Involvement of AMPKα and MAPK-ERK/-JNK Signals in Docetaxel-Induced Human Tongue Squamous Cell Carcinoma Cell Apoptosis
Abstract
1. Introduction
2. Results
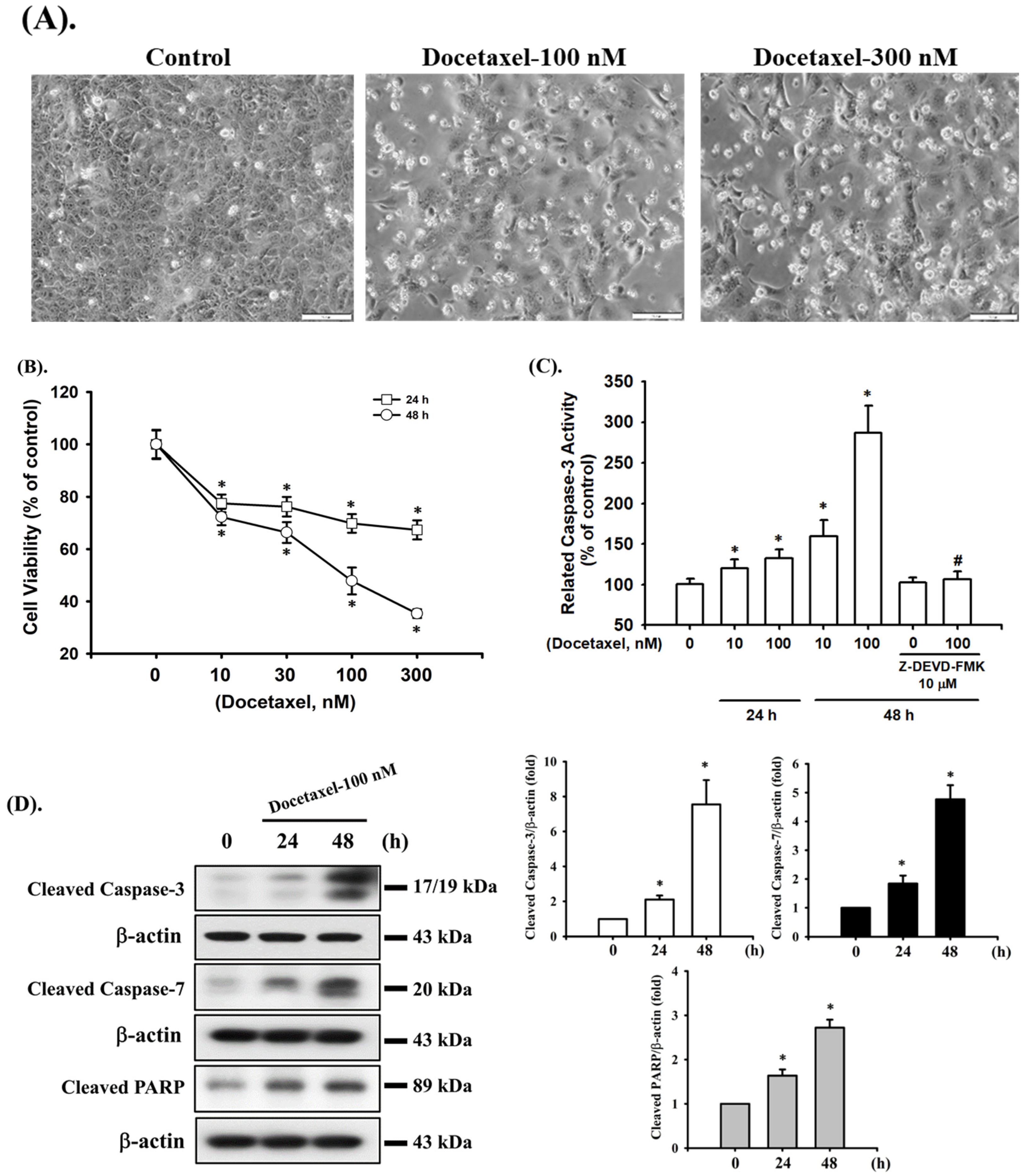
2.1. Docetaxel Induces Cytotoxicity and Apoptosis in Human Tongue SCC SAS Cells
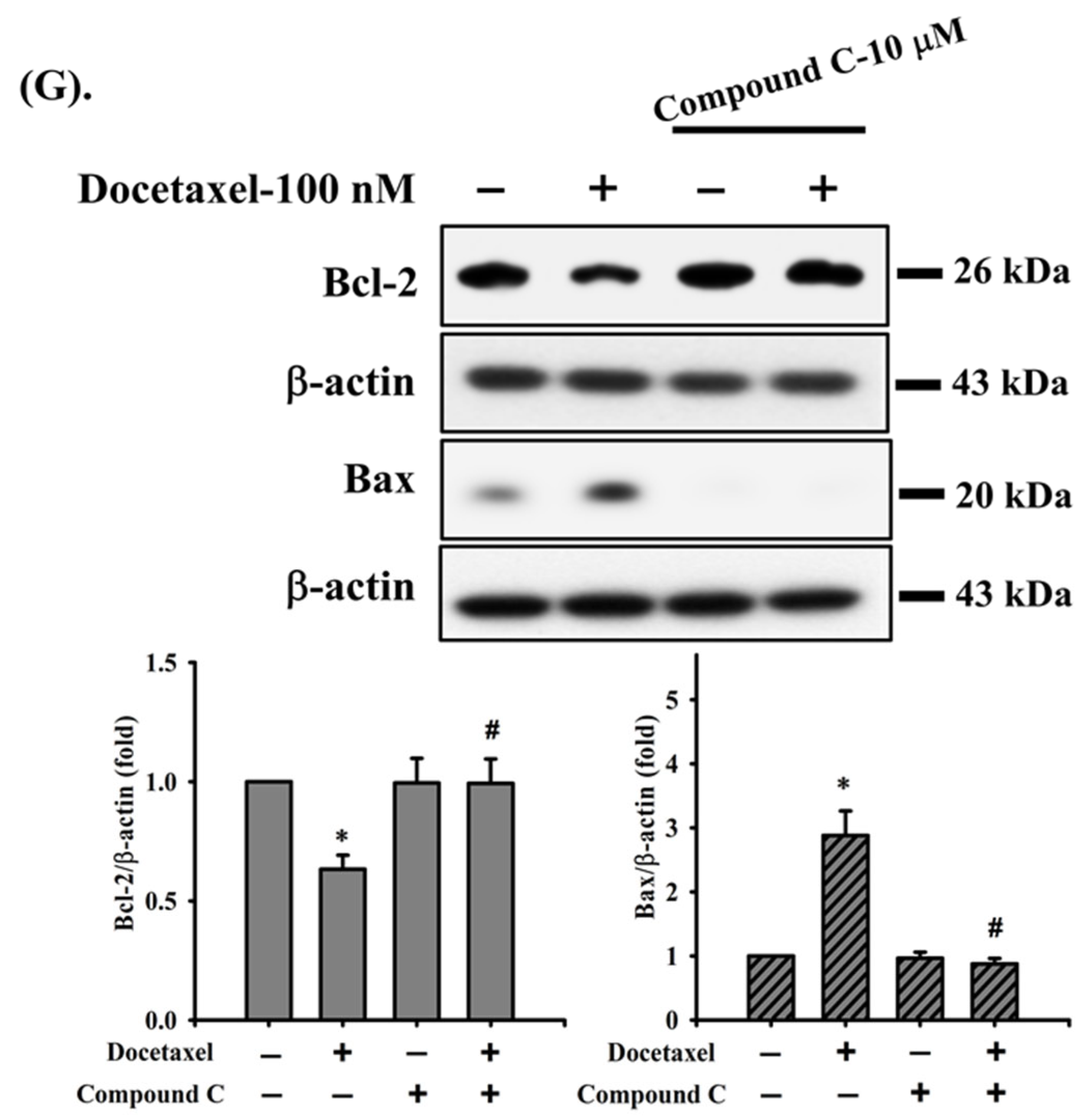
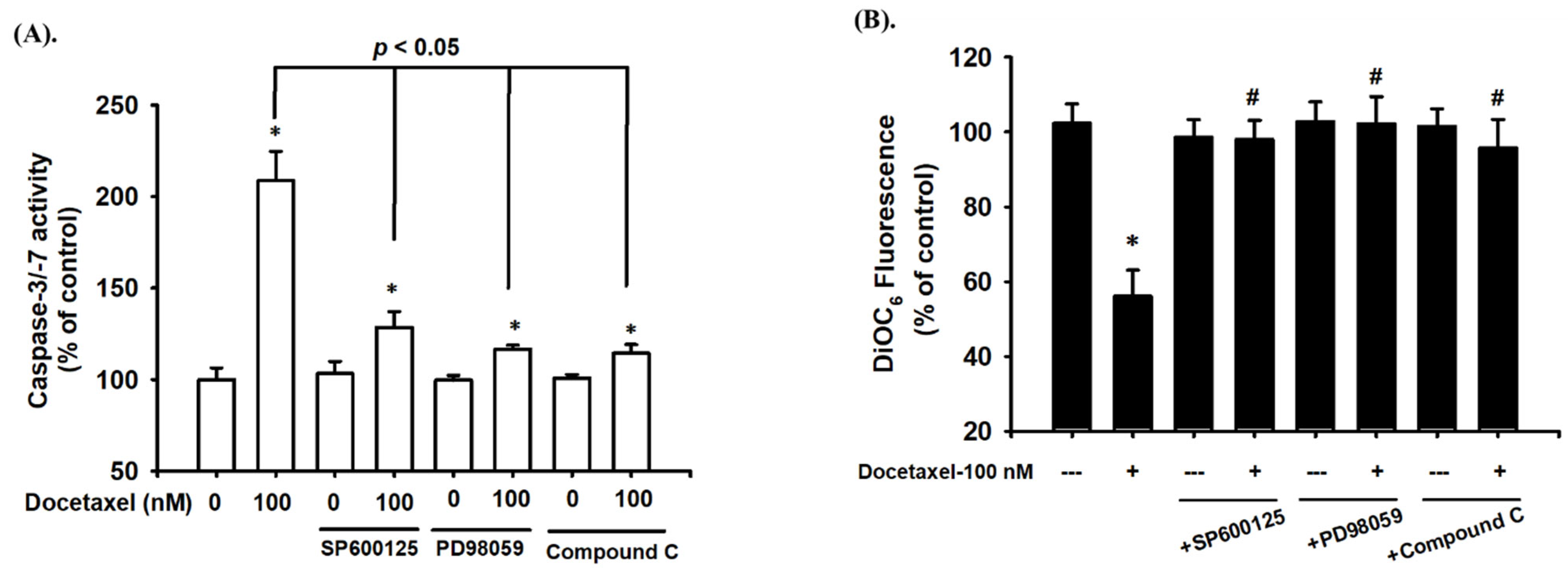
2.2. Docetaxel-Induced Apoptosis Is Mediated by a Mitochondria-Dependent Pathway in Human Tongue SCC SAS Cells
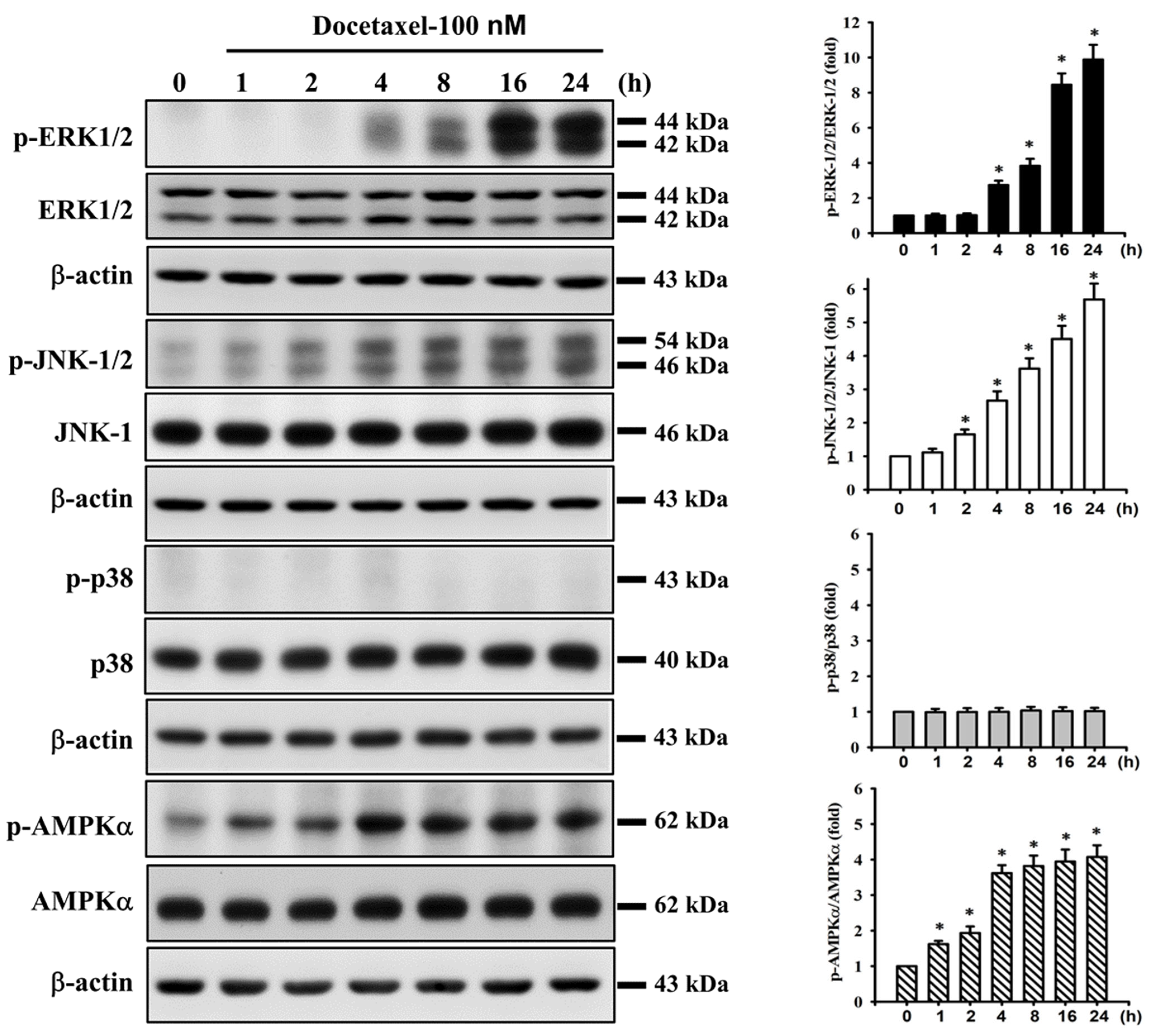
2.3. Docetaxel Induces Phosphorylation of MAPK-ERK/JNK and AMPKα in Human Tongue SCC SAS Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Morphological Analysis
4.4. Cytotoxicity Assay
4.5. Determination of Caspase-3 Activity
4.6. Detection of Mitochondrial Membrane Potential (MMP)
4.7. Caspase -3/-7 Activity Assay
4.8. Western Blot Analysis
4.9. Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction (RT-qRT-PCR) Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | adenosine monophosphate-activated protein kinase |
| ERK | extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MMP | mitochondrial membrane potential |
| PARP | poly (ADP-Ribose) polymerase |
| SCC | squamous cell carcinoma |
References
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kerawala, C.; Roques, T.; Jeannon, J.P.; Bisase, B. Oral cavity and lip cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S83–S89. [Google Scholar] [CrossRef] [PubMed]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e23–e29. [Google Scholar] [CrossRef]
- Dolens, E.D.S.; Dourado, M.R.; Almangush, A.; Salo, T.A.; Gurgel Rocha, C.A.; da Silva, S.D.; Brennan, P.A.; Coletta, R.D. The Impact of Histopathological Features on the Prognosis of Oral Squamous Cell Carcinoma: A Comprehensive Review and Meta-Analysis. Front. Oncol. 2021, 11, 784924. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Schmidt, B.L.; Jordan, R.C. Tongue and tonsil carcinoma: Increasing trends in the U.S. population ages 20–44 years. Cancer 2005, 103, 1843–1849. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Pitiyage, G.; Kumarasiri, P.V.; Liyanage, R.L.; Dias, K.D.; Tilakaratne, W.M. Clinical and histopathologic parameters in survival of oral squamous cell carcinoma. Oral. Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 518–525. [Google Scholar] [CrossRef]
- Koontongkaew, S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef]
- Dalianis, T. Human papillomavirus and oropharyngeal cancer, the epidemics, and significance of additional clinical biomarkers for prediction of response to therapy (Review). Int. J. Oncol. 2014, 44, 1799–1805. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Viet, C.T.; Schmidt, B.L. Biologic mechanisms of oral cancer pain and implications for clinical therapy. J. Dent. Res. 2012, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, L.P.; Carvalho, A.L. Natural history of untreated head and neck cancer. Eur. J. Cancer 2000, 36, 1032–1037. [Google Scholar] [CrossRef]
- Guneri, P.; Epstein, J.B. Late stage diagnosis of oral cancer: Components and possible solutions. Oral Oncol. 2014, 50, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Sone, K.; Oguri, T.; Ito, K.; Kitamura, Y.; Inoue, Y.; Takeuchi, A.; Fukuda, S.; Takakuwa, O.; Maeno, K.; Asano, T.; et al. Predictive Role of CYFRA21-1 and CEA for Subsequent Docetaxel in Non-small Cell Lung Cancer Patients. Anticancer Res. 2017, 37, 5125–5131. [Google Scholar] [CrossRef]
- Varnai, R.; Koskinen, L.M.; Mantyla, L.E.; Szabo, I.; FitzGerald, L.M.; Sipeky, C. Pharmacogenomic Biomarkers in Docetaxel Treatment of Prostate Cancer: From Discovery to Implementation. Genes 2019, 10, 599. [Google Scholar] [CrossRef]
- Lin, H.; Chang, J.; Li, J. Effects of Docetaxel Combined with Icotinib on Serum Tumor Markers and Quality of Life of Patients with Advanced Non-Small Cell Lung Cancer. Iran J. Public Health 2020, 49, 1885–1893. [Google Scholar] [CrossRef]
- Piechocki, M.P.; Lonardo, F.; Ensley, J.F.; Nguyen, T.; Kim, H.; Yoo, G.H. Anticancer activity of docetaxel in murine salivary gland carcinoma. Clin. Cancer Res. 2002, 8, 870–877. [Google Scholar]
- Fu, W.; Hong, Z.; You, X.; Din, J.; Chen, B.; Zhao, B.; Yuan, G.; Li, Q. Enhancement of anticancer activity of docetaxel by combination with Fuzheng Yiliu decoction in a mouse model of castration-resistant prostate cancer. Biomed. Pharmacother. 2019, 118, 109374. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, H.S.; Soong, Y.K. Paclitaxel-induced cell death: Where the cell cycle and apoptosis come together. Cancer 2000, 88, 2619–2628. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Stein, C.A. Taxol and estramustine-induced modulation of human prostate cancer cell apoptosis via alteration in bcl-xL and bak expression. Clin. Cancer Res. 1997, 3, 2039–2046. [Google Scholar]
- Jones, N.A.; Turner, J.; McIlwrath, A.J.; Brown, R.; Dive, C. Cisplatin- and paclitaxel-induced apoptosis of ovarian carcinoma cells and the relationship between bax and bak up-regulation and the functional status of p53. Mol. Pharmacol. 1998, 53, 819–826. [Google Scholar] [PubMed]
- Srivastava, R.K.; Srivastava, A.R.; Korsmeyer, S.J.; Nesterova, M.; Cho-Chung, Y.S.; Longo, D.L. Involvement of microtubules in the regulation of Bcl2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol. Cell Biol. 1998, 18, 3509–3517. [Google Scholar] [CrossRef] [PubMed]
- Carling, D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.C.; Chou, C.C.; Kulp, S.K.; Chen, C.S. AMPK as a potential anticancer target—friend or foe? Curr. Pharm. Des. 2014, 20, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Xia, Y.C.; Zha, J.H.; Sang, Y.H.; Yin, H.; Xu, G.Q.; Zhen, J.; Zhang, Y.; Yu, B.T. AMPK activation by ASP4132 inhibits non-small cell lung cancer cell growth. Cell Death Dis. 2021, 12, 365. [Google Scholar] [CrossRef]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef]
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.P.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33. [Google Scholar] [CrossRef]
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef]
- Luo, Z.; Zang, M.; Guo, W. AMPK as a metabolic tumor suppressor: Control of metabolism and cell growth. Future Oncol. 2010, 6, 457–470. [Google Scholar] [CrossRef]
- Hadad, S.M.; Baker, L.; Quinlan, P.R.; Robertson, K.E.; Bray, S.E.; Thomson, G.; Kellock, D.; Jordan, L.B.; Purdie, C.A.; Hardie, D.G.; et al. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer 2009, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Iyer, N.G.; Tan, M.H.; Edgren, G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck 2017, 39, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Nocini, R.; Lippi, G.; Mattiuzzi, C. Biological and epidemiologic updates on lip and oral cavity cancers. Ann. Cancer Epidemiol. 2020, 4, 1. [Google Scholar] [CrossRef]
- American Lung Association. Trends in Tobacco Use. American Lung Association Research and Program Services, Epidemiology and Statistics Unit: Washington, DC, USA, 2011. Available online: http://www.lung.org/assets/docu-ments/research/tobacco-trend-report.pdf (accessed on 1 January 2015).
- Vettore, A.L.; Ramnarayanan, K.; Poore, G.; Lim, K.; Ong, C.K.; Huang, K.K.; Leong, H.S.; Chong, F.T.; Lim, T.K.; Lim, W.K.; et al. Mutational landscapes of tongue carcinoma reveal recurrent mutations in genes of therapeutic and prognostic relevance. Genome Med. 2015, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.C.; Carpenter, W.R.; Tyree, S.; Couch, M.E.; Weissler, M.; Hackman, T.; Hayes, D.N.; Shores, C.; Chera, B.S. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J. Clin. Oncol. 2011, 29, 1488–1494. [Google Scholar] [CrossRef]
- Bissery, M.C.; Guenard, D.; Gueritte-Voegelein, F.; Lavelle, F. Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res. 1991, 51, 4845–4852. [Google Scholar]
- Couteau, C.; Chouaki, N.; Leyvraz, S.; Oulid-Aissa, D.; Lebecq, A.; Domenge, C.; Groult, V.; Bordessoule, S.; Janot, F.; De Forni, M.; et al. A phase II study of docetaxel in patients with metastatic squamous cell carcinoma of the head and neck. Br. J. Cancer 1999, 81, 457–462. [Google Scholar] [CrossRef]
- Fleisher, T.A. Apoptosis. Ann. Allergy Asthma Immunol. 1997, 78, 245–249, quiz 249–250. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- van Gurp, M.; Festjens, N.; van Loo, G.; Saelens, X.; Vandenabeele, P. Mitochondrial intermembrane proteins in cell death. Biochem. Biophys. Res. Commun. 2003, 304, 487–497. [Google Scholar] [CrossRef]
- Peng, X.; Gan, J.; Wang, Q.; Shi, Z.; Xia, X. 3-Monochloro-1,2-propanediol (3-MCPD) induces apoptosis via mitochondrial oxidative phosphorylation system impairment and the caspase cascade pathway. Toxicology 2016, 372, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Acehan, D.; Jiang, X.; Morgan, D.G.; Heuser, J.E.; Wang, X.; Akey, C.W. Three-dimensional structure of the apoptosome: Implications for assembly, procaspase-9 binding, and activation. Mol. Cell 2002, 9, 423–432. [Google Scholar] [CrossRef]
- Peng, Q.; Deng, Z.; Pan, H.; Gu, L.; Liu, O.; Tang, Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol. Lett. 2018, 15, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Burotto, M.; Chiou, V.L.; Lee, J.M.; Kohn, E.C. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef]
- Persons, D.L.; Yazlovitskaya, E.M.; Pelling, J.C. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J. Biol. Chem. 2000, 275, 35778–35785. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Choi, S.Y.; Yang, J.H. NMDA receptor-mediated ERK 1/2 pathway is involved in PFHxS-induced apoptosis of PC12 cells. Sci. Total Environ. 2014, 491–492, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Lee, K.I.; Chen, M.K.; Kuo, C.Y.; Tang, C.H.; Liu, S.H. Cantharidin Induced Oral Squamous Cell Carcinoma Cell Apoptosis via the JNK-Regulated Mitochondria and Endoplasmic Reticulum Stress-Related Signaling Pathways. PLoS ONE 2016, 11, e0168095. [Google Scholar] [CrossRef]
- You, J.; Cheng, J.; Yu, B.; Duan, C.; Peng, J. Baicalin, a Chinese Herbal Medicine, Inhibits the Proliferation and Migration of Human Non-Small Cell Lung Carcinoma (NSCLC) Cells, A549 and H1299, by Activating the SIRT1/AMPK Signaling Pathway. Med. Sci. Monit. 2018, 24, 2126–2133. [Google Scholar] [CrossRef]
- Wei, C.; Yao, X.; Jiang, Z.; Wang, Y.; Zhang, D.; Chen, X.; Fan, X.; Xie, C.; Cheng, J.; Fu, J.; et al. Cordycepin Inhibits Drug-resistance Non-small Cell Lung Cancer Progression by Activating AMPK Signaling Pathway. Pharmacol. Res. 2019, 144, 79–89. [Google Scholar] [CrossRef]
- Wu, Y.; Si, Y.; Xiang, Y.; Zhou, T.; Liu, X.; Wu, M.; Li, W.; Zhang, T.; Xiang, K.; Zhang, L.; et al. Polyphyllin I activates AMPK to suppress the growth of non-small-cell lung cancer via induction of autophagy. Arch. Biochem. Biophys. 2020, 687, 108285. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.Z.; Gao, Y.; Zhao, H.W.; Zhou, M.; Chen, D.L.; Tao, L.T.; Guo, W.; Sun, L.L.; Gu, C.Y.; Chen, H.R.; et al. Cordycepin Reverses Cisplatin Resistance in Non-small Cell Lung Cancer by Activating AMPK and Inhibiting AKT Signaling Pathway. Front. Cell Dev. Biol. 2020, 8, 609285. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Tsai, M.H.; Chiu, C.F.; Lu, C.C.; Kuo, S.C.; Chang, N.W.; Yang, J.S. AMPK-dependent signaling modulates the suppression of invasion and migration by fenofibrate in CAL 27 oral cancer cells through NF-κB pathway. Environ. Toxicol. 2016, 31, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Liu, S.H.; Ho, T.J.; Lee, K.I.; Fang, K.M.; Lo, W.C.; Liu, J.M.; Wu, C.C.; Su, C.C. Quercetin induces tongue squamous cell carcinoma cell apoptosis via the JNK activation-regulated ERK/GSK-3α/β-mediated mitochondria-dependent apoptotic signaling pathway. Oncol. Lett. 2022, 23, 78. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liu, S.H.; Su, C.C.; Fang, K.M.; Yang, T.Y.; Liu, J.M.; Chen, Y.W.; Chang, K.C.; Chuang, H.L.; Wu, C.T.; et al. Methylmercury Induces Mitochondria- and Endoplasmic Reticulum Stress-Dependent Pancreatic β-Cell Apoptosis via an Oxidative Stress-Mediated JNK Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 2858. [Google Scholar] [CrossRef]
- Nencioni, L.; De Chiara, G.; Sgarbanti, R.; Amatore, D.; Aquilano, K.; Marcocci, M.E.; Serafino, A.; Torcia, M.; Cozzolino, F.; Ciriolo, M.R.; et al. Bcl-2 expression and p38MAPK activity in cells infected with influenza A virus: Impact on virally induced apoptosis and viral replication. J. Biol. Chem. 2009, 284, 16004–16015. [Google Scholar] [CrossRef]
- Kotsafti, A.; Farinati, F.; Cardin, R.; Cillo, U.; Nitti, D.; Bortolami, M. Autophagy and apoptosis-related genes in chronic liver disease and hepatocellular carcinoma. BMC Gastroenterol. 2012, 12, 118. [Google Scholar] [CrossRef]
- Hasan, Z.; Ashraf, M.; Tayyebi, A.; Hussain, R.M. leprae inhibits apoptosis in THP-1 cells by downregulation of Bad and Bak and upregulation of Mcl-1 gene expression. BMC Microbiol. 2006, 6, 78. [Google Scholar] [CrossRef][Green Version]
- Zhou, Z.; Zhu, C.; Cai, Z.; Zhao, F.; He, L.; Lou, X.; Qi, X. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol. Lett. 2018, 15, 7319–7327. [Google Scholar] [CrossRef]
- Lim, C.; Lee, P.C.W.; Shim, S.; Jang, S.W. HS-1793 inhibits cell proliferation in lung cancer by interfering with the interaction between p53 and MDM2. Oncol. Lett. 2022, 24, 290. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.-C.; Lin, J.-W.; Chang, K.-Y.; Wu, C.-T.; Liu, S.-H.; Chang, K.-C.; Liu, J.-M.; Lee, K.-I.; Fang, K.-M.; Chen, Y.-W. Involvement of AMPKα and MAPK-ERK/-JNK Signals in Docetaxel-Induced Human Tongue Squamous Cell Carcinoma Cell Apoptosis. Int. J. Mol. Sci. 2022, 23, 13857. https://doi.org/10.3390/ijms232213857
Su C-C, Lin J-W, Chang K-Y, Wu C-T, Liu S-H, Chang K-C, Liu J-M, Lee K-I, Fang K-M, Chen Y-W. Involvement of AMPKα and MAPK-ERK/-JNK Signals in Docetaxel-Induced Human Tongue Squamous Cell Carcinoma Cell Apoptosis. International Journal of Molecular Sciences. 2022; 23(22):13857. https://doi.org/10.3390/ijms232213857
Chicago/Turabian StyleSu, Chin-Chuan, Jhe-Wei Lin, Kai-Yao Chang, Cheng-Tien Wu, Shing-Hwa Liu, Kai-Chih Chang, Jui-Ming Liu, Kuan-I Lee, Kai-Min Fang, and Ya-Wen Chen. 2022. "Involvement of AMPKα and MAPK-ERK/-JNK Signals in Docetaxel-Induced Human Tongue Squamous Cell Carcinoma Cell Apoptosis" International Journal of Molecular Sciences 23, no. 22: 13857. https://doi.org/10.3390/ijms232213857
APA StyleSu, C.-C., Lin, J.-W., Chang, K.-Y., Wu, C.-T., Liu, S.-H., Chang, K.-C., Liu, J.-M., Lee, K.-I., Fang, K.-M., & Chen, Y.-W. (2022). Involvement of AMPKα and MAPK-ERK/-JNK Signals in Docetaxel-Induced Human Tongue Squamous Cell Carcinoma Cell Apoptosis. International Journal of Molecular Sciences, 23(22), 13857. https://doi.org/10.3390/ijms232213857





