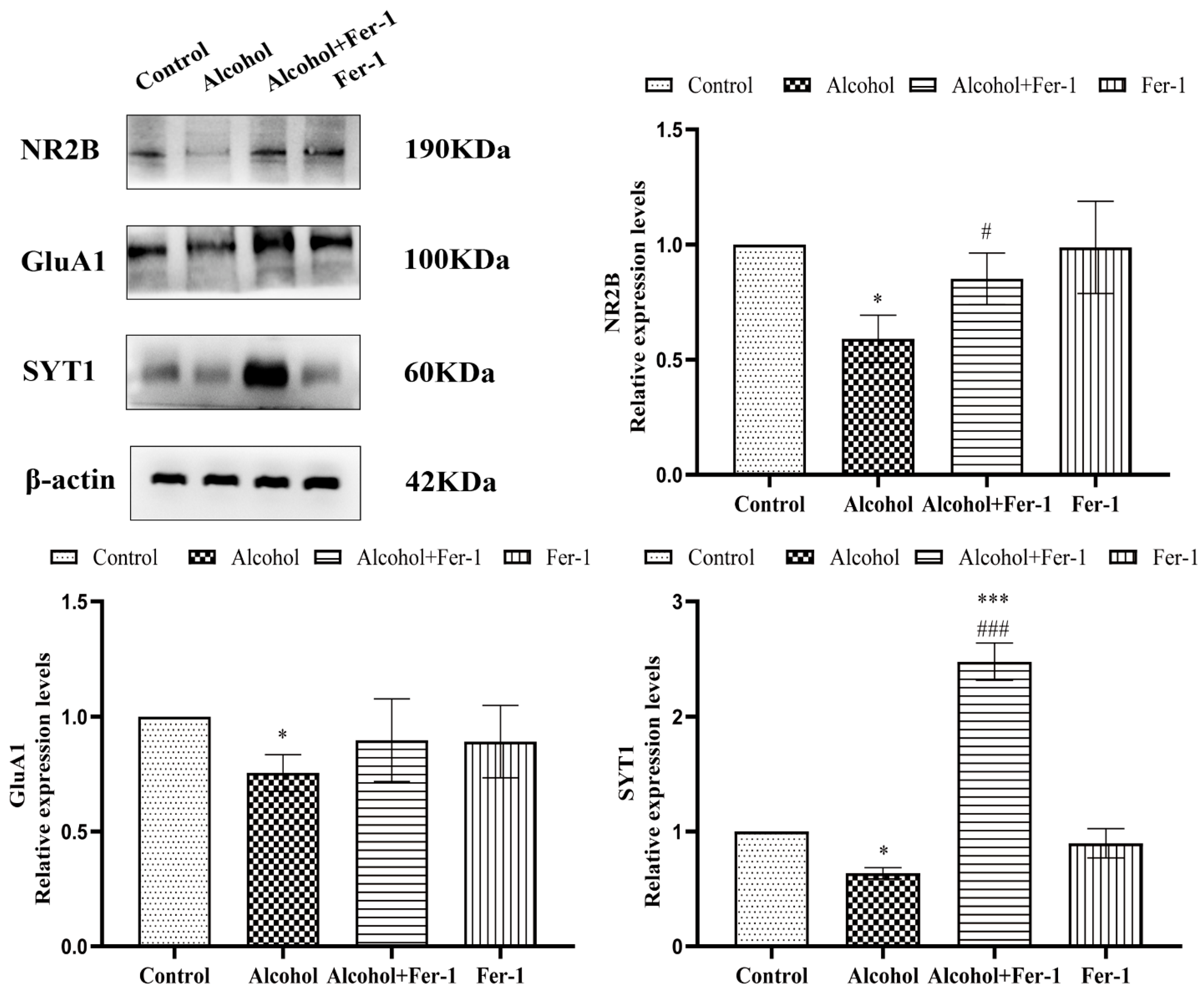
2.6. Inhibition of Ferroptosis Ameliorated Synaptic Plasticity
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Animal Treatment
4.3. Behavioral Testing
4.3.1. Zero Maze Test
4.3.2. Novelty-Suppressed Feeding Test
4.3.3. Open Field Test
4.3.4. Tail Suspension Test
4.3.5. Sucrose Preference Test
4.4. Histology
4.4.1. Nissl Staining
4.4.2. Iron Staining
4.4.3. Immunohistochemistry
4.5. Western Blot Analysis
4.6. Cell Treatment
4.6.1. Primary Cultures of Prefrontal Cortical Neural Cells
4.6.2. Cultures of N2A Cells
4.7. Measurement of Cell Viability, MDA Contents and GSH Contents
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Status Report on Alcohol and Health 2018; WHO: Geneva, Switzerland, 2018.
- Bloch, S.; Rinker, J.A.; Marcus, M.M.; Mulholland, P.J. Absence of effects of intermittent access to alcohol on negative affective and anxiety-like behaviors in male and female C57BL/6J mice. Alcohol 2020, 88, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, T.M.; Lown, E.A.; Li, C.; Tonning Olsson, I.; Marchak, J.G.; Stuber, M.L.; Vuotto, S.; Srivastava, D.; Nathan, P.C.; Leisenring, W.M.; et al. Alcohol consumption behaviors and neurocognitive dysfunction and emotional distress in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Addiction 2019, 114, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kavalali, E.T.; Monteggia, L.M. Targeting Homeostatic Synaptic Plasticity for Treatment of Mood Disorders. Neuron 2020, 106, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Heller, H.C. The Function(s) of Sleep. Handb. Exp. Pharmacol. 2019, 253, 3–34. [Google Scholar] [CrossRef]
- Lau, C.G.; Zukin, R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007, 8, 413–426. [Google Scholar] [CrossRef]
- Guire, E.S.; Oh, M.C.; Soderling, T.R.; Derkach, V.A. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J. Neurosci. 2008, 28, 6000–6009. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Jackson, A.C.; Bjorgan, K.; During, M.J.; Sprengel, R.; Seeburg, P.H.; Nicoll, R.A. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 2009, 62, 254–268. [Google Scholar] [CrossRef]
- Yoshiya, M.; Komatsuzaki, Y.; Hojo, Y.; Ikeda, M.; Mukai, H.; Hatanaka, Y.; Murakami, G.; Kawata, M.; Kimoto, T.; Kawato, S. Corticosterone rapidly increases thorns of CA3 neurons via synaptic/extranuclear glucocorticoid receptor in rat hippocampus. Front. Neural Circuits 2013, 7, 191. [Google Scholar] [CrossRef]
- Zhou, H.Y.; He, J.G.; Hu, Z.L.; Xue, S.G.; Xu, J.F.; Cui, Q.Q.; Gao, S.Q.; Zhou, B.; Wu, P.F.; Long, L.H.; et al. A-Kinase Anchoring Protein 150 and Protein Kinase A Complex in the Basolateral Amygdala Contributes to Depressive-like Behaviors Induced by Chronic Restraint Stress. Biol. Psychiatry 2019, 86, 131–142. [Google Scholar] [CrossRef]
- Aleksandrova, L.R.; Phillips, A.G.; Wang, Y.T. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. 2017, 42, 222–229. [Google Scholar] [CrossRef]
- Henter, I.D.; de Sousa, R.T.; Zarate, C.A., Jr. Glutamatergic Modulators in Depression. Harv. Rev. Psychiatry 2018, 26, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Draganov, M.; Vives-Gilabert, Y.; de Diego-Adelino, J.; Vicent-Gil, M.; Puigdemont, D.; Portella, M.J. Glutamatergic and GABA-ergic abnormalities in First-episode depression. A 1-year follow-up 1H-MR spectroscopic study. J. Affect. Disord. 2020, 266, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Phillips, C.; Trillo, L.; De Miguel, Z.; Das, D.; Salehi, A. The role of NMDA receptors in the pathophysiology and treatment of mood disorders. Neurosci. Biobehav. Rev. 2014, 47, 336–358. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Manji, H.K.; Zarate, C.A. The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders. Neuroscientist 2009, 15, 525–539. [Google Scholar] [CrossRef]
- Kim, J.; Farchione, T.; Potter, A.; Chen, Q.; Temple, R. Esketamine for Treatment-Resistant Depression—First FDA-Approved Antidepressant in a New Class. N. Engl. J. Med. 2019, 381, 1–4. [Google Scholar] [CrossRef]
- Wills, T.A.; Baucum, A.J., 2nd; Holleran, K.M.; Chen, Y.; Pasek, J.G.; Delpire, E.; Tabb, D.L.; Colbran, R.J.; Winder, D.G. Chronic intermittent alcohol disrupts the GluN2B-associated proteome and specifically regulates group I mGlu receptor-dependent long-term depression. Addict. Biol. 2017, 22, 275–290. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Gerhard, D.M.; Wohleb, E.S.; Duman, R.S. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov. Today 2016, 21, 454–464. [Google Scholar] [CrossRef]
- Marsden, W.N. Synaptic plasticity in depression: Molecular, cellular and functional correlates. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 168–184. [Google Scholar] [CrossRef]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Kohgo, Y.; Ikuta, K.; Ohtake, T.; Torimoto, Y.; Kato, J. Iron overload and cofactors with special reference to alcohol, hepatitis C virus infection and steatosis/insulin resistance. World J. Gastroenterol. 2007, 13, 4699–4706. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Dominitz, J.A.; Weiss, N.S.; Heagerty, P.J.; Kowdley, K.V. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology 2004, 126, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Ferroptosis Mechanisms Involved in Hippocampal-Related Diseases. Int. J. Mol. Sci. 2021, 22, 9902. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, S.J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef]
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Chou, S.P.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015, 72, 757–766. [Google Scholar] [CrossRef]
- Parsons, O.A.; Schaeffer, K.W.; Glenn, S.W. Does neuropsychological test performance predict resumption of drinking in posttreatment alcoholics? Addict. Behav. 1990, 15, 297–307. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Li, M.; Shen, Q.; Li, X.; Rong, X.; Peng, Y. Efficacy of pharmacotherapeutics for patients comorbid with alcohol use disorders and depressive symptoms—A Bayesian network meta-analysis. CNS Neurosci. Ther. 2020, 26, 1185–1197. [Google Scholar] [CrossRef]
- Coccaro, E.F. New Hope for Patients with Major Depressive Disorder? N. Engl. J. Med. 2019, 381, 980–981. [Google Scholar] [CrossRef]
- Yan, H.C.; Cao, X.; Das, M.; Zhu, X.H.; Gao, T.M. Behavioral animal models of depression. Neurosci. Bull. 2010, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Trucco, E.M.; Kopera, M.; Kobylinski, P.; Suszek, H.; Fudalej, S.; Brower, K.J.; Wojnar, M. The association between impulsivity, emotion regulation, and symptoms of alcohol use disorder. J. Subst. Abuse Treat. 2018, 91, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bloodgood, D.W.; Hardaway, J.A.; Stanhope, C.M.; Pati, D.; Pina, M.M.; Neira, S.; Desai, S.; Boyt, K.M.; Palmiter, R.D.; Kash, T.L. Kappa opioid receptor and dynorphin signaling in the central amygdala regulates alcohol intake. Mol. Psychiatry 2021, 26, 2187–2199. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, R.M.; Brissett, D.I.; Whistler, J.L. Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety. J. Pharmacol. Exp. Ther. 2010, 335, 133–139. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.D.; Narasimhan, A.; Thatcher, D.L.; Keshavan, M.S.; Soloff, P.; Clark, D.B. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin. Exp. Res. 2005, 29, 1590–1600. [Google Scholar] [CrossRef]
- Nagel, B.J.; Schweinsburg, A.D.; Phan, V.; Tapert, S.F. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005, 139, 181–190. [Google Scholar] [CrossRef]
- Geil, C.R.; Hayes, D.M.; McClain, J.A.; Liput, D.J.; Marshall, S.A.; Chen, K.Y.; Nixon, K. Alcohol and adult hippocampal neurogenesis: Promiscuous drug, wanton effects. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 103–113. [Google Scholar] [CrossRef]
- Ge, Y.; Dong, Z.; Bagot, R.C.; Howland, J.G.; Phillips, A.G.; Wong, T.P.; Wang, Y.T. Hippocampal long-term depression is required for the consolidation of spatial memory. Proc. Natl. Acad. Sci. USA 2010, 107, 16697–16702. [Google Scholar] [CrossRef]
- Dong, Z.; Bai, Y.; Wu, X.; Li, H.; Gong, B.; Howland, J.G.; Huang, Y.; He, W.; Li, T.; Wang, Y.T. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology 2013, 64, 65–73. [Google Scholar] [CrossRef]
- Gross, J.J. Handbook of Emotion Regulation, 2nd ed.; Guilford Press: New York, NY, USA, 2014. [Google Scholar]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model—Are we there yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Bagot, R.C.; Parise, E.M.; Peña, C.J.; Zhang, H.X.; Maze, I.; Chaudhury, D.; Persaud, B.; Cachope, R.; Bolaños-Guzmán, C.A.; Cheer, J.F.; et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 2015, 6, 7062. [Google Scholar] [CrossRef] [PubMed]
- Van Beugen, B.J.; Qiao, X.; Simmons, D.H.; De Zeeuw, C.I.; Hansel, C. Enhanced AMPA receptor function promotes cerebellar long-term depression rather than potentiation. Learn. Mem. 2014, 21, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Siddoway, B.; Hou, H.; Xia, H. Molecular mechanisms of homeostatic synaptic downscaling. Neuropharmacology 2014, 78, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.H.; Park, B.S.; Adermark, L.; Lovinger, D.M. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur. J. Neurosci. 2007, 25, 3226–3232. [Google Scholar] [CrossRef]
- Kroener, S.; Mulholland, P.J.; New, N.N.; Gass, J.T.; Becker, H.C.; Chandler, L.J. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS ONE 2012, 7, e37541. [Google Scholar] [CrossRef]
- Wang, J.; Carnicella, S.; Phamluong, K.; Jeanblanc, J.; Ronesi, J.A.; Chaudhri, N.; Janak, P.H.; Lovinger, D.M.; Ron, D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: Implications for alcohol drinking behavior. J. Neurosci. 2007, 27, 3593–3602. [Google Scholar] [CrossRef]
- Munoz, B.; Fritz, B.M.; Yin, F.; Atwood, B.K. Alcohol exposure disrupts mu opioid receptor-mediated long-term depression at insular cortex inputs to dorsolateral striatum. Nat. Commun. 2018, 9, 1318. [Google Scholar] [CrossRef]
- Fu, R.; Zuo, W.; Shiwalkar, N.; Mei, Q.; Fan, Q.; Chen, X.; Li, J.; Bekker, A.; Ye, J.H. Alcohol withdrawal drives depressive behaviors by activating neurons in the rostromedial tegmental nucleus. Neuropsychopharmacology 2019, 44, 1464–1475. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, C.; Ewing, A.G. Single-Vesicle Electrochemistry Following Repetitive Stimulation Reveals a Mechanism for Plasticity Changes with Iron Deficiency. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200716. [Google Scholar] [CrossRef]
- Singh, N.; Haldar, S.; Tripathi, A.K.; Horback, K.; Wong, J.; Sharma, D.; Beserra, A.; Suda, S.; Anbalagan, C.; Dev, S.; et al. Brain iron homeostasis: From molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid. Redox Signal. 2014, 20, 1324–1363. [Google Scholar] [CrossRef]
- Haacke, E.M.; Cheng, N.Y.; House, M.J.; Liu, Q.; Neelavalli, J.; Ogg, R.J.; Khan, A.; Ayaz, M.; Kirsch, W.; Obenaus, A. Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging 2005, 23, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Neves, P.; Gozzelino, R. Multilevel Impacts of Iron in the Brain: The Cross Talk between Neurophysiological Mechanisms, Cognition, and Social Behavior. Pharmaceuticals 2019, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Chiou, B.; Neal, E.H.; Bowman, A.B.; Lippmann, E.S.; Simpson, I.A.; Connor, J.R. Endothelial cells are critical regulators of iron transport in a model of the human blood–brain barrier. J. Cereb. Blood Flow Metab. 2019, 39, 2117–2131. [Google Scholar] [CrossRef]
- Duck, K.A.; Neely, E.B.; Simpson, I.A.; Connor, J.R. A role for sex and a common HFE gene variant in brain iron uptake. J. Cereb. Blood Flow Metab. 2018, 38, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Ke, Y. Brain iron transport. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1672–1684. [Google Scholar] [CrossRef]
- Biasiotto, G.; Di Lorenzo, D.; Archetti, S.; Zanella, I. Iron and Neurodegeneration: Is Ferritinophagy the Link? Mol. Neurobiol. 2016, 53, 5542–5574. [Google Scholar] [CrossRef]
- Möller, H.E.; Bossoni, L.; Connor, J.R.; Crichton, R.R.; Does, M.D.; Ward, R.J.; Zecca, L.; Zucca, F.A.; Ronen, I. Iron, Myelin, and the Brain: Neuroimaging Meets Neurobiology. Trends Neurosci. 2019, 42, 384–401. [Google Scholar] [CrossRef]
- Horowitz, M.P.; Greenamyre, J.T. Mitochondrial iron metabolism and its role in neurodegeneration. J. Alzheimers Dis. 2010, 20 (Suppl. S2), S551–S568. [Google Scholar] [CrossRef]
- Melis, J.P.; van Steeg, H.; Luijten, M. Oxidative DNA damage and nucleotide excision repair. Antioxid. Redox Signal. 2013, 18, 2409–2419. [Google Scholar] [CrossRef]
- Hare, D.J.; Double, K.L. Iron and dopamine: A toxic couple. Brain 2016, 139, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, J.; Mao, A.; Zhang, R.; Guan, S. Autophagy Inhibition Plays a Protective Role in Ferroptosis Induced by Alcohol via the p62-Keap1-Nrf2 Pathway. J. Agric. Food Chem. 2021, 69, 9671–9683. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Kong, B.; Qin, T.; Xiao, Z.; Fang, J.; Gong, Y.; Zhu, J.; Liu, Q.; Fu, H.; Meng, H.; et al. Inhibition of ferroptosis reduces susceptibility to frequent excessive alcohol consumption-induced atrial fibrillation. Toxicology 2022, 465, 153055. [Google Scholar] [CrossRef]
- Brissot, P.; Pietrangelo, A.; Adams, P.C.; de Graaff, B.; McLaren, C.E.; Loreal, O. Haemochromatosis. Nat. Rev. Dis. Prim. 2018, 4, 18016. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wang, M.; Yu, H.M.; Han, F.X.; Wu, Q.S.; Cai, X.J.; Kurihara, H.; Chen, Y.X.; Li, Y.F.; He, R.R. Ferroptosis is involved in alcohol-induced cell death in vivo and in vitro. Biosci. Biotechnol. Biochem. 2020, 84, 1621–1628. [Google Scholar] [CrossRef]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019, 133, 221–233. [Google Scholar] [CrossRef]
- Duce, J.A.; Tsatsanis, A.; Cater, M.A.; James, S.A.; Robb, E.; Wikhe, K.; Leong, S.L.; Perez, K.; Johanssen, T.; Greenough, M.A.; et al. Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 2010, 142, 857–867. [Google Scholar] [CrossRef]
- Ayton, S.; Lei, P.; Hare, D.J.; Duce, J.A.; George, J.L.; Adlard, P.A.; McLean, C.; Rogers, J.T.; Cherny, R.A.; Finkelstein, D.I.; et al. Parkinson’s disease iron deposition caused by nitric oxide-induced loss of β-amyloid precursor protein. J. Neurosci. 2015, 35, 3591–3597. [Google Scholar] [CrossRef]
- Ayton, S.; Wang, Y.; Diouf, I.; Schneider, J.A.; Brockman, J.; Morris, M.C.; Bush, A.I. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry 2020, 25, 2932–2941. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, J.; Hao, X.; Li, H.; Zhang, G.; Liu, X.; Li, X.; Zhao, C.; Kuang, W.; Chen, D.; et al. FTH1 Inhibits Ferroptosis Through Ferritinophagy in the 6-OHDA Model of Parkinson’s Disease. Neurotherapeutics 2020, 17, 1796–1812. [Google Scholar] [CrossRef]
- Guiney, S.J.; Adlard, P.A.; Bush, A.I.; Finkelstein, D.I.; Ayton, S. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem. Int. 2017, 104, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Gao, X.; Xu, H.; Cui, Y.; Zhang, Y.; Gou, X. The Emerging Roles of Ferroptosis in Huntington’s Disease. Neuromol. Med. 2019, 21, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Ke, Y. Hepcidin and its therapeutic potential in neurodegenerative disorders. Med. Res. Rev. 2020, 40, 633–653. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Marks, E.; Lai, B.; Zhang, Z.; Duce, J.A.; Lam, L.Q.; Volitakis, I.; Bush, A.I.; Hersch, S.; Fox, J.H. Iron accumulates in Huntington’s disease neurons: Protection by deferoxamine. PLoS ONE 2013, 8, e77023. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Weiland, A.; Wang, Y.; Wu, W.; Lan, X.; Han, X.; Li, Q.; Wang, J. Ferroptosis and Its Role in Diverse Brain Diseases. Mol. Neurobiol. 2019, 56, 4880–4893. [Google Scholar] [CrossRef]
- Jiao, H.; Yang, H.; Yan, Z.; Chen, J.; Xu, M.; Jiang, Y.; Liu, Y.; Xue, Z.; Ma, Q.; Li, X.; et al. Traditional Chinese Formula Xiaoyaosan Alleviates Depressive-like Behavior in CUMS Mice by Regulating PEBP1-GPX4-Mediated Ferroptosis in the Hippocampus. Neuropsychiatr. Dis. Treat. 2021, 17, 1001–1019. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xin, Y.; Zhang, J.; Wang, S.; Yang, Z.; Liu, C. Hydrogen sulfide alleviates the anxiety-like and depressive-like behaviors of type 1 diabetic mice via inhibiting inflammation and ferroptosis. Life Sci. 2021, 278, 119551. [Google Scholar] [CrossRef]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Codazzi, F.; Pelizzoni, I.; Zacchetti, D.; Grohovaz, F. Iron entry in neurons and astrocytes: A link with synaptic activity. Front. Mol. Neurosci. 2015, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Yanatori, I.; Tabuchi, M.; Kawai, Y.; Yasui, Y.; Akagi, R.; Kishi, F. Heme and non-heme iron transporters in non-polarized and polarized cells. BMC Cell Biol. 2010, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Pelizzoni, I.; Zacchetti, D.; Smith, C.P.; Grohovaz, F.; Codazzi, F. Expression of divalent metal transporter 1 in primary hippocampal neurons: Reconsidering its role in non-transferrin-bound iron influx. J. Neurochem. 2012, 120, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Humeres, A.; Elgueta, C.; Kirkwood, A.; Hidalgo, C.; Núñez, M.T. Iron mediates N-methyl-d-aspartate receptor-dependent stimulation of calcium-induced pathways and hippocampal synaptic plasticity. J. Biol. Chem. 2011, 286, 13382–13392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yan, Z.F.; Gao, J.H.; Sun, L.; Huang, X.Y.; Liu, Z.; Yu, S.Y.; Cao, C.J.; Zuo, L.J.; Chen, Z.J.; et al. Role and mechanism of microglial activation in iron-induced selective and progressive dopaminergic neurodegeneration. Mol. Neurobiol. 2014, 49, 1153–1165. [Google Scholar] [CrossRef]
- Song, L.M.; Xiao, Z.X.; Zhang, N.; Yu, X.Q.; Cui, W.; Xie, J.X.; Xu, H.M. Apoferritin improves motor deficits in MPTP-treated mice by regulating brain iron metabolism and ferroptosis. iScience 2021, 24, 102431. [Google Scholar] [CrossRef]
- Tucker, L.B.; McCabe, J.T. Behavior of Male and Female C57BL/6J Mice Is More Consistent with Repeated Trials in the Elevated Zero Maze than in the Elevated Plus Maze. Front. Behav. Neurosci. 2017, 11, 13. [Google Scholar] [CrossRef]
- Lee, K.M.; Coehlo, M.; McGregor, H.A.; Waltermire, R.S.; Szumlinski, K.K. Binge alcohol drinking elicits persistent negative affect in mice. Behav. Brain Res. 2015, 291, 385–398. [Google Scholar] [CrossRef]
- Cannella, N.; Kallupi, M.; Li, H.W.; Stopponi, S.; Cifani, C.; Ciccocioppo, R.; Ubaldi, M. Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats. Psychopharmacology 2016, 233, 2915–2924. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wei, H.J.; Wu, L.; Liu, S.M.; Tang, Y.Y.; Zou, W.; Wang, C.Y.; Zhang, P.; Tang, X.Q. BDNF-TrkB pathway mediates antidepressant-like roles of H2S in diabetic rats via promoting hippocampal autophagy. Clin. Exp. Pharmacol. Physiol. 2020, 47, 302–312. [Google Scholar] [CrossRef]
- Ru, Q.; Xiong, Q.; Zhou, M.; Chen, L.; Tian, X.; Xiao, H.; Li, C.; Li, Y. Withdrawal from chronic treatment with methamphetamine induces anxiety and depression-like behavior in mice. Psychiatry Res. 2019, 271, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, S.; Ghiasyzadeh, F.; Darvishi, M.; Karimi, E.; Ghaneialvar, H.; Alizadeh, R.; Moayeri, A.; Abbasi, N. The Effect of Prosopis farcta and Its Bioactive Luteolin on the Hippocampus of Mice after Induced Ischemia Reperfusion. Evid. Based Complement. Alternat. Med. 2022, 2022, 8157948. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Tian, X.; Xu, C.; Ma, B.; Liu, W.; Sun, B.; Ru, Q.; Shu, X. PM2.5 exposure-induced ferroptosis in neuronal cells via inhibiting ERK/CREB pathway. Environ. Toxicol. 2022, 37, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Wang, Z.; Zhang, X.; Qian, D.; Wang, Y.; Jiang, S.; Liang, S.; Wang, B. HCMV-encoded IE2 induces anxiety-depression and cognitive impairment in UL122 genetically modified mice. Int. J. Clin. Exp. Pathol. 2019, 12, 4087–4095. [Google Scholar] [PubMed]
- Zeng, Q.; Xiong, Q.; Lin, K.; Liang, Z.; Zhou, M.; Tian, X.; Xu, C.; Ru, Q. Terminalia chebula extracts ameliorate methamphetamine-induced memory deficits via activating the ERK and Nrf2 pathway. Brain Res. Bull. 2022, 184, 76–87. [Google Scholar] [CrossRef]
- Mu, X.; Li, W.; Ze, X.; Li, L.; Wang, G.; Hong, F.; Ze, Y. Molecular mechanism of nanoparticulate TiO2 induction of axonal development inhibition in rat primary cultured hippocampal neurons. Environ. Toxicol. 2020, 35, 895–905. [Google Scholar] [CrossRef]
- Xiong, Q.; Ru, Q.; Tian, X.; Zhou, M.; Chen, L.; Li, Y.; Li, C. Krill oil protects PC12 cells against methamphetamine-induced neurotoxicity by inhibiting apoptotic response and oxidative stress. Nutr. Res. 2018, 58, 84–94. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Xiong, Q.; Tian, X.; Liu, W.; Sun, B.; Ru, Q.; Shu, X. Alcohol Exposure Induces Depressive and Anxiety-like Behaviors via Activating Ferroptosis in Mice. Int. J. Mol. Sci. 2022, 23, 13828. https://doi.org/10.3390/ijms232213828
Xu C, Xiong Q, Tian X, Liu W, Sun B, Ru Q, Shu X. Alcohol Exposure Induces Depressive and Anxiety-like Behaviors via Activating Ferroptosis in Mice. International Journal of Molecular Sciences. 2022; 23(22):13828. https://doi.org/10.3390/ijms232213828
Chicago/Turabian StyleXu, Congyue, Qi Xiong, Xiang Tian, Wei Liu, Binlian Sun, Qin Ru, and Xiji Shu. 2022. "Alcohol Exposure Induces Depressive and Anxiety-like Behaviors via Activating Ferroptosis in Mice" International Journal of Molecular Sciences 23, no. 22: 13828. https://doi.org/10.3390/ijms232213828
APA StyleXu, C., Xiong, Q., Tian, X., Liu, W., Sun, B., Ru, Q., & Shu, X. (2022). Alcohol Exposure Induces Depressive and Anxiety-like Behaviors via Activating Ferroptosis in Mice. International Journal of Molecular Sciences, 23(22), 13828. https://doi.org/10.3390/ijms232213828




