In Silico Study of the Acquired Resistance Caused by the Secondary Mutations of KRAS G12C Protein Using Long Time Molecular Dynamics Simulation and Markov State Model Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Dynamics Simulations
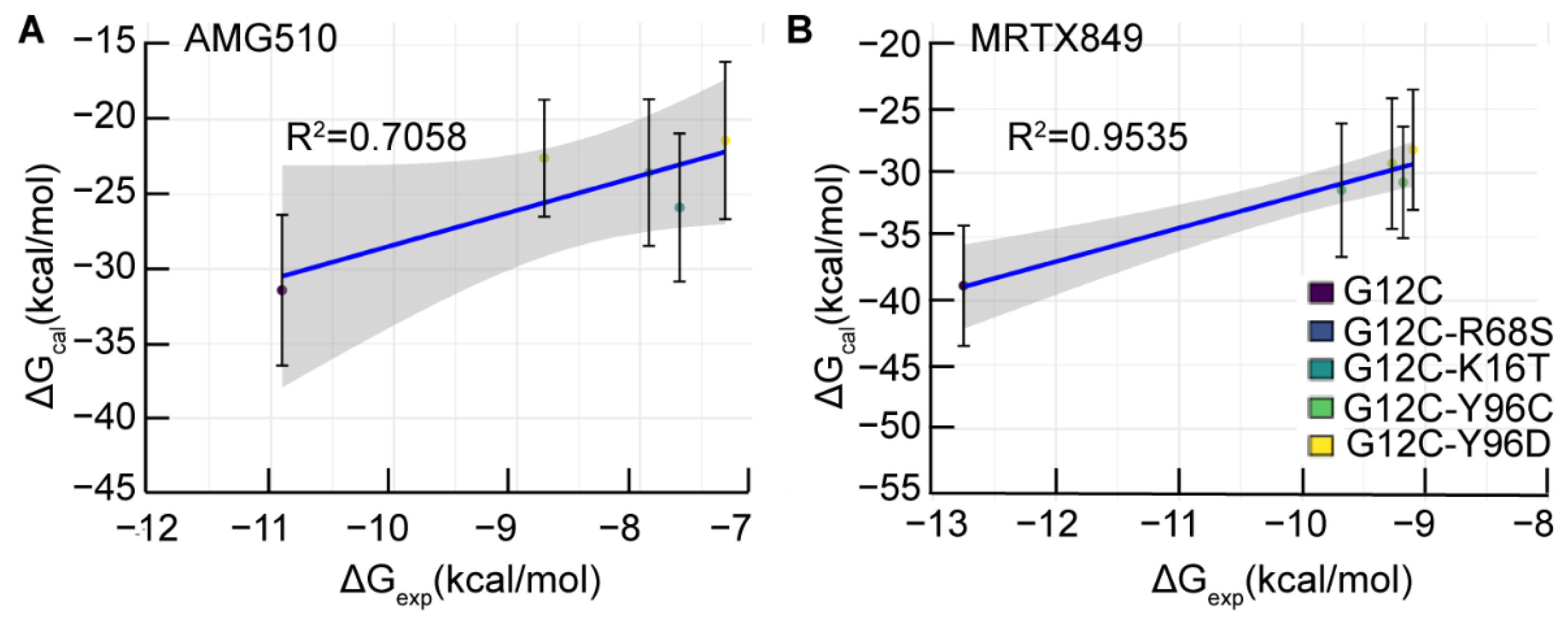
2.2. Binding Free Energy of AMG510 and MRTX849 to Mutant KRAS
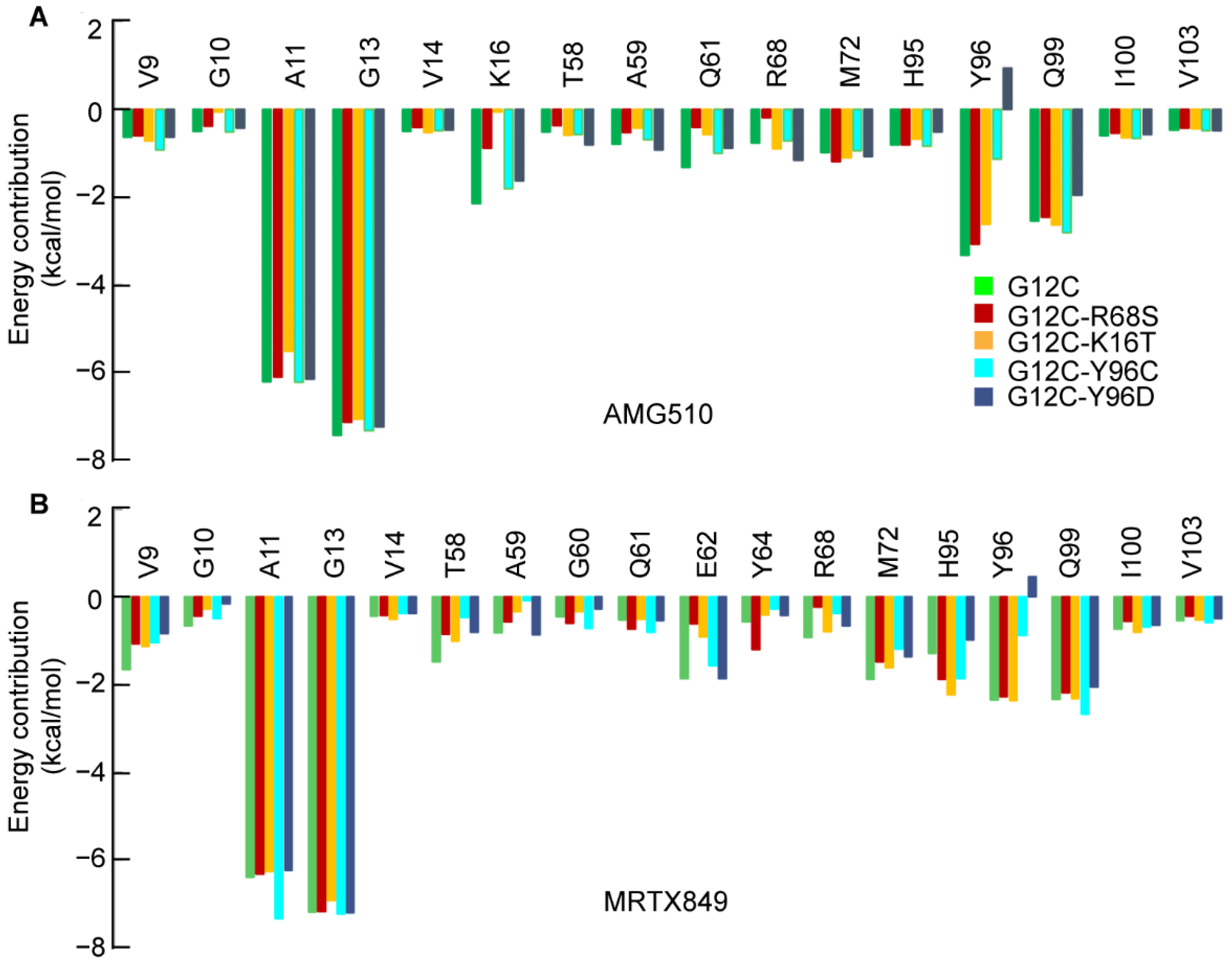
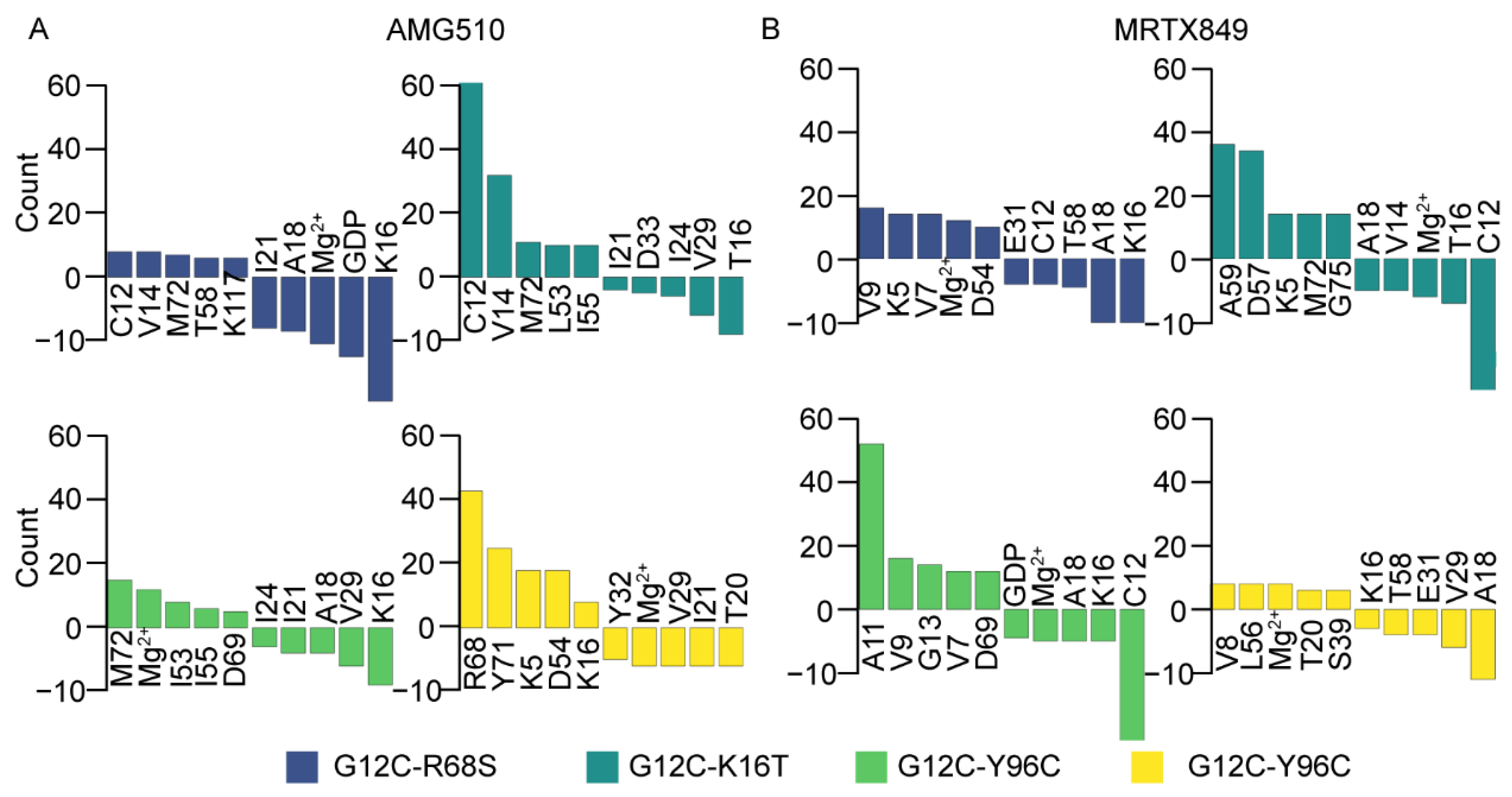
2.3. Insight from the Per-Residue Binding Free Energy Calculation
2.3.1. Per-Residue Energy Decomposition for AMG510
2.3.2. Per-Residue Energy-Decomposition for MRTX849
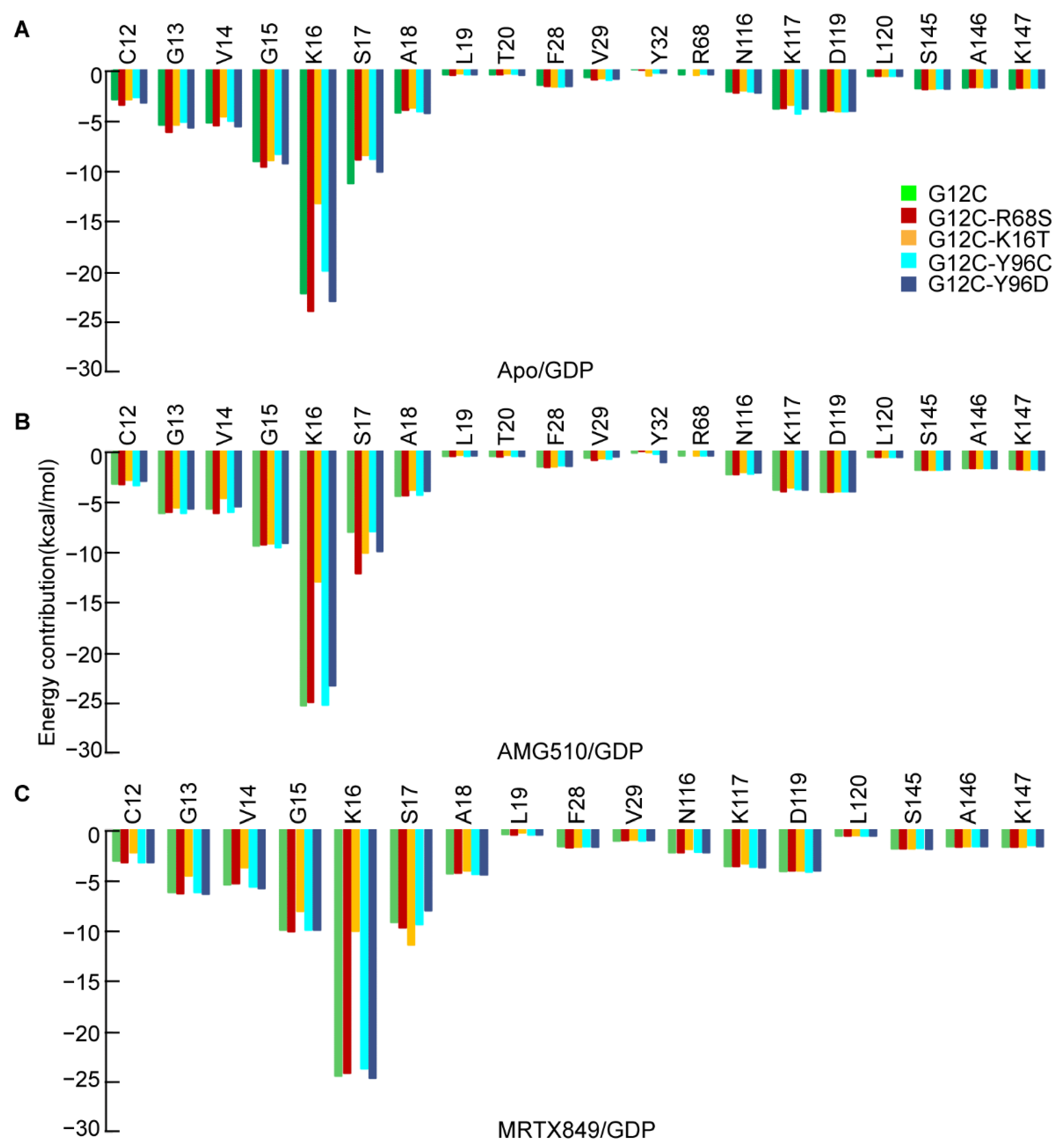
2.4. Binding Free Energy of Mutated KRAS G12C and GDP
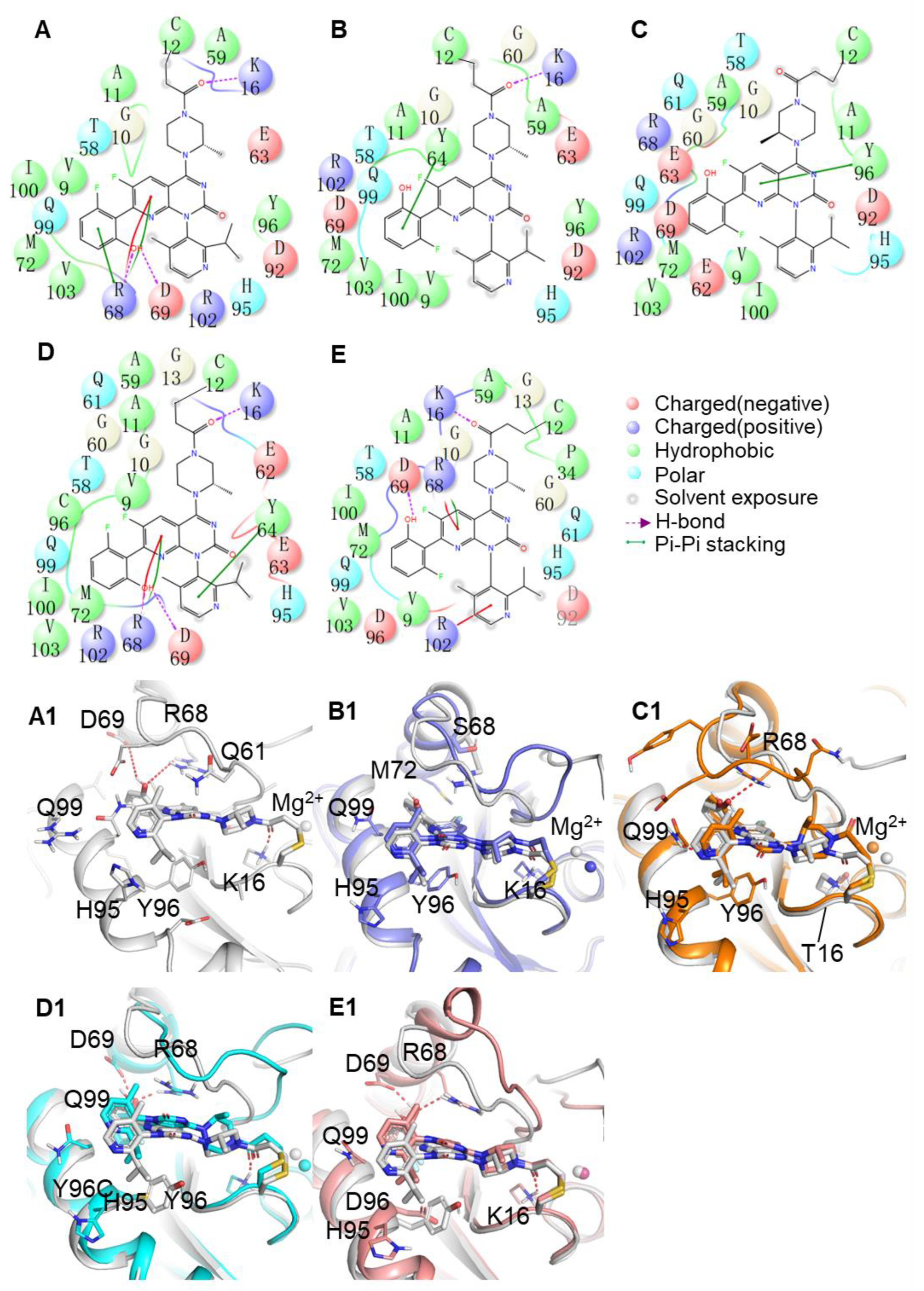
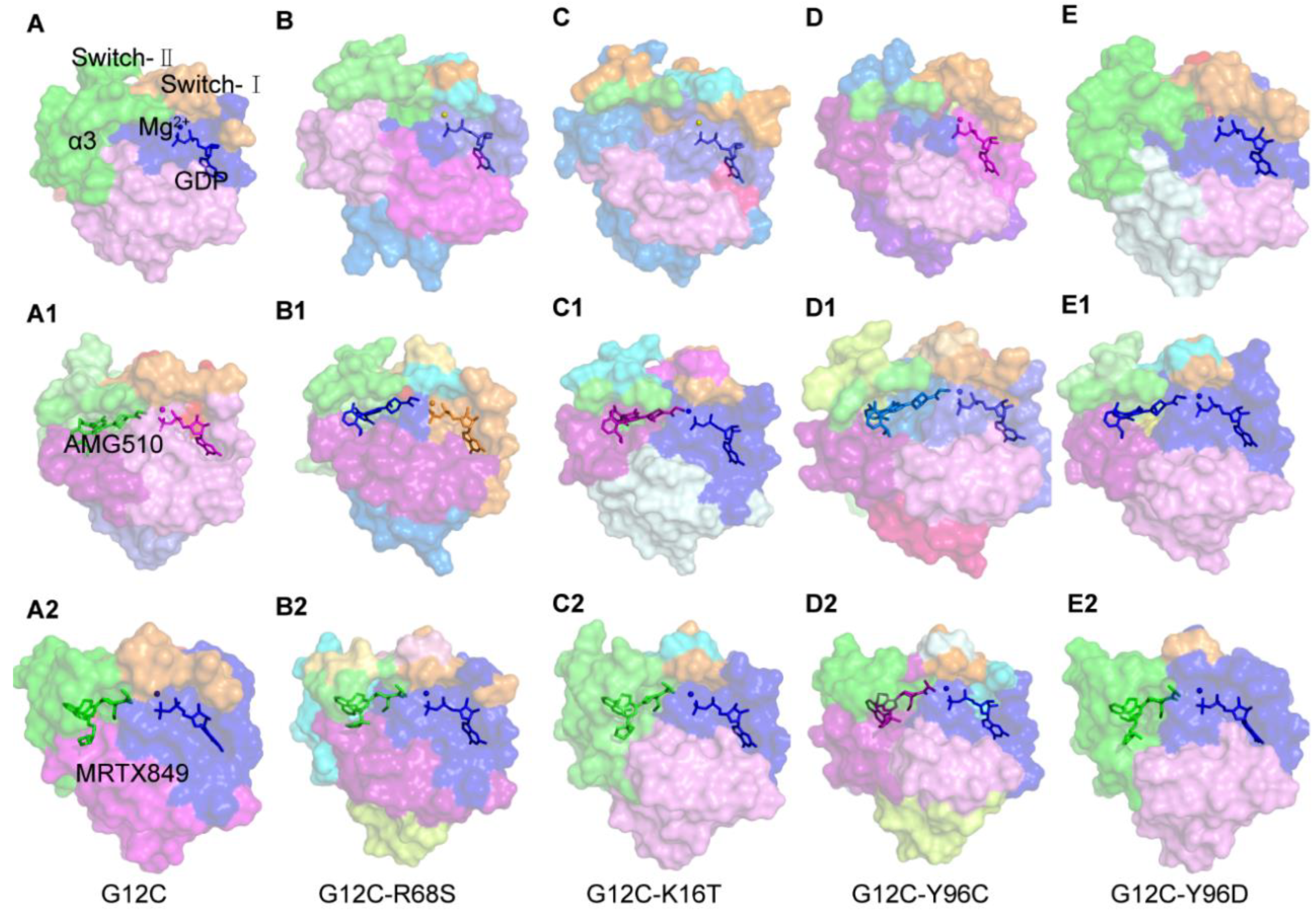
2.5. Binding Mode between AMG510 and MRTX849 with G12C and Double Mutant KRAS
2.6. Conformational Dynamics Comparison between the Apo Form KRAS and Inhibitor Bind to KRAS Complex
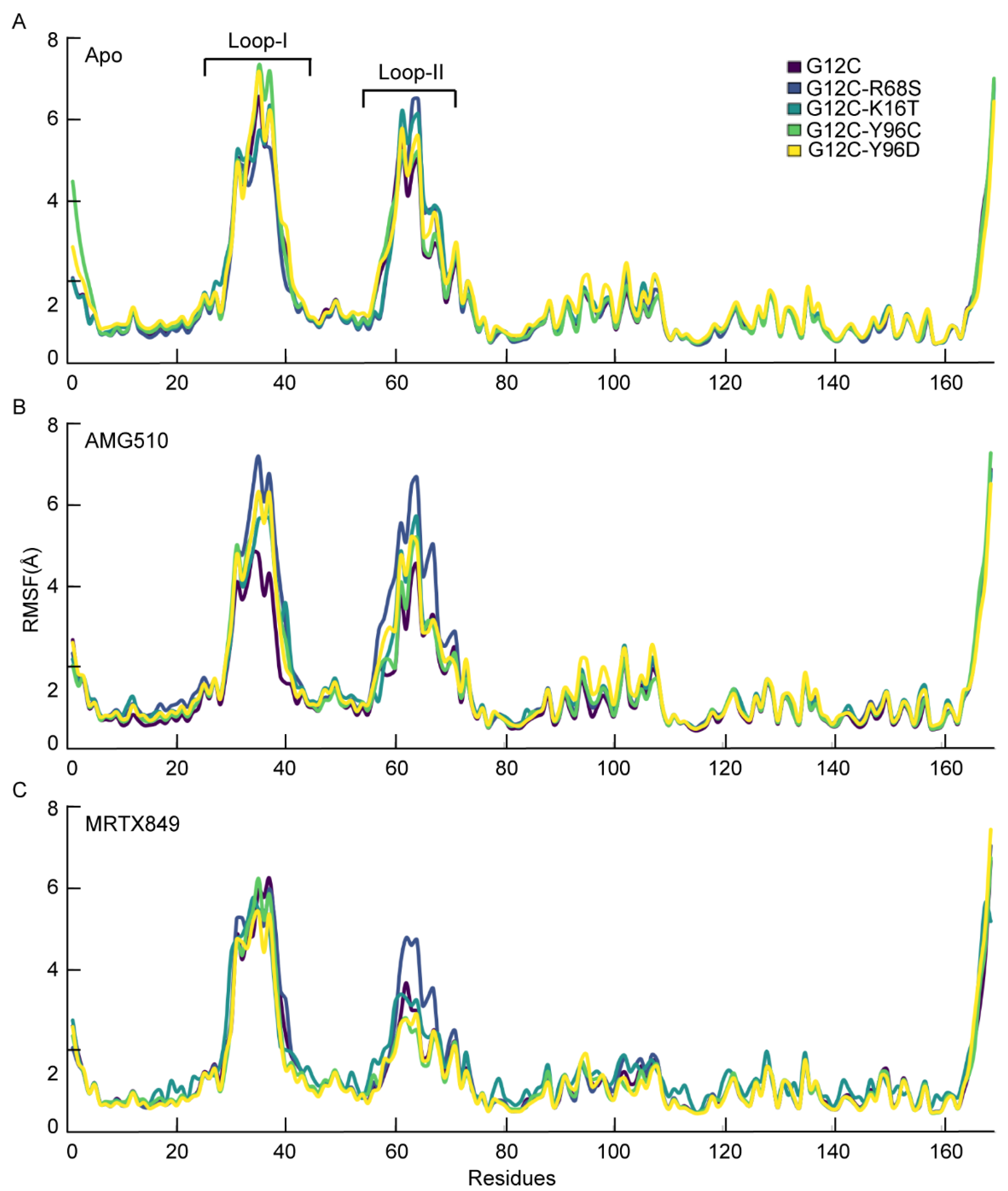
2.6.1. Structural Fluctuation for the Apo and Inhibitor Complex
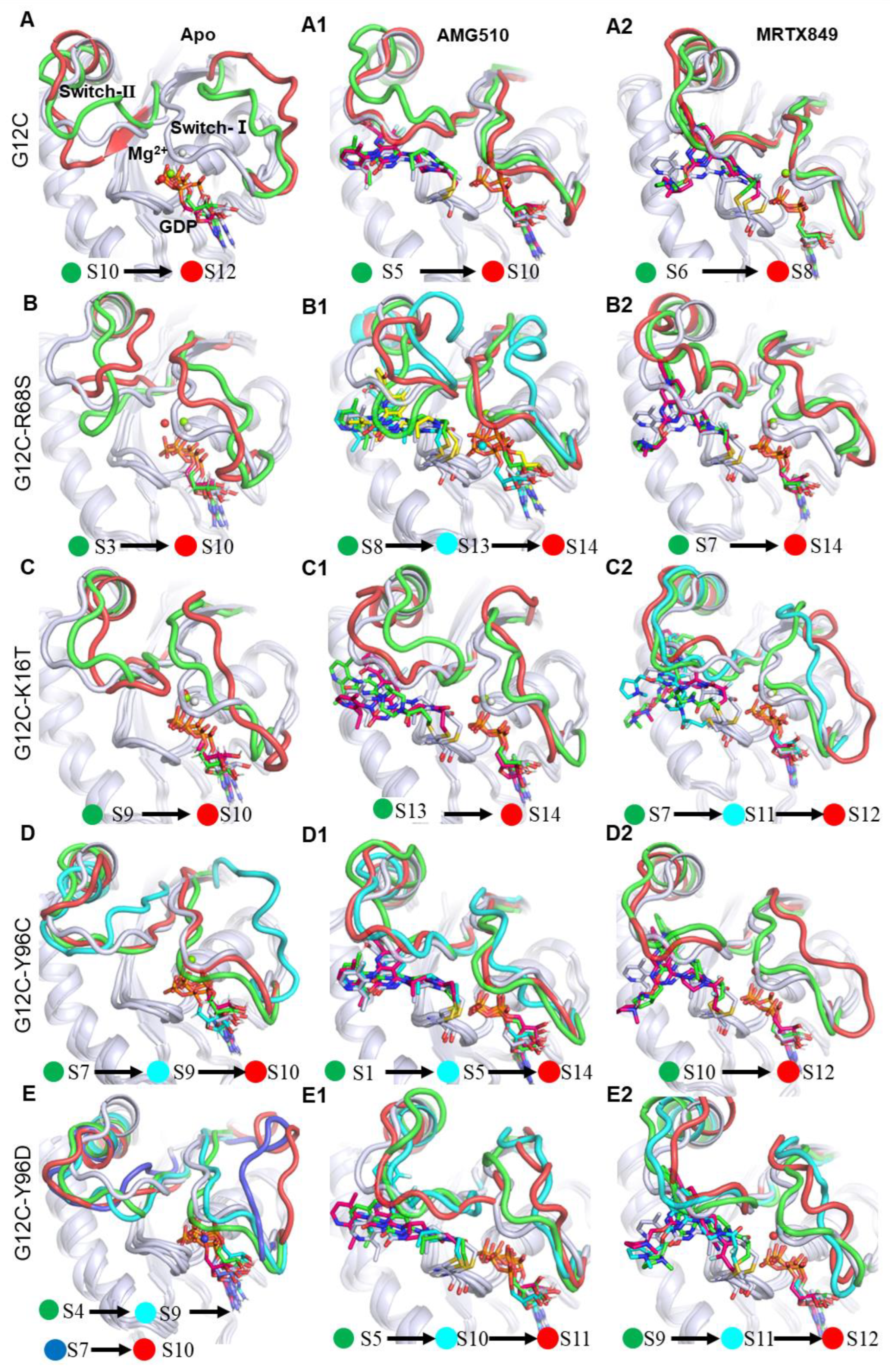
2.6.2. Results of MSM
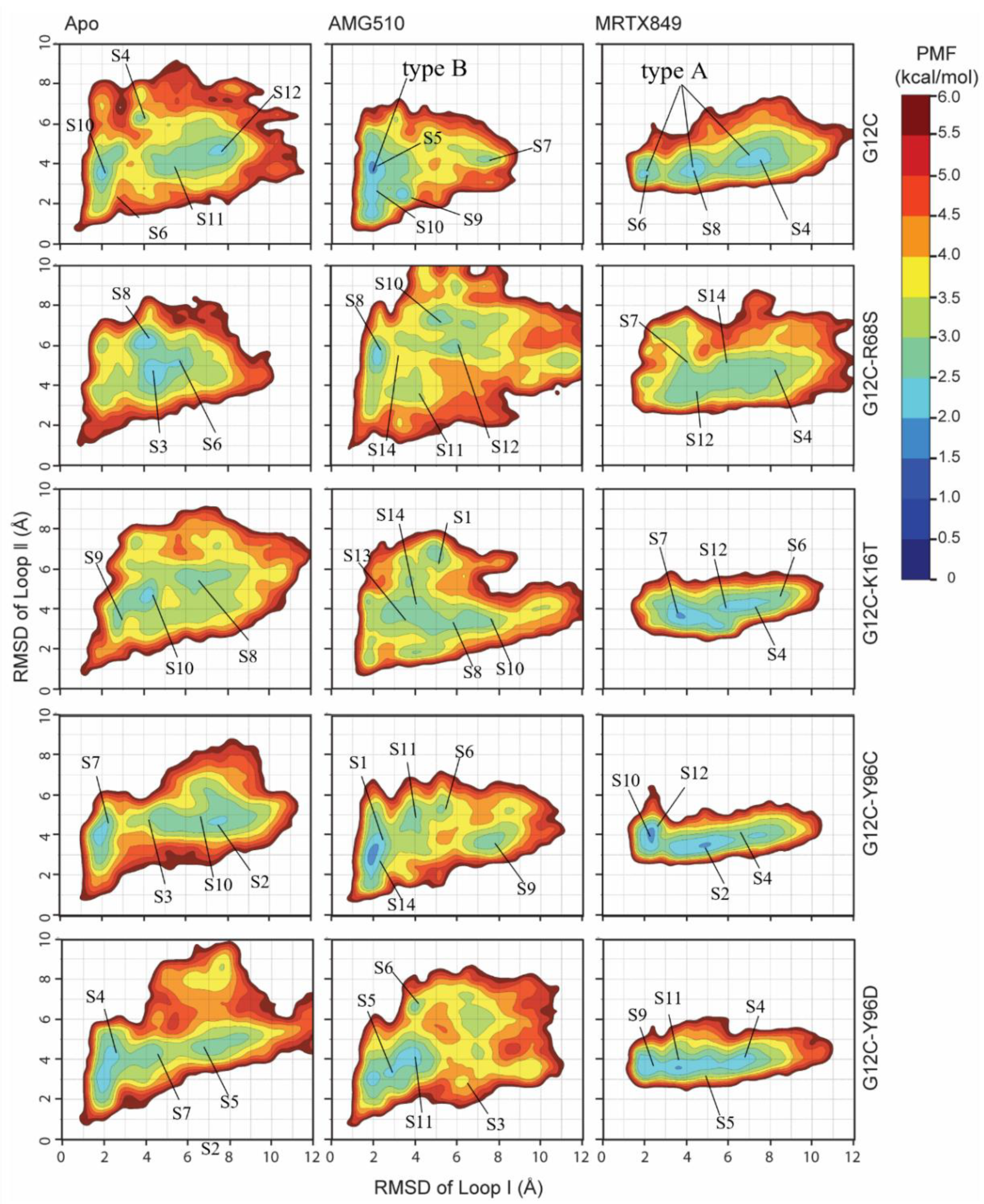
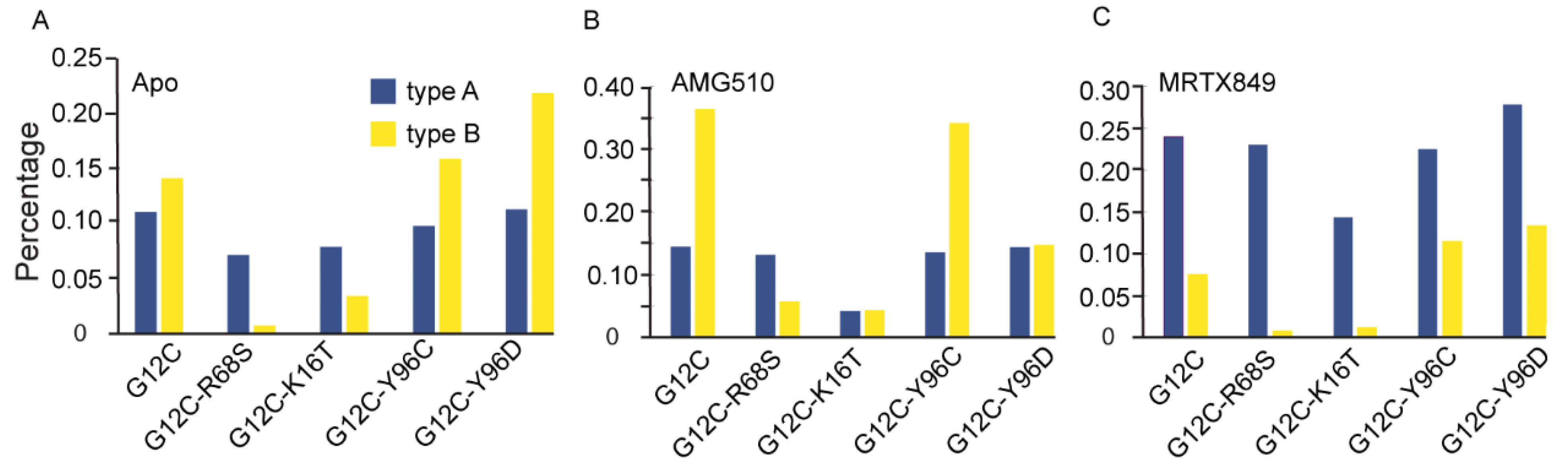
2.6.3. Mutation-Mediated Impacts on Free Energy Profiles of Apo and Inhibitor KRAS Complex
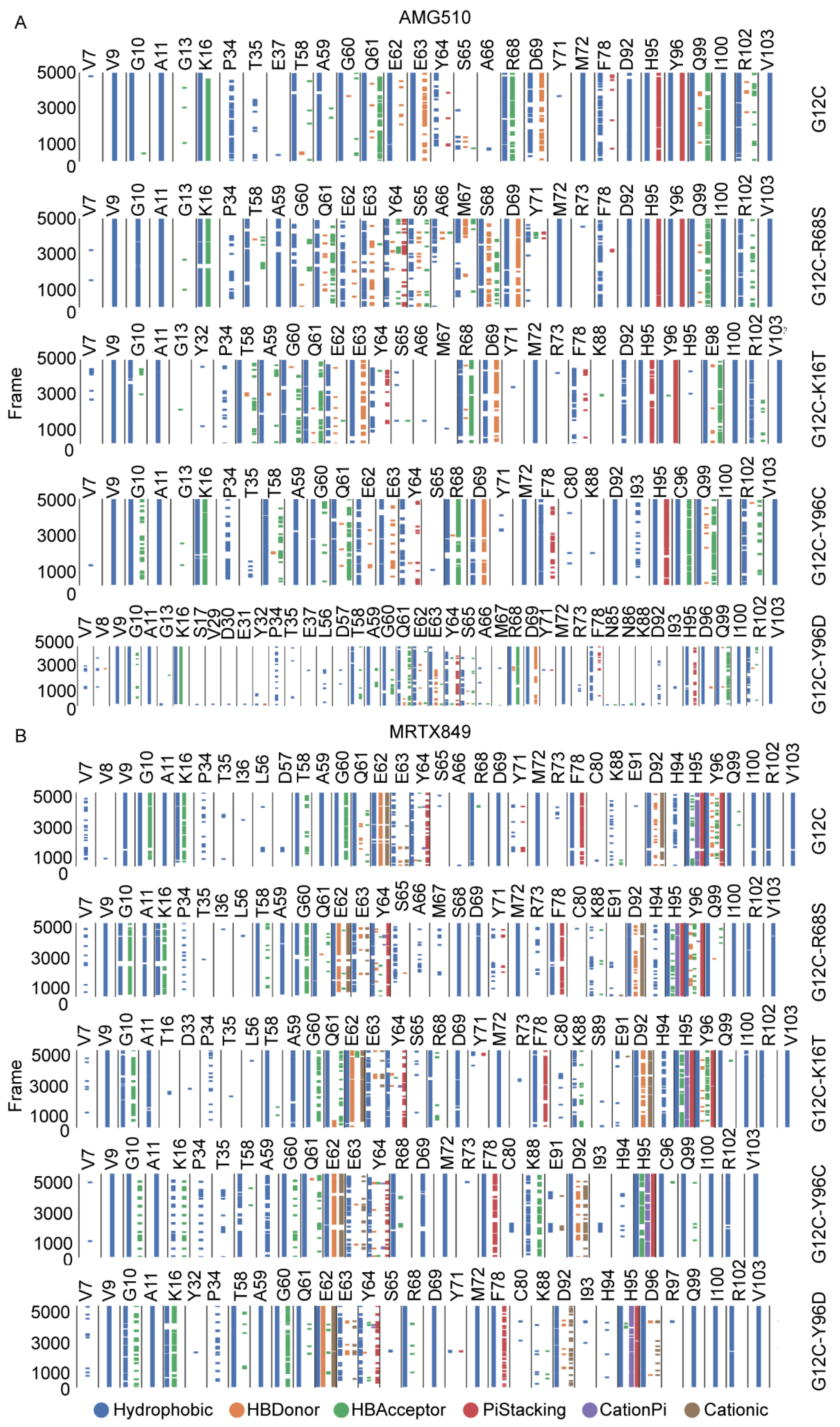
2.7. Comparison of Protein-Ligand Interaction Fingerprint between KRAS G12C and Double-Mutant KRAS
2.8. Dynamics Network Analysis
3. Materials and Methods
3.1. Construction of Simulated Systems
3.2. Molecular Dynamics Simulation
3.3. Thermodynamics Analysis
3.4. Construction and Verification of Markov State Models
3.5. Potential of Mean Force Analysis
3.6. Interaction Fingerprints Analysis
3.7. Dynamic Network Analysis
3.8. Pharmacophore Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, G.A.; Wittinghofer, A.; Der, C.J. Selective Targeting of the KRAS G12C Mutant: Kicking KRAS When It’s Down. Cancer Cell 2016, 29, 251–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef] [Green Version]
- McCormick, F. KRAS as a Therapeutic Target. Clin. Cancer Res. 2015, 21, 1797–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef] [Green Version]
- Ostrem, J.M.; Shokat, K.M. Direct small-molecule inhibitors of KRAS: From structural insights to mechanism-based design. Nat. Rev. Drug Discov. 2016, 15, 771–785. [Google Scholar] [CrossRef]
- Collins, M.A.; Pasca di Magliano, M. Kras as a key oncogene and therapeutic target in pancreatic cancer. Front. Physiol. 2013, 4, 407. [Google Scholar] [CrossRef] [Green Version]
- Pantsar, T. The current understanding of KRAS protein structure and dynamics. Comput. Struct. Biotechnol. J. 2020, 18, 189–198. [Google Scholar] [CrossRef]
- Mullard, A. Cracking KRAS. Nat. Rev. Drug Discov. 2019, 18, 887–891. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Liu, Q.; Fan, X.X.; Leung, E.L.; Yao, X.J.; Liu, L. Resistance looms for KRAS G12C inhibitors and rational tackling strategies. Pharmacol. Ther. 2022, 229, 108050. [Google Scholar] [CrossRef]
- Ghimessy, A.; Radeczky, P.; Laszlo, V.; Hegedus, B.; Renyi-Vamos, F.; Fillinger, J.; Klepetko, W.; Lang, C.; Dome, B.; Megyesfalvi, Z. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev. 2020, 39, 1159–1177. [Google Scholar] [CrossRef]
- Kwan, A.K.; Piazza, G.A.; Keeton, A.B.; Leite, C.A. The path to the clinic: A comprehensive review on direct KRAS(G12C) inhibitors. J. Exp. Clin. Cancer Res. 2022, 41, 27. [Google Scholar] [CrossRef]
- Ou, S.-H.I.; Jänne, P.A.; Leal, T.A.; Rybkin, I.I.; Sabari, J.K.; Barve, M.A.; Bazhenova, L.; Johnson, M.L.; Velastegui, K.L.; Cilliers, C. First-in-Human Phase I/IB Dose-Finding Study of Adagrasib (MRTX849) in Patients With Advanced KRAS G12C Solid Tumors (KRYSTAL-1). J. Clin. Oncol. 2022, 40, 2530. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, R.B. Improved Synthesis of New FDA-Approved Treatment for KRAS G12C Mutation in Non-small Cell Lung Cancer. ACS. Med. Chem. Lett. 2021, 12, 1186–1187. [Google Scholar] [CrossRef]
- Awad, M.M.; Liu, S.; Rybkin, I.I.; Arbour, K.C.; Dilly, J.; Zhu, V.W.; Johnson, M.L.; Heist, R.S.; Patil, T.; Riely, G.J.; et al. Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N. Engl. J. Med. 2021, 384, 2382–2393. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Suda, K.; Fujino, T.; Ohara, S.; Hamada, A.; Nishino, M.; Chiba, M.; Shimoji, M.; Takemoto, T.; Arita, T.; et al. KRAS Secondary Mutations That Confer Acquired Resistance to KRAS G12C Inhibitors, Sotorasib and Adagrasib, and Overcoming Strategies: Insights From In Vitro Experiments. J. Thorac. Oncol. 2021, 16, 1321–1332. [Google Scholar] [CrossRef]
- Tanaka, N.; Lin, J.J.; Li, C.; Ryan, M.B.; Zhang, J.; Kiedrowski, L.A.; Michel, A.G.; Syed, M.U.; Fella, K.A.; Sakhi, M.; et al. Clinical Acquired Resistance to KRAS(G12C) Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS-MAPK Reactivation. Cancer Discov. 2021, 11, 1913–1922. [Google Scholar] [CrossRef]
- Xue, W.; Jin, X.; Ning, L.; Wang, M.; Liu, H.; Yao, X. Exploring the molecular mechanism of cross-resistance to HIV-1 integrase strand transfer inhibitors by molecular dynamics simulation and residue interaction network analysis. J. Chem. Inf. Model. 2013, 53, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Qi, J.; Yang, Y.; Jin, X.; Liu, H.; Yao, X. Understanding the effect of drug-resistant mutations of HIV-1 intasome on raltegravir action through molecular modeling study. Mol. Biosyst. 2012, 8, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, T.; Wang, W.; Liu, J.S. Detecting and understanding combinatorial mutation patterns responsible for HIV drug resistance. Proc. Natl. Acad. Sci. USA 2010, 107, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Yu, R. Molecular dynamics and free energy studies on the wild-type and double mutant HIV-1 protease complexed with amprenavir and two amprenavir-related inhibitors: Mechanism for binding and drug resistance. J. Med. Chem. 2007, 50, 1177–1188. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Ban, Y.; Liu, H.; Yao, X. Computational study on the drug resistance mechanism against HCV NS3/4A protease inhibitors vaniprevir and MK-5172 by the combination use of molecular dynamics simulation, residue interaction network, and substrate envelope analysis. J. Chem. Inf. Model. 2014, 54, 621–633. [Google Scholar] [CrossRef]
- Li, Y.; Han, L.; Zhang, Z. Understanding the influence of AMG 510 on the structure of KRAS(G12C) empowered by molecular dynamics simulation. Comput. Struct. Biotechnol. J. 2022, 20, 1056–1067. [Google Scholar] [CrossRef]
- Li, X.; Dai, J.; Ni, D.; He, X.; Zhang, H.; Zhang, J.; Fu, Q.; Liu, Y.; Lu, S. Insight into the mechanism of allosteric activation of PI3Kalpha by oncoprotein K-Ras4B. Int. J. Biol. Macromol. 2020, 144, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Paul, F.; Wehmeyer, C.; Noe, F. Multiensemble Markov models of molecular thermodynamics and kinetics. Proc. Natl. Acad. Sci. USA 2016, 113, E3221–E3230. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Du, K.; Wang, Y.; Fan, J.; Li, M.; Ni, D.; Lu, S.; Bian, X.; Liu, Y. Autopromotion of K-Ras4B Feedback Activation Through an SOS-Mediated Long-Range Allosteric Effect. Front. Mol. Biosci. 2022, 9, 860962. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Song, K.; Zhang, J.; Lu, S. Molecular Dynamics Simulations and Dynamic Network Analysis Reveal the Allosteric Unbinding of Monobody to H-Ras Triggered by R135K Mutation. Int. J. Mol. Sci. 2017, 18, 2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef]
- Fell, J.B.; Fischer, J.P.; Baer, B.R.; Blake, J.F.; Bouhana, K.; Briere, D.M.; Brown, K.D.; Burgess, L.E.; Burns, A.C.; Burkard, M.R.; et al. Identification of the Clinical Development Candidate MRTX849, a Covalent KRAS(G12C) Inhibitor for the Treatment of Cancer. J. Med. Chem. 2020, 63, 6679–6693. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Kang, R.; Tang, D. The KRAS-G12C inhibitor: Activity and resistance. Cancer Gene. Ther. 2022, 29, 875–878. [Google Scholar] [CrossRef]
- Milburn, M.V.; Tong, L.; deVos, A.M.; Brunger, A.; Yamaizumi, Z.; Nishimura, S.; Kim, S.H. Molecular switch for signal transduction: Structural differences between active and inactive forms of protooncogenic ras proteins. Science 1990, 247, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Trozzi, F.; Zoltowski, B.D.; Tao, P. Deciphering the Allosteric Process of the Phaeodactylum tricornutum Aureochrome 1a LOV Domain. J. Phys. Chem. B 2020, 124, 8960–8972. [Google Scholar] [CrossRef]
- Lu, S.; Ni, D.; Wang, C.; He, X.; Lin, H.; Wang, Z.; Zhang, J. Deactivation pathway of Ras GTPase underlies conformational substates as targets for drug design. ACS Catal. 2019, 9, 7188–7196. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PyMOL Molecular Graphics System, Version 1.3; Schrödinger, LLC: New York, NY, USA, 2010.
- Release, S. 4: BioLuminate, Version 3.3; Schrödinger, LLC: New York, NY, USA, 2018.
- AMBER, Version 18; University of California: San Francisco, CA, USA, 2018.
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Version 5.0; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Meagher, K.L.; Redman, L.T.; Carlson, H.A. Development of polyphosphate parameters for use with the AMBER force field. J. Comput. Chem. 2003, 24, 1016–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Tsui, V.; Case, D.A. Theory and applications of the generalized Born solvation model in macromolecular simulations. Biopolymers 2000, 56, 275–291. [Google Scholar] [CrossRef]
- Wang, C.; Greene, D.; Xiao, L.; Qi, R.; Luo, R. Recent Developments and Applications of the MMPBSA Method. Front. Mol. Biosci. 2017, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert. Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Sitkoff, D.; Sharp, K.A.; Honig, B. Accurate calculation of hydration free energies using macroscopic solvent models. J. Phys. Chem. 1994, 98, 1978–1988. [Google Scholar] [CrossRef]
- Miller, B.R., 3rd; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Metzner, P.; Schütte, C.; Vanden-Eijnden, E. Transition path theory for Markov jump processes. Multiscale Model. Simul. 2009, 7, 1192–1219. [Google Scholar] [CrossRef]
- Vanden-Eijnden, E. Towards a theory of transition paths. J. Stat. Phys. 2006, 123, 503–523. [Google Scholar]
- Scherer, M.K.; Trendelkamp-Schroer, B.; Paul, F.; Perez-Hernandez, G.; Hoffmann, M.; Plattner, N.; Wehmeyer, C.; Prinz, J.H.; Noe, F. PyEMMA 2: A Software Package for Estimation, Validation, and Analysis of Markov Models. J. Chem. Theory Comput. 2015, 11, 5525–5542. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications; Elsevier: Amsterdam, The Netherlands, 2001; Volume 1. [Google Scholar]
- Bouysset, C.; Fiorucci, S. ProLIF: A library to encode molecular interactions as fingerprints. J. Cheminform. 2021, 13, 72. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Leung, E.L.; Yao, X. In silico study of intrinsic dynamics of full-length apo-ACE2 and RBD-ACE2 complex. Comput. Struct. Biotechnol. J. 2021, 19, 5455–5465. [Google Scholar] [CrossRef]
- Maestro, Version 9.0; Schrodinger, LLC: New York, NY, USA, 2009.


| Energy Components (kcal/mol) | AMG510-Bound System | ||||
|---|---|---|---|---|---|
| G12C | G12C-R68S | G12C-K16T | G12C-Y96C | G12C-Y96D | |
| ΔEvdW | −62.48 ± 4.92 | −51.83 ± 6.46 | −58.18 ± 7.21 | −56.68 ± 6.67 | −52.55 ± 6.45 |
| ΔEelec | −152.11 ± 7.46 | −147.85 ± 10.66 | −146.96 ± 10.80 | −148.16 ± 9.23 | −145.39 ± 10.94 |
| ΔEcovalent | 89.06 ± 1.51 | 89.12 ± 1.50 | 89.83 ± 1.42 | 88.94 ± 1.51 | 89.32 ± 1.48 |
| ΔGsolv | 59.13 ± 6.30 | 52.20 ± 9.51 | 55.93 ± 8.12 | 54.48 ± 7.36 | 52.01 ± 8.88 |
| a ΔEbind | −67.39 ± 5.15 | −58.36 ± 6.97 | −59.38 ± 6.52 | −61.42 ± 6.8 | −56.59 ± 6.11 |
| b−TΔS | 35.96 ± 5.51 | 35.78 ± 3.92 | 35.84 ± 4.91 | 35.54 ± 4.96 | 35.19 ± 5.27 |
| c ΔGcal | −31.43 | −22.58 | −23.54 | −25.88 | −21.40 |
| d ΔΔGcal | 8.85 | 7.89 | 5.55 | 10.03 | |
| e ΔGexp | −10.91 | −8.78 | −7.93 | −7.68 | −7.31 |
| IC50(nM) | 19.81 | 643.27 | 2524 | 3814 | 6920.67 |
| Energy Components (kcal/mol) | MRTX849-Bound System | ||||
|---|---|---|---|---|---|
| G12C | G12C-R68S | G12C-K16T | G12C-Y96C | G12C-Y96D | |
| ΔEvdW | −71.53 ± 3.94 | −62.38 ± 8.77 | −63.40 ± 6.77 | −62.05 ± 4.79 | −56.34 ± 9.84 |
| ΔEelec | −306.89 ± 29.32 | −299.89 ± 28.64 | −319.38 ± 29.57 | −281.80 ± 21.47 | −308.99 ± 22.98 |
| ΔEcovalent | 89.99 ± 1.48 | 89.71 ± 1.44 | 90.35 ± 1.56 | 89.67 ± 1.42 | 89.64 ± 1.49 |
| ΔGsolv | 211.95 ± 27.82 | 204.29 ± 28.15 | 226.33 ± 28.39 | 187.00 ± 20.53 | 215.42 ± 22.71 |
| a ΔEbind | −76.48 ± 7.28 | −68.28 ± 7.70 | −66.10 ± 8.22 | −67.18 ± 5.27 | −60.39 ± 8.62 |
| b−TΔS | 37.67 ± 4.70 | 36.94 ± 5.22 | 36.84 ± 5.11 | 36.45 ± 4.36 | 34.81 ± 4.78 |
| c ΔGcal | −38.81 | −31.34 | −29.26 | −30.73 | −28.18 |
| d ΔΔGcal | 7.47 | 9.55 | 8.08 | 10.63 | |
| e ΔGexp | −12.75 | −9.68 | −9.27 | −9.18 | −9.10 |
| IC50(nM) | 1.01 | 147.07 | 289.47 | 332.90 | 376.64 |
| Residues | Interaction Type | Frequency (%) | ||||
|---|---|---|---|---|---|---|
| G12C | G12-R68S | G12C-K16T | G12C-Y96C | G12C-Y96D | ||
| V9 | Hydrophobic | 93.78 | 77.52 | 77.42 | 95.44 | 68.04 |
| G10 | Hydrophobic | 67.94 | 64.06 | 0.00 | 68.78 | 32.80 |
| A11 | Hydrophobic | 72.70 | 73.30 | 39.42 | 79.40 | 56.64 |
| 16 | Hydrophobic | 77.04 | 73.72 | 0.00 | 68.30 | 54.02 |
| HBAcceptor | 85.84 | 84.16 | 0.00 | 78.74 | 69.06 | |
| T58 | Hydrophobic | 84.54 | 71.74 | 80.60 | 84.74 | 67.60 |
| A59 | Hydrophobic | 83.12 | 70.82 | 56.18 | 78.66 | 77.70 |
| G60 | Hydrophobic | 72.16 | 37.82 | 57.28 | 64.00 | 60.34 |
| Q61 | Hydrophobic | 78.12 | 40.56 | 54.40 | 62.24 | 55.86 |
| E62 | Hydrophobic | 33.34 | 0.00 | 0.00 | 0.00 | 0.00 |
| E63 | Hydrophobic | 56.62 | 33.72 | 57.86 | 43.26 | 51.84 |
| 68 | Hydrophobic | 61.20 | 0.00 | 76.62 | 64.30 | 84.44 |
| M72 | Hydrophobic | 99.56 | 99.02 | 98.84 | 98.92 | 95.28 |
| H95 | Hydrophobic | 99.66 | 99.36 | 97.86 | 97.52 | 60.00 |
| 96 | Hydrophobic | 100.00 | 100.00 | 99.96 | 98.44 | 85.82 |
| Q99 | Hydrophobic | 99.98 | 99.90 | 99.96 | 99.94 | 95.22 |
| I100 | Hydrophobic | 43.96 | 43.20 | 62.14 | 76.82 | 70.48 |
| V103 | Hydrophobic | 86.10 | 81.74 | 84.32 | 84.30 | 70.14 |
| Residues | Interaction Type | Frequency (%) | ||||
|---|---|---|---|---|---|---|
| G12C | G12C-R68S | G12C-K16T | G12C-Y96C | G12C-Y96D | ||
| V9 | Hydrophobic | 89.20 | 83.46 | 90.76 | 98.82 | 93.42 |
| G10 | Hydrophobic | 75.92 | 72.84 | 52.52 | 43.82 | 0.00 |
| HBAcceptor | 48.92 | 54.86 | 0.00 | 0.00 | 0.00 | |
| A11 | Hydrophobic | 32.34 | 0.00 | 41.06 | 76.36 | 41.62 |
| 16 | Hydrophobic | 60.90 | 50.58 | 0.00 | 0.00 | 41.76 |
| T58 | Hydrophobic | 91.06 | 84.84 | 74.02 | 40.08 | 80.42 |
| A59 | Hydrophobic | 71.84 | 78.16 | 56.96 | 32.46 | 77.54 |
| G60 | Hydrophobic | 87.62 | 77.00 | 79.66 | 89.52 | 86.02 |
| Q61 | Hydrophobic | 87.02 | 83.64 | 84.38 | 88.62 | 90.98 |
| E62 | Hydrophobic | 82.60 | 80.66 | 80.72 | 86.26 | 97.26 |
| HBDonor | 45.70 | 0.00 | 37.46 | 0.00 | 33.48 | |
| Cationic | 44.36 | 0.00 | 36.42 | 0.00 | 32.46 | |
| Y64 | Hydrophobic | 0.00 | 60.16 | 32.82 | 0.00 | 0.00 |
| PiStacking | 0.00 | 35.76 | 0.00 | 0.00 | 0.00 | |
| 68 | Hydrophobic | 84.12 | 52.88 | 84.32 | 64.46 | 87.56 |
| D69 | Hydrophobic | 36.64 | 59.32 | 0.00 | 0.00 | 0.00 |
| M72 | Hydrophobic | 99.60 | 99.12 | 99.78 | 99.64 | 98.42 |
| F78 | Hydrophobic | 43.60 | 39.94 | 42.22 | 0.00 | 0.00 |
| D92 | Hydrophobic | 59.72 | 59.96 | 66.50 | 61.32 | 0.00 |
| H95 | Hydrophobic | 90.84 | 94.90 | 91.56 | 93.08 | 74.84 |
| PiStacking | 0.00 | 0.00 | 44.22 | 52.08 | 0.00 | |
| 96 | Hydrophobic | 98.08 | 99.26 | 99.66 | 93.30 | 86.82 |
| PiStacking | 38.52 | 33.04 | 50.26 | 0.00 | 0.00 | |
| Q99 | Hydrophobic | 95.84 | 98.80 | 99.92 | 100.00 | 99.44 |
| I100 | Hydrophobic | 83.80 | 80.96 | 82.80 | 90.06 | 87.86 |
| V103 | Hydrophobic | 86.48 | 86.82 | 88.26 | 82.16 | 86.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, G.; Liu, Q.; Qiu, Y.; Leung, E.L.-H.; Yao, X. In Silico Study of the Acquired Resistance Caused by the Secondary Mutations of KRAS G12C Protein Using Long Time Molecular Dynamics Simulation and Markov State Model Analysis. Int. J. Mol. Sci. 2022, 23, 13845. https://doi.org/10.3390/ijms232213845
Tu G, Liu Q, Qiu Y, Leung EL-H, Yao X. In Silico Study of the Acquired Resistance Caused by the Secondary Mutations of KRAS G12C Protein Using Long Time Molecular Dynamics Simulation and Markov State Model Analysis. International Journal of Molecular Sciences. 2022; 23(22):13845. https://doi.org/10.3390/ijms232213845
Chicago/Turabian StyleTu, Gao, Qing Liu, Yue Qiu, Elaine Lai-Han Leung, and Xiaojun Yao. 2022. "In Silico Study of the Acquired Resistance Caused by the Secondary Mutations of KRAS G12C Protein Using Long Time Molecular Dynamics Simulation and Markov State Model Analysis" International Journal of Molecular Sciences 23, no. 22: 13845. https://doi.org/10.3390/ijms232213845
APA StyleTu, G., Liu, Q., Qiu, Y., Leung, E. L.-H., & Yao, X. (2022). In Silico Study of the Acquired Resistance Caused by the Secondary Mutations of KRAS G12C Protein Using Long Time Molecular Dynamics Simulation and Markov State Model Analysis. International Journal of Molecular Sciences, 23(22), 13845. https://doi.org/10.3390/ijms232213845








