Isopropyl Amino Acid Esters Ionic Liquids as Vehicles for Non-Steroidal Anti-Inflammatory Drugs in Potential Topical Drug Delivery Systems with Antimicrobial Activity
Abstract
:1. Introduction
2. Results
3. Materials and Methods
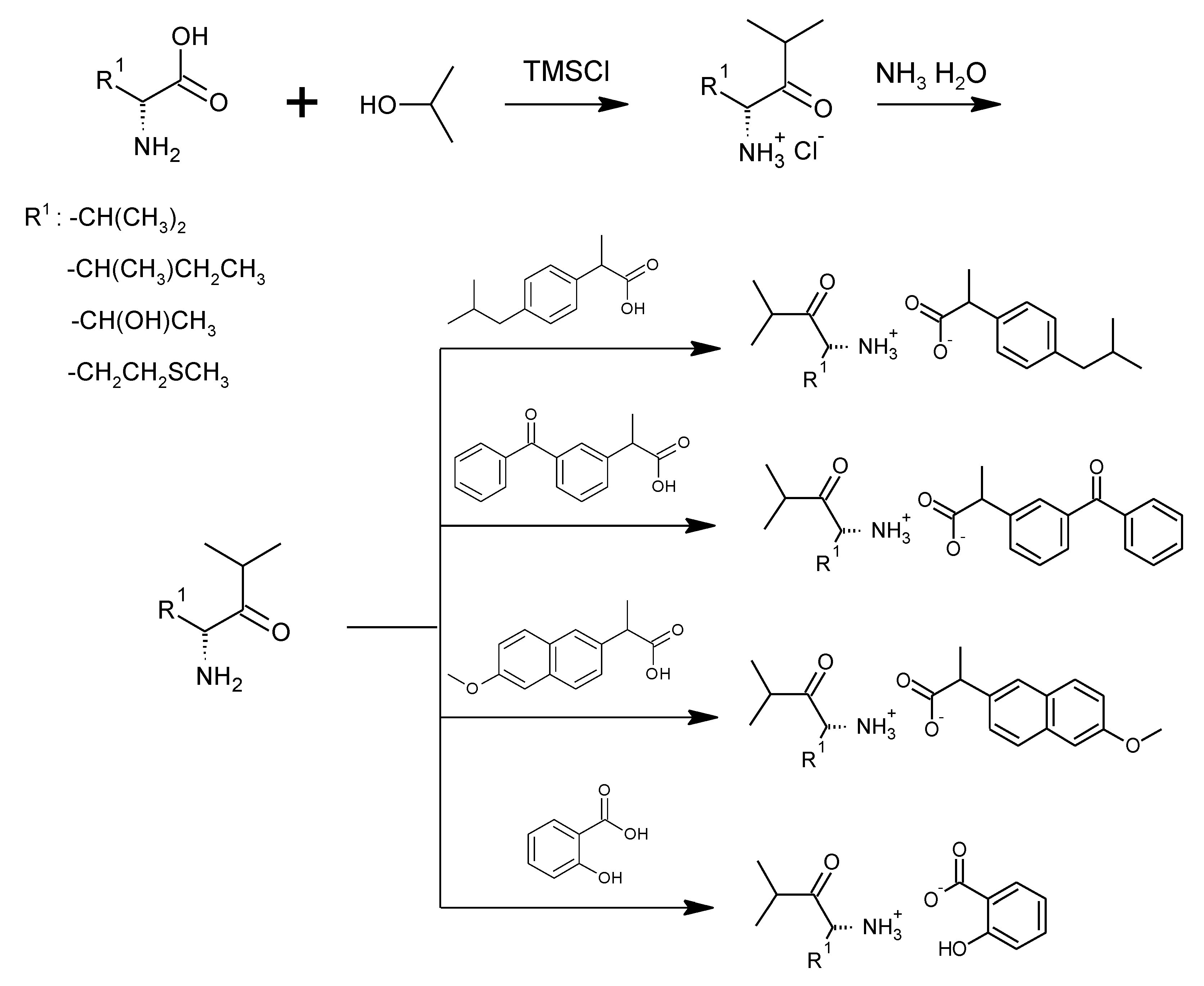
3.1. Synthesis of Amino Acid Isopropyl Ester Salts
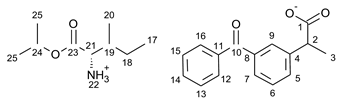

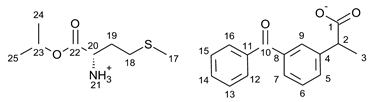

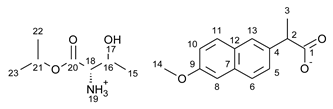

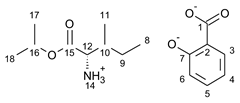

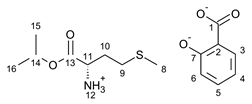
3.2. Antimicrobial Susceptibility Test by Disc Diffusion Technique
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lou, Y.; Zhu, J. Carboxylic Acid Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). In Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals; Lamberth, C., Dinges, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 221–236. ISBN 978-3-527-33947-1. [Google Scholar]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-G.; Leung, W.K. Potential Strategies in the Prevention of Nonsteroidal Anti-Inflammatory Drugs-Associated Adverse Effects in the Lower Gastrointestinal Tract. Gut Liver 2020, 14, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse Effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs, Aspirin and Coxibs) on Upper Gastrointestinal Tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Bardou, M.; Barkun, A.N. Preventing the Gastrointestinal Adverse Effects of Nonsteroidal Anti-Inflammatory Drugs: From Risk Factor Identification to Risk Factor Intervention. Jt. Bone Spine 2010, 77, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Qandil, A. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), More Than Meets the Eye: A Critical Review. Int. J. Mol. Sci. 2012, 13, 17244–17274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.K.; Pati, K.; Bose, A.; Dey, S.; De, A.; Bose, S.; De, A. Various Ester Prodrugs of NSAIDs with Low Ulcerogenic Activity. Int. J. Pharm. Sci. Rev. Res. 2019, 54, 45–49. [Google Scholar]
- Huang, M.-M.; Jiang, Y.; Sasisanker, P.; Driver, G.W.; Weingärtner, H. Static Relative Dielectric Permittivities of Ionic Liquids at 25 °C. J. Chem. Eng. Data 2011, 56, 1494–1499. [Google Scholar] [CrossRef]
- Das, N.; Dhanawat, M.; Dash, B.; Nagarwal, R.C.; Shrivastava, S.K. Codrug: An Efficient Approach for Drug Optimization. Eur. J. Pharm. Sci. 2010, 41, 571–588. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Lin, C.-F.; Chen, C.-H.; Fang, J.-Y. The Codrug Approach for Facilitating Drug Delivery and Bioactivity. Expert Opin. Drug Deliv. 2016, 13, 1311–1325. [Google Scholar] [CrossRef]
- Sehajpal, S.; Prasad, D.N.; Singh, R.K. Novel Ketoprofen–Antioxidants Mutual Codrugs as Safer Nonsteroidal Anti-inflammatory Drugs: Synthesis, Kinetic and Pharmacological Evaluation. Arch. Pharm. Chem. Life Sci. 2019, 352, 1800339. [Google Scholar] [CrossRef]
- de Oliveira Pedrosa Rolim, M.; de Almeida, A.R.; da Rocha Pitta, M.G.; de Melo Rêgo, M.J.B.; Quintans-Júnior, L.J.; de Souza Siqueira Quintans, J.; Heimfarth, L.; Scotti, L.; Scotti, M.T.; da Cruz, R.M.D.; et al. Design, Synthesis and Pharmacological Evaluation of CVIB, a Codrug of Carvacrol and Ibuprofen as a Novel Anti-Inflammatory Agent. Int. Immunopharmacol. 2019, 76, 105856. [Google Scholar] [CrossRef] [PubMed]
- Redasani, V.K.; Bari, S.B. Synthesis and Evaluation of Mutual Prodrugs of Ibuprofen with Menthol, Thymol and Eugenol. Eur. J. Med. Chem. 2012, 56, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.S. Carboxylic Acid Counterions in FDA-Approved Pharmaceutical Salts. Pharm. Res. 2021, 38, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
- Handa, M.; Almalki, W.H.; Shukla, R.; Afzal, O.; Altamimi, A.S.A.; Beg, S.; Rahman, M. Active Pharmaceutical Ingredients (APIs) in Ionic Liquids: An Effective Approach for API Physiochemical Parameter Optimization. Drug Discov. Today 2022, 27, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Brain, P.; Leyva, R.; Doyle, G.; Kellstein, D. Onset of Analgesia and Efficacy of Ibuprofen Sodium in Postsurgical Dental Pain: A Randomized, Placebo-Controlled Study Versus Standard Ibuprofen. Clin. J. Pain 2015, 31, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanphui, P.; Bolla, G.; Nangia, A. High Solubility Piperazine Salts of the Nonsteroidal Anti-Inflammatory Drug (NSAID) Meclofenamic Acid. Cryst. Growth Des. 2012, 12, 2023–2036. [Google Scholar] [CrossRef]
- Kuczyńska, J.; Nieradko-Iwanicka, B. Future Prospects of Ketoprofen in Improving the Safety of the Gastric Mucosa. Biomed. Pharmacother. 2021, 139, 111608. [Google Scholar] [CrossRef]
- Martin, W.; Koselowske, G.; Töberich, H.; Kerkmann, T.; Mangold, B.; Augustin, J. Pharmacokinetics and Absolute Bioavailability of Ibuprofen after Oral Administration of Ibuprofen Lysine in Man. Biopharm. Drug Dispos. 1990, 11, 265–278. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Q.; Chen, Z.; Wu, W.; Lu, Y.; Qi, J. Ionic Liquids as a Useful Tool for Tailoring Active Pharmaceutical Ingredients. J. Control. Release 2021, 338, 268–283. [Google Scholar] [CrossRef]
- Curreri, A.M.; Mitragotri, S.; Tanner, E.E.L. Recent Advances in Ionic Liquids in Biomedicine. Adv. Sci. 2021, 8, 2004819. [Google Scholar] [CrossRef] [PubMed]
- Moshikur, R.M.d.; Chowdhury, M.R.; Wakabayashi, R.; Tahara, Y.; Moniruzzaman, M.; Goto, M. Characterization and Cytotoxicity Evaluation of Biocompatible Amino Acid Esters Used to Convert Salicylic Acid into Ionic Liquids. Int. J. Pharm. 2018, 546, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sintra, T.E.; Shimizu, K.; Ventura, S.P.M.; Shimizu, S.; Canongia Lopes, J.N.; Coutinho, J.A.P. Enhanced Dissolution of Ibuprofen Using Ionic Liquids as Catanionic Hydrotropes. Phys. Chem. Chem. Phys. 2018, 20, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Balk, A.; Wiest, J.; Widmer, T.; Galli, B.; Holzgrabe, U.; Meinel, L. Transformation of Acidic Poorly Water Soluble Drugs into Ionic Liquids. Eur. J. Pharm. Biopharm. 2015, 94, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Abraham, W.; Jasti, B. Transdermal and Topical Drug Delivery Systems. In Theory and Practice of Contemporary Pharmaceutics; Jasti, B., Ghosh, T., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 423–455. ISBN 978-0-415-28863-7. [Google Scholar]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic Liquids as a Potential Tool for Drug Delivery Systems. Med. Chem. Commun. 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Wang, J.; Ren, S.; Song, Y.; Quan, P.; Fang, L. Ionic Liquids in Transdermal Drug Delivery System: Current Applications and Future Perspectives. Chin. Chem. Lett. 2022, S1001841722006301. [Google Scholar] [CrossRef]
- Dobler, D.; Schmidts, T.; Klingenhöfer, I.; Runkel, F. Ionic Liquids as Ingredients in Topical Drug Delivery Systems. Int. J. Pharm. 2013, 441, 620–627. [Google Scholar] [CrossRef]
- Lu, B.; Liu, T.; Wang, H.; Wu, C.; Chen, H.; Liu, Z.; Zhang, J. Ionic Liquid Transdermal Delivery System: Progress, Prospects, and Challenges. J. Mol. Liq. 2022, 351, 118643. [Google Scholar] [CrossRef]
- Yang, D.; Liu, C.; Ding, D.; Quan, P.; Fang, L. The Molecular Design of Drug-Ionic Liquids for Transdermal Drug Delivery: Mechanistic Study of Counterions Structure on Complex Formation and Skin Permeation. Int. J. Pharm. 2021, 602, 120560. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Shamshina, J.; Cojocaru, O.A.; Janikowski, J.; MacFarlane, D.R.; Davis, J.H.; Rogers, R.D. Simultaneous Membrane Transport of Two Active Pharmaceutical Ingredients by Charge Assisted Hydrogen Bond Complex Formation. Chem. Sci. 2014, 5, 3449–3456. [Google Scholar] [CrossRef]
- Miwa, Y.; Hamamoto, H.; Ishida, T. Lidocaine Self-Sacrificially Improves the Skin Permeation of the Acidic and Poorly Water-Soluble Drug Etodolac via Its Transformation into an Ionic Liquid. Eur. J. Pharm. Biopharm. 2016, 102, 92–100. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Neves, B.M.; Sèbe, G.; Coma, V.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Design of Nonsteroidal Anti-Inflammatory Drug-Based Ionic Liquids with Improved Water Solubility and Drug Delivery. ACS Sustain. Chem. Eng. 2019, 7, 14126–14134. [Google Scholar] [CrossRef]
- Pereira, R.; Silva, S.G.; Pinheiro, M.; Reis, S.; Vale, M.L. do Current Status of Amino Acid-Based Permeation Enhancers in Transdermal Drug Delivery. Membranes 2021, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Janůšová, B.; Školová, B.; Tükörová, K.; Wojnarová, L.; Šimůnek, T.; Mladěnka, P.; Filipský, T.; Říha, M.; Roh, J.; Palát, K.; et al. Amino Acid Derivatives as Transdermal Permeation Enhancers. J. Control. Release 2013, 165, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Ferreira, A.; Matos, J.; Fresco, P.; Gouveia, M. Amino Acids in the Development of Prodrugs. Molecules 2018, 23, 2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-Type Amino Acid Transporter 1 as a Target for Drug Delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef]
- Vig, B.S.; Huttunen, K.M.; Laine, K.; Rautio, J. Amino Acids as Promoieties in Prodrug Design and Development. Adv. Drug Deliv. Rev. 2013, 65, 1370–1385. [Google Scholar] [CrossRef]
- Beauchamp, L.M.; Orr, G.F.; de Miranda, P.; Bumette, T.; Krenitsky, T.A. Amino Acid Ester Prodrugs of Acyclovir. Antivir. Chem. Chemother. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Perry, C.M.; Faulds, D. Valaciclovir: A Review of Its Antiviral Activity, Pharmacokinetic Properties and Therapeutic Efficacy in Herpesvirus Infections. Drugs 1996, 52, 754–772. [Google Scholar] [CrossRef] [PubMed]
- Ormrod, D.; Scott, L.J.; Perry, C.M. Valaciclovir: A Review of Its Long Term Utility in the Management of Genital Herpes Simplex Virus and Cytomegalovirus Infections. Drugs 2000, 59, 839–863. [Google Scholar] [CrossRef]
- Sanchez, C.J.; Akers, K.S.; Romano, D.R.; Woodbury, R.L.; Hardy, S.K.; Murray, C.K.; Wenke, J.C. Amino Acids Enhance the Activity of Antimicrobials against Biofilms of Clinical Wound Isolates of Staphylococcus Aureus and Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 4353–4361. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Mohammad, A.R.; Karodia, N.; Rahman, A. Multimodal Role of Amino Acids in Microbial Control and Drug Development. Antibiotics 2020, 9, 330. [Google Scholar] [CrossRef]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of Ibuprofen Solubility and Skin Permeation by Conjugation with L-Valine Alkyl Esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef] [Green Version]
- Ossowicz, P.; Klebeko, J.; Janus, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. The Effect of Alcohols as Vehicles on the Percutaneous Absorption and Skin Retention of Ibuprofen Modified with L -Valine Alkyl Esters. RSC Adv. 2020, 10, 41727–41740. [Google Scholar] [CrossRef] [PubMed]
- Ossowicz, P.; Kardaleva, P.; Guncheva, M.; Klebeko, J.; Świątek, E.; Janus, E.; Yancheva, D.; Angelov, I. Ketoprofen-Based Ionic Liquids: Synthesis and Interactions with Bovine Serum Albumin. Molecules 2020, 25, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossowicz, P.; Janus, E.; Klebeko, J.; Światek, E.; Kardaleva, P.; Taneva, S.; Krachmarova, E.; Rangelov, M.; Todorova, N.; Guncheva, M. Modulation of the Binding Affinity of Naproxen to Bovine Serum Albumin by Conversion of the Drug into Amino Acid Ester Salts. J. Mol. Liq. 2020, 319, 114283. [Google Scholar] [CrossRef]
- Klebeko, J.; Ossowicz-Rupniewska, P.; Świątek, E.; Szachnowska, J.; Janus, E.; Taneva, S.G.; Krachmarova, E.; Guncheva, M. Salicylic Acid as Ionic Liquid Formulation May Have Enhanced Potency to Treat Some Chronic Skin Diseases. Molecules 2021, 27, 216. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Klebeko, J.; Świątek, E.; Bilska, K.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Struk, Ł.; Wenelska, K.; Klimowicz, A.; et al. Influence of the Type of Amino Acid on the Permeability and Properties of Ibuprofenates of Isopropyl Amino Acid Esters. Int. J. Mol. Sci. 2022, 23, 4158. [Google Scholar] [CrossRef]
- Katritzky, A.R. A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures By Noel P.G. Roeges (Katholieke Industriele Hogeschool O-VI). Wiley: New York. 1994. x + 340 pp. $69.95. ISBN 0-471-93998-6. J. Am. Chem. Soc. 1996, 118, 3543. [Google Scholar] [CrossRef]
- Vairam, S.; Premkumar, T.; Govindarajan, S. Trimellitate Complexes of Divalent Transition Metals with Hydrazinium Cation: Thermal and Spectroscopic Studies. J. Therm. Anal. Calorim. 2010, 100, 955–960. [Google Scholar] [CrossRef]
- Kolev, T.; Spiteller, M.; Koleva, B. Spectroscopic and Structural Elucidation of Amino Acid Derivatives and Small Peptides: Experimental and Theoretical Tools. Amino Acids 2010, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Calvar, N.; Domínguez, Á. Thermal Behaviour of Pure Ionic Liquids. In Ionic Liquids—Current State of the Art; Handy, S., Ed.; InTech: Rijeka, Croatia, 2015; ISBN 978-953-51-2122-0. [Google Scholar]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Updated and Enl. Edition; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-32473-6. [Google Scholar]
- Bastin, R.J.; Bowker, M.J.; Slater, B.J. Salt Selection and Optimisation Procedures for Pharmaceutical New Chemical Entities. Org. Process Res. Dev. 2000, 4, 427–435. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–Base Crystalline Complexes and the PKa Rule. CrystEngComm 2012, 14, 6362. [Google Scholar] [CrossRef]
- Brittain, H.G. Strategy for the Prediction and Selection of Drug Substance Salt Forms. Pharm. Tech. 2007, 31, 78–88. [Google Scholar]
- Amrati, F.E.-Z.; Bourhia, M.; Saghrouchni, H.; Slighoua, M.; Grafov, A.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Bari, A.; Ibenmoussa, S.; et al. Caralluma europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions. Molecules 2021, 26, 636. [Google Scholar] [CrossRef]
- Le, K.Y.; Park, M.D.; Otto, M. Immune Evasion Mechanisms of Staphylococcus Epidermidis Biofilm Infection. Front. Microbiol. 2018, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Bhatia, D.; Dave, V.; Sutariya, V.; Varghese Gupta, S. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Molecules 2018, 23, 1719. [Google Scholar] [CrossRef] [Green Version]
- Gannesen, A.V.; Schelkunov, M.I.; Geras’kina, O.V.; Makarova, N.E.; Sukhacheva, M.V.; Danilova, N.D.; Ovcharova, M.A.; Mart’yanov, S.V.; Pankratov, T.A.; Muzychenko, D.S.; et al. Epinephrine Affects Gene Expression Levels and Has a Complex Effect on Biofilm Formation in Micrococcus Luteus Strain C01 Isolated from Human Skin. Biofilm 2021, 3, 100058. [Google Scholar] [CrossRef]
- Leão, C.; Borges, A.; Simões, M. NSAIDs as a Drug Repurposing Strategy for Biofilm Control. Antibiotics 2020, 9, 591. [Google Scholar] [CrossRef]
- Senthilraj, R.; Swetha, V.; Kavitha, S.; Haripriya, A.; Tharunkumar, M.; Santhosh, A. Screening of Antibacterial Activity of Nonsteroidal Anti-Inflammatory Drugs against Selected Pathogens. Drug Invent. Today 2020, 14, 692–696. [Google Scholar]
- Paes Leme, R.C.; da Silva, R.B. Antimicrobial Activity of Non-Steroidal Anti-Inflammatory Drugs on Biofilm: Current Evidence and Potential for Drug Repurposing. Front. Microbiol. 2021, 12, 707629. [Google Scholar] [CrossRef] [PubMed]
- Obad, J.; Šušković, J.; Kos, B. Antimicrobial Activity of Ibuprofen: New Perspectives on an “Old” Non-Antibiotic Drug. Eur. J. Pharm. Sci. 2015, 71, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S. Escherichia Coli. Biochem. Mol. Biol. Educ. 2009, 37, 325–332. [Google Scholar] [CrossRef]
- Jones, C.M.; Hernández Lozada, N.J.; Pfleger, B.F. Efflux Systems in Bacteria and Their Metabolic Engineering Applications. Appl. Microbiol. Biotechnol. 2015, 99, 9381–9393. [Google Scholar] [CrossRef] [Green Version]
- Van Dyk, T.K. Bacterial Efflux Transport in Biotechnology. Adv. Appl. Microbiol. 2008, 63, 231–247. [Google Scholar] [PubMed]
- Beavers, W.N.; DuMont, A.L.; Monteith, A.J.; Maloney, K.N.; Tallman, K.A.; Weiss, A.; Christian, A.H.; Toste, F.D.; Chang, C.J.; Porter, N.A.; et al. Staphylococcus Aureus Peptide Methionine Sulfoxide Reductases Protect from Human Whole-Blood Killing. Infect. Immun. 2021, 89, e00146-21. [Google Scholar] [CrossRef]
- Lichev, A.; Angelov, A.; Cucurull, I.; Liebl, W. Amino Acids as Nutritional Factors and (p)PpGpp as an Alarmone of the Stringent Response Regulate Natural Transformation in Micrococcus Luteus. Sci. Rep. 2019, 9, 11030. [Google Scholar] [CrossRef] [Green Version]
- Draskovic, I.; Dubnau, D. Biogenesis of a Putative Channel Protein, ComEC, Required for DNA Uptake: Membrane Topology, Oligomerization and Formation of Disulphide Bonds: Channel Protein ComEC. Mol. Microbiol. 2004, 55, 881–896. [Google Scholar] [CrossRef]
- Torasso Kasem, E.J.; Angelov, A.; Werner, E.; Lichev, A.; Vanderhaeghen, S.; Liebl, W. Identification of New Chromosomal Loci Involved in Com Genes Expression and Natural Transformation in the Actinobacterial Model Organism Micrococcus Luteus. Genes 2021, 12, 1307. [Google Scholar] [CrossRef]
- Antimicrobial Susceptibility Testing, EUCAST Disk Diffusion Method (Version 10.0); European Committee on Antimicrobial Susceptibility Testing (EUCAST), European Society of Clinical Microbiology and Infectious Diseases: Basel, Switzerland, 2022.
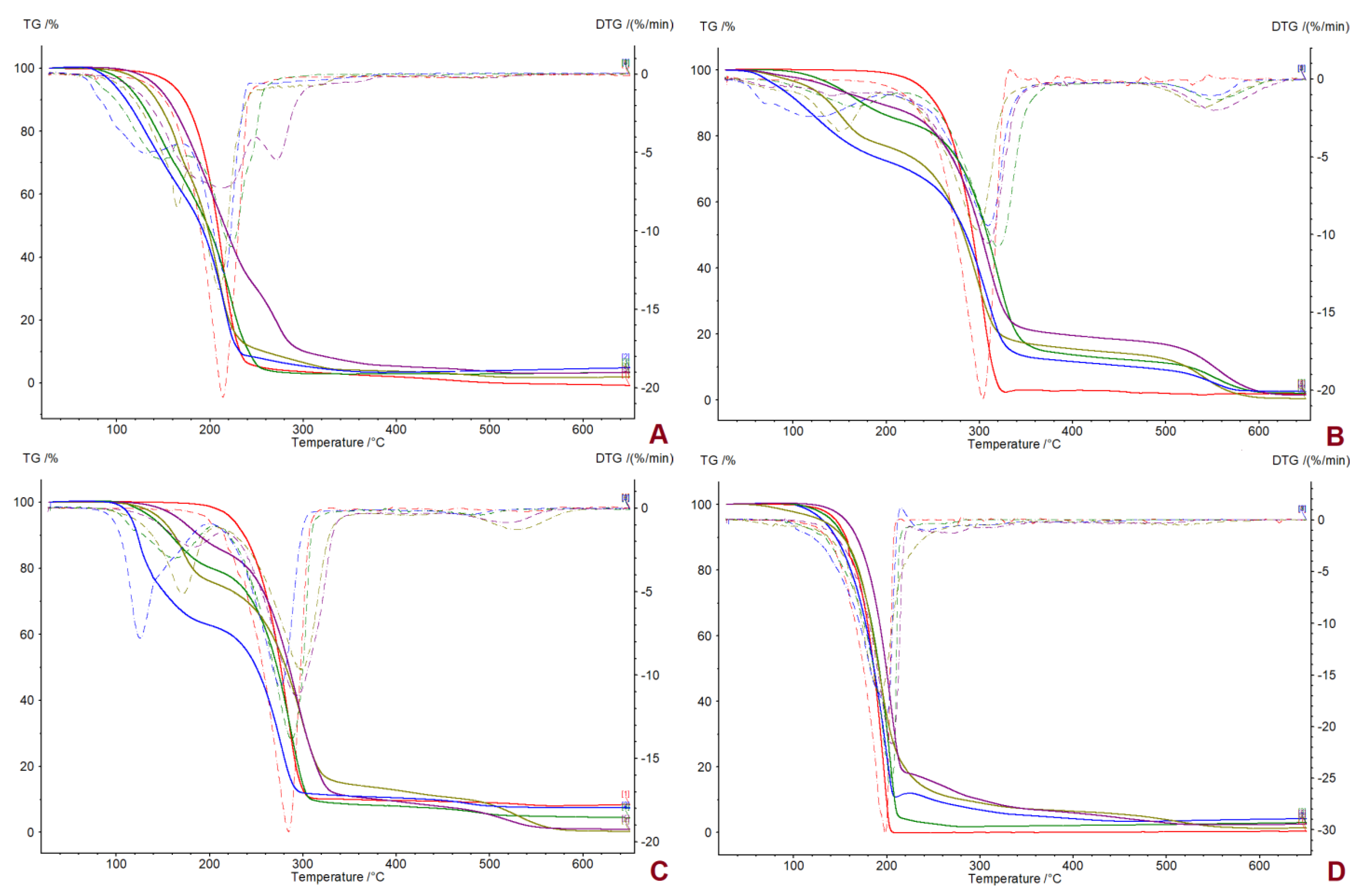

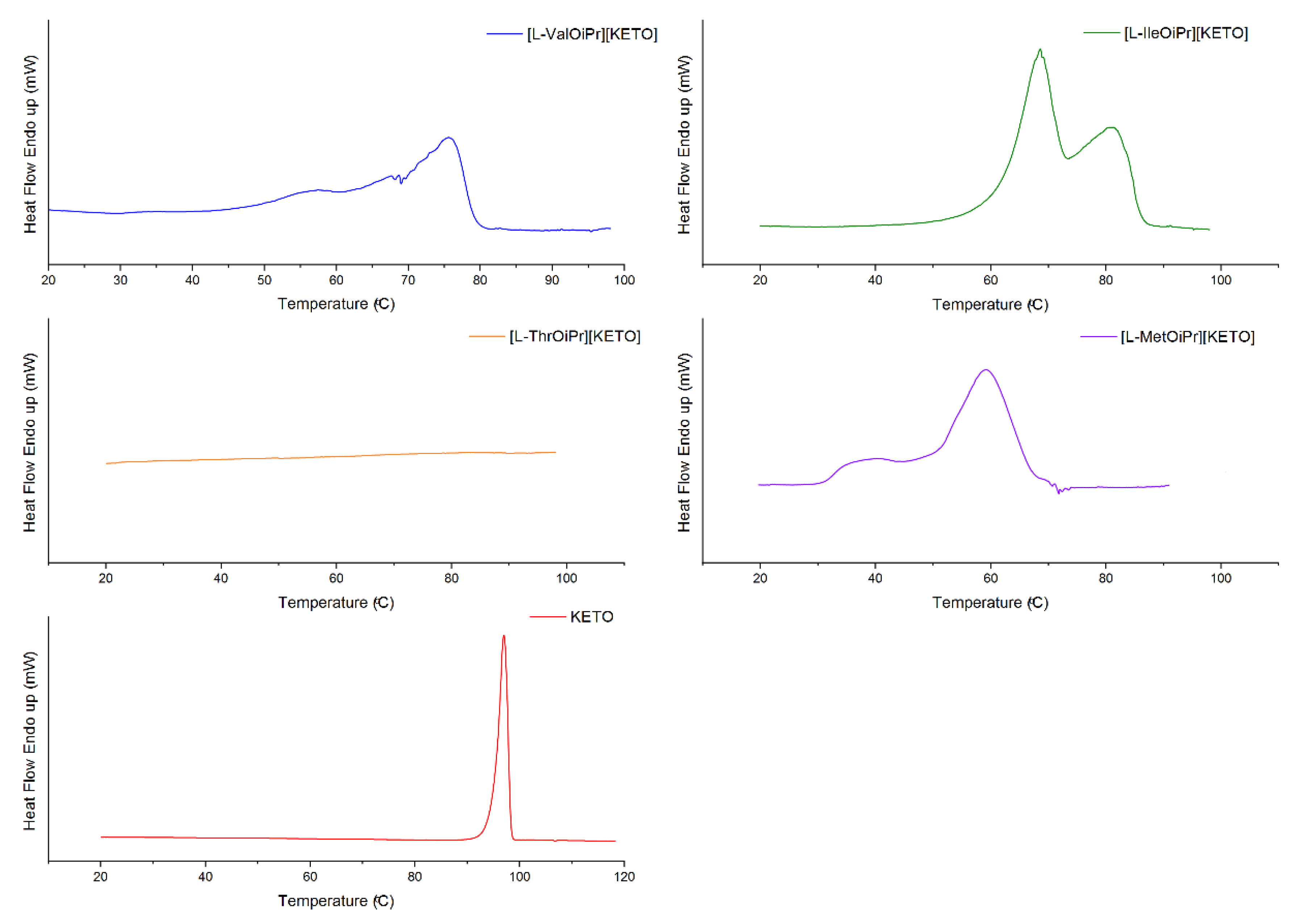
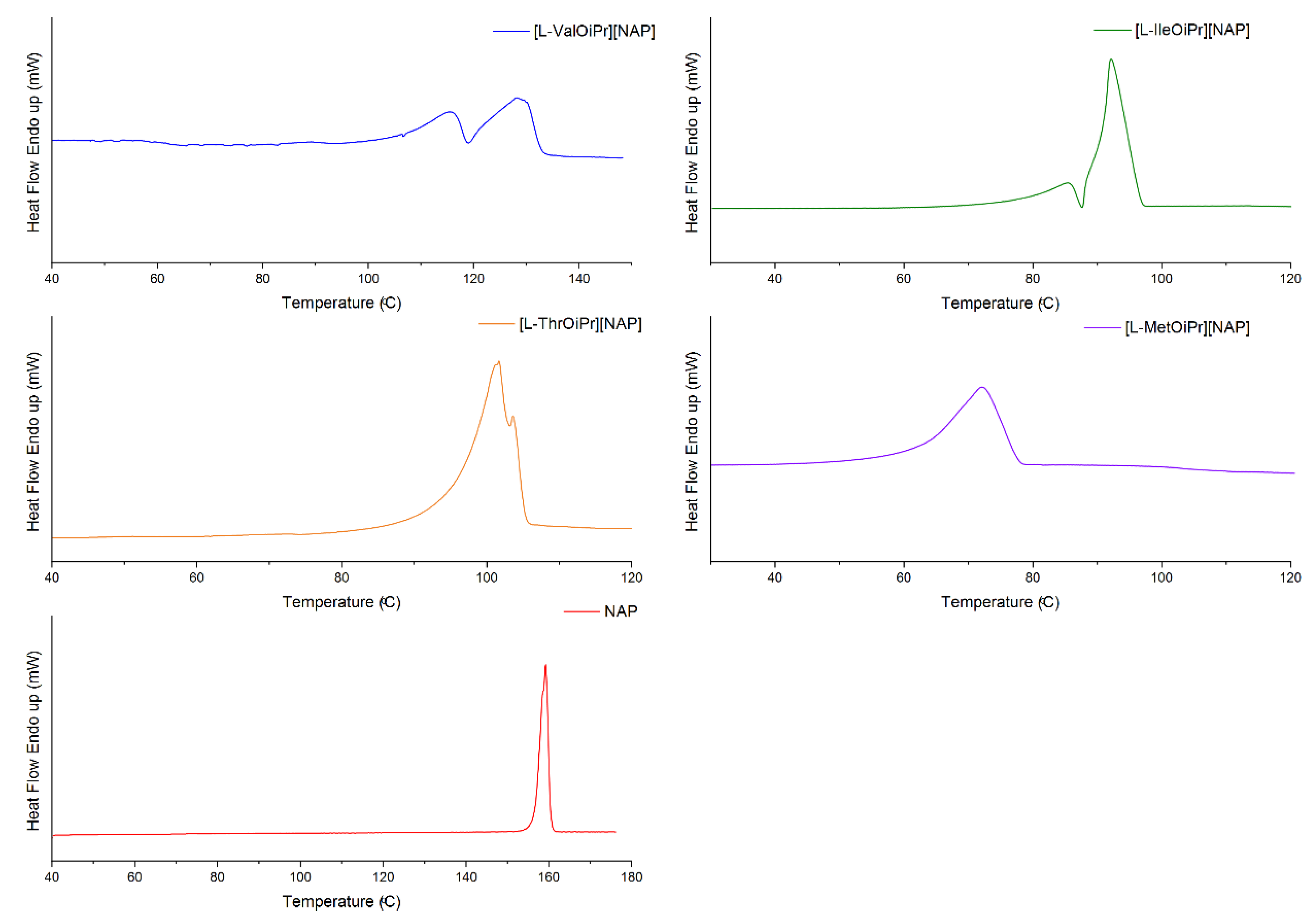
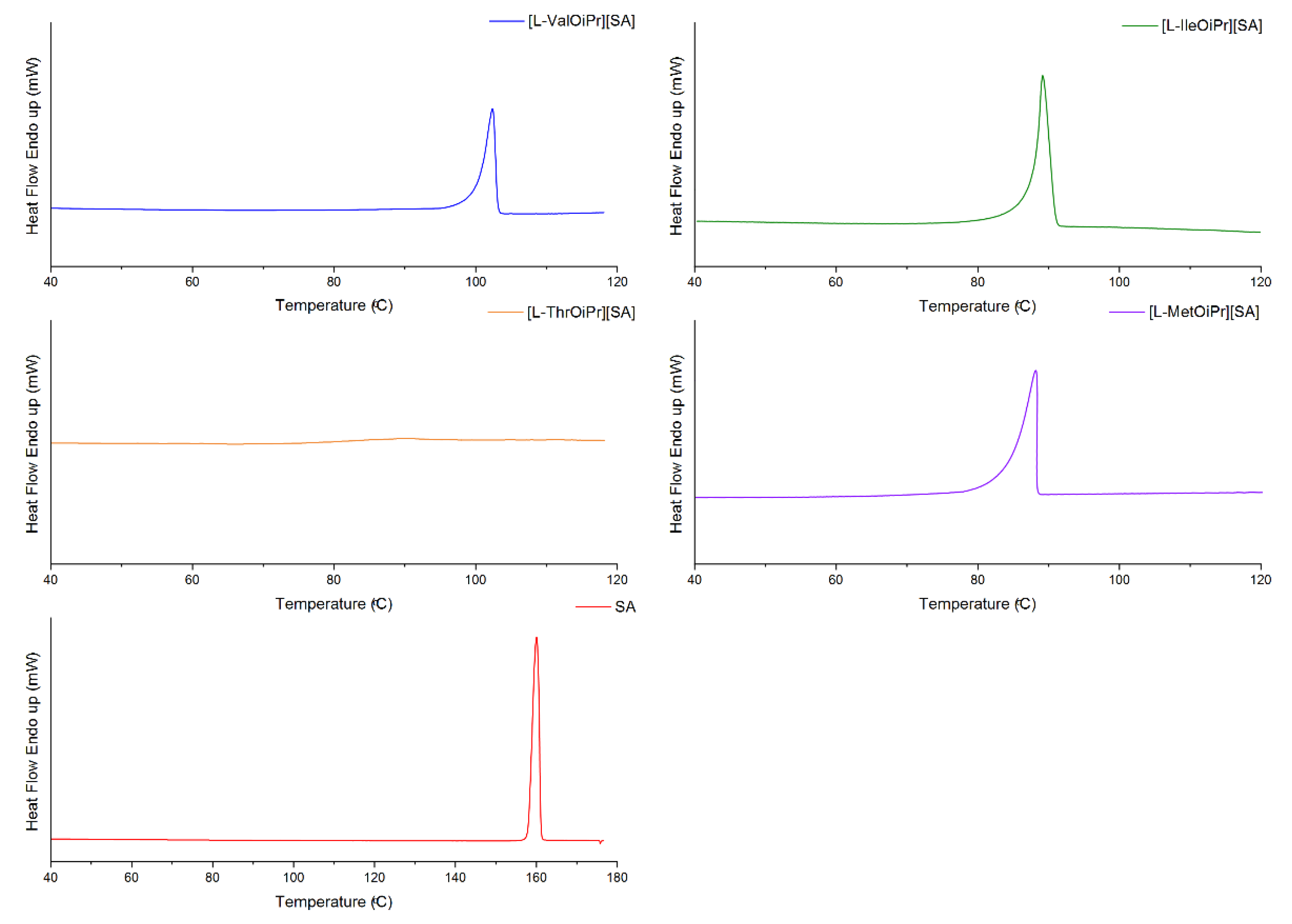

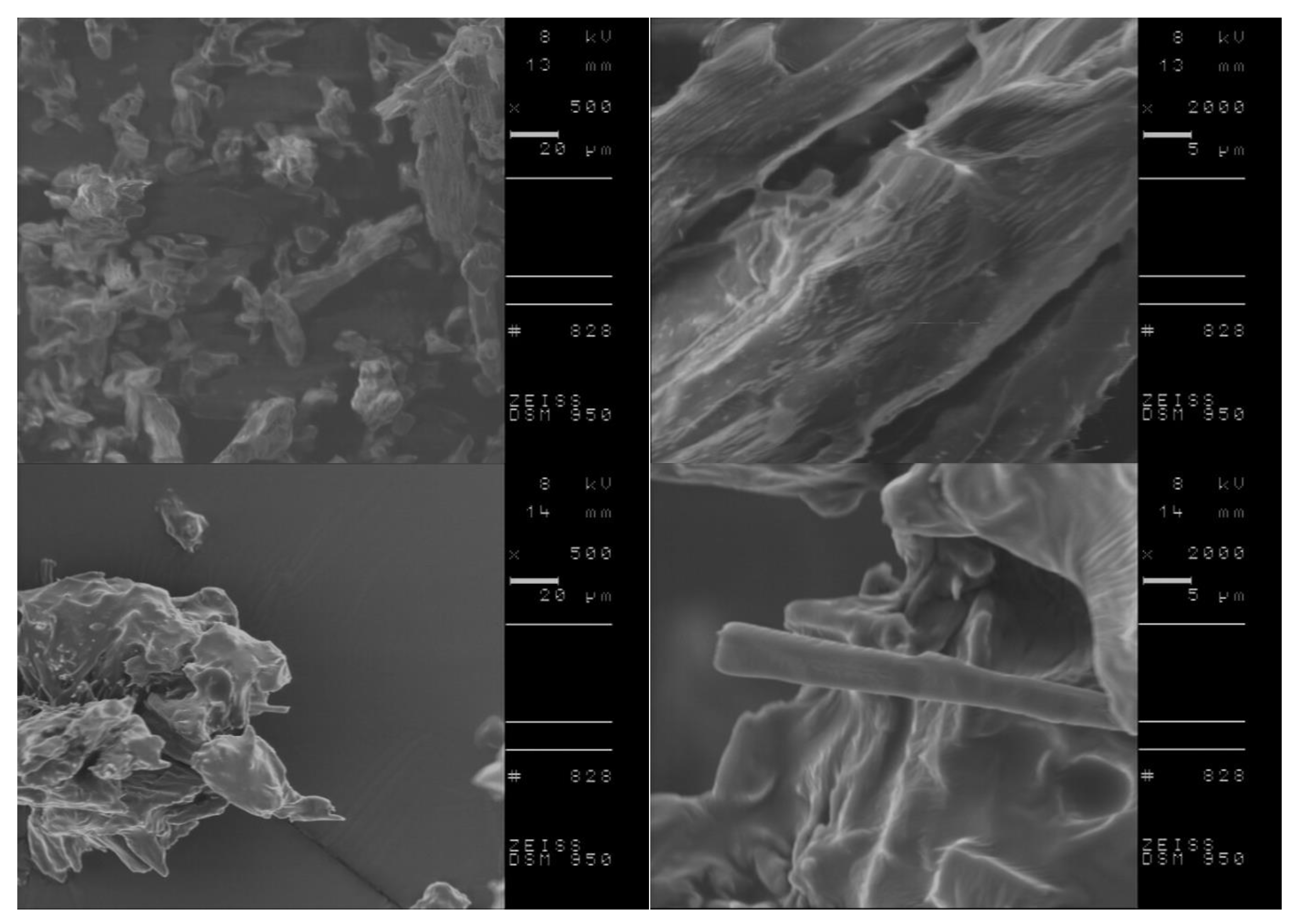
| Compound | TDSConset (°C) | TDSCmax (°C) | TC (°C) | TTGonset (°C) | TDTGmax (°C) | ||
|---|---|---|---|---|---|---|---|
| IBU | 76.23 | 78.58 | - | 186.2 | 218.8 | - | - |
| [L-ValOiPr][IBU] | 65.84 | 91.15 | 66.33 | 90.2 [50] | 215.5 [50] | +11.852 [50] | +43.313 [50] |
| [L-IleOiPr][IBU] | 73.68/ 79.98 | 77.24/ 82.56 | 52.67 | 105.2 [50] | 221.9 [50] | +15.779 [50] | +59.887 [50] |
| [L-ThrOiPr][IBU] | 44.62 | 53.17 | - | 129.6 [50] | 209.9 [50] | −1.538 [50] | −5.654 [50] |
| [L-MetOiPr][IBU] | 60.42 | 65.09 | 47.04 | 146.2 [50] | 209.9 [50] | +3.137 [50] | +12.472 [50] |
| KETO | 95.14 | 97.03 | - | 241.4 | 304.9 | - | - |
| [L-ValOiPr][KETO] | 69.84 | 75.65 | - | 60.7 [47] | 307.6 [47] | +8.083 [47] | +33.424 [47] |
| [L-IleOiPr][KETO] | 61.70/ 73.38 | 68.73/ 81.36 | - | 116.6 | 319.6 | +9.053 | +38.407 |
| [L-ThrOiPr][KETO] | - | - | - | 82.4 | 295.1 | −9.742 | −40.475 |
| [L-MetOiPr][KETO] | 49.62 | 61.67 | - | 98.0 | 306.5 | +9.182 | +40.912 |
| NAP | 155.98 | 158.41 | 98.60 | 224.5 | 284.0 | +50.988 | +117.405 |
| [L-ValOiPr][NAP] | 107.23/ 119.16 | 115.32/ 128.19 | 81.52 | 112.2 [48] | 275.8 [48] | +30.740 [48] | +119.729 [48] |
| [L-IleOiPr][NAP] | 74.50/ 86.79 | 82.58/ 92.13 | 56.74 | 127.0 | 287.4 | +34.343 | +138.578 |
| [L-ThrOiPr][NAP] | 87.16 | 91.97 | - | 145.8 | 295.3 | +26.608 | +104.159 |
| [L-MetOiPr][NAP] | 71.00 | 83.79 | 55.66 | 149.6 | 293.2 | +26.693 | +112.524 |
| SA | 158.05 | 160.04 | 107.83 | 146.2 | 198.8 | - | - |
| [L-ValOiPr][SA] | 100.13 | 102.33 | 83.01 | 124.6 [49] | 197.6 [49] | +12.457 [49] | +37.040 [49] |
| [L-IleOiPr][SA] | 86.10 | 88.50 | 67.26 | 144.2 | 202.4 | +18.939 | +58.780 |
| [L-ThrOiPr][SA] | - | - | - | 127.2 | 191.4 | −4.105 | −12.245 |
| [L-MetOiPr][SA] | 85.39 | 89.09 | 51.62 | 161.1 | 205.0 | +10.575 | +34.729 |
| Compound | Ethanol (59.1) | DMSO (45.1) | Dichloromethane (40.7) | Chloroform (39.1) | Ethyl Acetate (38.1) | Diethyl Ether (34.5) | Toluene (33.9) | Hexane (31.0) |
|---|---|---|---|---|---|---|---|---|
| IBU | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● |
| [ValOiPr][IBU] | ○ [45] | ○ [45] | ○ [45] | ○ [45] | ◐ [45] | ◐ [45] | ○ [45] | ● [45] |
| [IleOiPr][IBU] | ○ [50] | ○ [50] | ○ [50] | ○ [50] | ○ [50] | ◐ [50] | ○ [50] | ● [50] |
| [ThrOiPr][IBU] | ○ [50] | ○ [50] | ○ [50] | ○ [50] | ◐ [50] | ◐ [50] | ◐ [50] | ● [50] |
| [MetOiPr][IBU] | ○ [50] | ○ [50] | ○ [50] | ○ [50] | ○ [50] | ○ [50] | ○ [50] | ● [50] |
| KETO | ○ | ○ | ○ | ○ | ◐ | ◐ | ● | ● |
| [ValOiPr][KETO] | ○ [47] | ○ [47] | ○ [47] | ◐ [47] | ● [47] | ○ [47] | ○ [47] | ● [47] |
| [IleOiPr][KETO] | ○ | ○ | ○ | ○ | ● | ● | ○ | ● |
| [ThrOiPr][KETO] | ○ | ○ | ◐ | ○ | ● | ● | ○ | ● |
| [MetOiPr][KETO] | ◐ | ○ | ○ | ○ | ● | ● | ○ | ● |
| NAP | ○ | ○ | ○ | ○ | ◐ | ◐ | ● | ● |
| [ValOiPr][NAP] | ◐ [48] | ◌ [48] | ◌ [48] | ◌ [48] | ● [48] | ◐ [48] | ● [48] | ● [48] |
| [IleOiPr][NAP] | ○ | ○ | ○ | ○ | ● | ● | ◐ | ● |
| [ThrOiPr][NAP] | ○ | ○ | ○ | ○ | ● | ● | ◐ | ● |
| [MetOiPr][NAP] | ○ | ○ | ○ | ○ | ● | ● | ○ | ● |
| SA | ○ | ○ | ○ | ◐ | ○ | ○ | ◐ | ● |
| [ValOiPr][SA] | ○ [49] | ○ [49] | ○ [49] | ○ [49] | ◐ [49] | ◐ [49] | ○ [49] | ● [49] |
| [IleOiPr][SA] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● |
| [ThrOiPr][SA] | ○ | ○ | ○ | ○ | ○ | ● | ● | ● |
| [MetOiPr][SA] | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● |
| Compound | Solubility in Water | Solubility in Phosphate Buffer | ||||
|---|---|---|---|---|---|---|
| pH = 5.4 | pH = 7.4 | |||||
| g dm−3 | gAS dm−3 | g dm−3 | gAS dm−3 | g dm−3 | gAS dm−3 | |
| IBU | 0.076 ± 0.001 [45] | 0.076 ± 0.001 [45] | 0.082 ± 0.001 [45] | 0.082 ± 0.001 [45] | 0.432 ± 0.001 [45] | 0.432 ± 0.001 [45] |
| [L-ValOiPr][IBU] | 3.468 ± 0.007 [45] | 1.957 ± 0.007 [45] | 2.351 ± 0.027 [45] | 1.326 ± 0.027 [45] | 4.998 ± 0.018 [45] | 2.821 ± 0.018 [45] |
| [L-IleOiPr][IBU] | 2.729 ± 0.180 [50] | 1.483 ± 0.098 [50] | 1.161 ± 0.002 | 0.631 ± 0.002 | 4.038 ± 0.021 | 2.195 ± 0.021 |
| [L-ThrOiPr][IBU] | 5.005 ± 0.007 [50] | 2.809 ± 0.004 [50] | 4.931 ± 0.013 | 2.768 ± 0.013 | 8.839 ± 0.005 | 4.962 ± 0.005 |
| [L-MetOiPr][IBU] | 1.191 ± 0.056 [50] | 0.618 ± 0.029 [50] | 0.932 ± 0.011 | 0.483 ± 0.011 | 3.376 ± 0.001 | 1.752 ± 0.001 |
| KETO | 0.013 ± 0.001 | 0.013 ± 0.001 | 0.024 ± 0.001 | 0.024 ± 0.001 | 0.079 ± 0.003 | 0.079 ± 0.003 |
| [L-ValOiPr][KETO] | 0.445 ± 0.014 | 0.274 ± 0.014 | 0.162 ± 0.054 | 0.100 ± 0.054 | 1.447 ± 0.011 | 0.890 ± 0.011 |
| [L-IleOiPr][KETO] | 0.577 ± 0.006 | 0.343 ± 0.006 | 0.344 ± 0.032 | 0.205 ± 0.032 | 1.173 ± 0.024 | 0.698 ± 0.024 |
| [L-ThrOiPr][KETO] | 1.012 ± 0.001 | 0.619 ± 0.001 | 0.823 ± 0.027 | 0.504 ± 0.027 | 2.566 ± 0.036 | 1.570 ± 0.036 |
| [L-MetOiPr][KETO] | 0.289 ± 0.004 | 0.165 ± 0.004 | 0.171 ± 0.058 | 0.098 ± 0.058 | 1.086 ± 0.007 | 0.622 ± 0.007 |
| NAP | 0.147 ± 0.001 | 0.147 ± 0.001 | 0.221 ± 0.015 | 0.221 ± 0.015 | 1.379 ± 0.021 | 1.379 ± 0.021 |
| [L-ValOiPr][NAP] | 3.988 ± 0.020 | 2.358 ± 0.020 | 2.323 ± 0.033 | 1.320 ± 0.033 | 5.484 ± 0.012 | 3.241 ± 0.012 |
| [L-IleOiPr][NAP] | 5.048 ± 0.027 | 2.881 ± 0.027 | 3.206 ± 0.017 | 1.829 ± 0.017 | 7.736 ± 0.030 | 4.415 ± 0.030 |
| [L-ThrOiPr][NAP] | 6.457 ± 0.019 | 3.798 ± 0.019 | 4.381 ± 0.008 | 2.585 ± 0.008 | 8.711 ± 0.029 | 5.137 ± 0.029 |
| [L-MetOiPr][NAP] | 3.011 ± 0.038 | 1.645 ± 0.038 | 1.509 ± 0.015 | 0.824 ± 0.015 | 4.404 ± 0.033 | 2.405 ± 0.033 |
| SA | 3.795 ± 0.004 | 3.795 ± 0.004 | 3.862 ± 0.005 | 3.862 ± 0.005 | 5.757 ± 0.024 | 5.757 ± 0.024 |
| [L-ValOiPr][SA] | 16.708 ± 2.044 | 7.761 ± 2.044 | 11.322 ± 0.014 | 5.259 ± 0.014 | 18.733 ± 0.027 | 8.702 ± 0.027 |
| [L-IleOiPr][SA] | 26.500 ± 0.422 | 11.793 ± 0.422 | 19.281 ± 0.015 | 8.581 ± 0.015 | 29.548 ± 0.041 | 13.149 ± 0.041 |
| [L-ThrOiPr][SA] | 38.714 ± 0.241 | 17.925 ± 0.241 | 30.189 ± 0.018 | 13.978 ± 0.018 | 40.757 ± 0.125 | 18.777 ± 0.125 |
| [L-MetOiPr][SA] | 16.046 ± 0.533 | 6.749 ± 0.533 | 10.550 ± 0.007 | 4.437 ± 0.007 | 18.036 ± 0.046 | 7.586 ± 0.046 |
| Compound | LogP | |||
|---|---|---|---|---|
| [IBU] | [KETO] | [NAP] | [SA] | |
| Unmodified acid | 3.208 ± 0.002 [50] | 1.577 ± 0.010 | 2.119 ± 0.021 | 1.321 ± 0.018 |
| [L-ValOiPr] | 1.154 ± 0.004 [45] | 0.570 ± 0.006 | 1.254 ± 0.016 | 1.610 ± 0.057 |
| [L-IleOiPr] | 1.652 ± 0.008 [50] | 0.897 ± 0.004 | 1.188 ± 0.033 | 1.601 ± 0.034 |
| [L-ThrOiPr] | 0.998 ± 0.001 [50] | 0.516 ± 0.001 | 0.089 ± 0.005 | 1.555 ± 0.021 |
| [L-MetOiPr] | 1.509 ± 0.001 [50] | 1.227 ± 0.015 | 1.372 ± 0.005 | 1.784 ± 0.035 |
| Compound | pKB of the Base | Log(KS) | |||
|---|---|---|---|---|---|
| [IBU] | [KETO] | [NAP] | [SA] | ||
| [L-ValOiPr] | 10.210 | 5.56 | 6.10 | 5.89 | 7.03 |
| [L-IleOiPr] | 10.430 | 5.78 | 6.32 | 6.11 | 7.25 |
| [L-ThrOiPr] | 11.118 | 6.47 | 6.80 | 6.80 | 7.94 |
| [L-MetOiPr] | 10.176 | 5.53 | 5.86 | 6.07 | 7.00 |
| Compound | Concentration of Solution Transferred to Disc [mg cm−3] | ||||||
|---|---|---|---|---|---|---|---|
| 1000 | 600 | 400 | 200 | 100 | 50 | 25 | |
| IBU | - | - | - | - | - | - | - |
| [L-ValOiPr][IBU] | 7.1 ± 0.3 | 6.8 ± 0.5 | 6.3 ± 0.2 | 6.2 ± 0.1 | - | - | - |
| [L-IleOiPr][IBU] | 7.8 ± 0.3 | 7.1 ± 0.2 | 6.4 ± 0.3 | 6.3 ± 0.1 | - | - | - |
| [L-ThrOiPr][IBU] | - | - | - | - | - | - | - |
| [L-MetOiPr][IBU] | 8.1 ± 0.1 | 7.6 ± 0.2 | 7.1 ± 0.3 | 6.5 ± 0.2 | 6.2 ± 0.1 | 6.1 ± 0.1 | - |
| KETO | - | - | - | - | - | - | - |
| [L-ValOiPr][KETO] | 8.3 ± 0.4 | 7.6 ± 0.3 | 7.0 ± 0.2 | 6.7 ± 0.3 | - | - | - |
| [L-IleOiPr][KETO] | - | - | - | - | - | - | - |
| [L-ThrOiPr][KETO] | - | - | - | - | - | - | - |
| [L-MetOiPr][KETO] | 12.9 ± 0.4 | 10.6 ± 0.7 | 8.9 ± 0.5 | 8.0 ± 0.6 | 7.3 ± 0.5 | - | - |
| NAP | - | - | - | - | - | - | - |
| [L-ValOiPr][NAP] | - | - | - | - | - | - | - |
| [L-IleOiPr][NAP] | - | - | - | - | - | - | - |
| [L-ThrOiPr][NAP] | - | - | - | - | - | - | - |
| [L-MetOiPr][NAP] | 7.7 ± 0.2 | 7.3 ± 0.2 | 7.1 ± 0.1 | 6.4 ± 0.3 | - | - | - |
| SA | 13.1 ± 1.2 | 11.0 ± 0.8 | 6.4 ± 0.1 | 6.1 ± 0.1 | - | - | - |
| [L-ValOiPr][SA] | 16.5 ± 1.0 | 12.6 ± 0.3 | 8.9 ± 0.4 | 7.5 ± 0.1 | 6.3 ± 0.2 | 6.2 ± 0.1 | - |
| [L-IleOiPr][SA] | 14.8 ± 0.7 | 13.7 ± 0.7 | 7.3 ± 0.3 | 6.2 ± 0.1 | - | - | - |
| [L-ThrOiPr][SA] | 13.2 ± 0.8 | 9.3 ± 0.4 | 7.3 ± 0.2 | - | - | - | - |
| [L-MetOiPr][SA] | 21.8 ± 0.7 | 18.8 ± 0.4 | 16.4 ± 0.3 | 14.4 ± 0.8 | 7.8 ± 0.2 | - | - |
| Compound | Concentration of Solution Transferred to Disc [mg cm−3] | |||||||
|---|---|---|---|---|---|---|---|---|
| 1000 | 800 | 600 | 400 | 200 | 100 | 50 | 25 | |
| IBU | 20.6 ± 0.2 | 18.6 ± 0.2 | 17.6 ± 0.2 | 16.4 ± 0.2 | 15.3 ± 0.3 | 14.9 ± 0.3 | 11.5 ± 0.3 | 10.8 ± 0.3 |
| [L-ValOiPr][IBU] | 18.9 ± 0.3 | 18.4 ± 0.2 | 17.7 ± 0.3 | 16.3 ± 0.3 | 13.2 ± 0.2 | 12.8 ± 0.2 | 10.4 ± 0.1 | 7.3 ± 0.2 |
| [L-IleOiPr][IBU] | 19.3 ± 0.1 | 18.0 ± 0.1 | 17.5 ± 0.1 | 17.0 ± 0.2 | 15.4 ± 0.4 | 14.4 ± 0.2 | 9.5 ± 0.4 | 7.5 ± 0.2 |
| [L-ThrOiPr][IBU] | 23.2 ± 0.1 | 19.8 ± 1.4 | 18.3 ± 0.2 | 17.5 ± 0.2 | 16.9 ± 0.2 | 15.4 ± 0.1 | 10.5 ± 0.2 | 7.5 ± 0.2 |
| [L-MetOiPr][IBU] | 17.7 ± 0.3 | 17.0 ± 0.2 | 15.4 ± 0.2 | 14.7 ± 0.2 | 13.6 ± 0.2 | 8.3 ± 0.1 | - | - |
| KETO | - | - | - | - | - | - | - | - |
| [L-ValOiPr][KETO] | 6.9 ± 0.2 | 6.3 ± 0.2 | 6.3 ± 0.1 | 6.2 ± 0.1 | - | - | - | - |
| [L-IleOiPr][KETO] | 10.4 ± 0.2 | 8.3 ± 0.1 | 7.3 ± 0.2 | 6.2 ± 0.1 | - | - | - | - |
| [L-ThrOiPr][KETO] | - | - | - | - | - | - | - | - |
| [L-MetOiPr][KETO] | 7.4 ± 0.2 | - | - | - | - | - | - | - |
| NAP | - | - | - | - | - | - | - | - |
| [L-ValOiPr][NAP] | 7.4 ± 0.1 | 6.6 ± 0.3 | - | - | - | - | - | - |
| [L-IleOiPr][NAP] | 13.5 ± 0.3 | 12.4 ± 0.2 | 12.0 ± 0.3 | - | - | - | - | - |
| [L-ThrOiPr][NAP] | - | - | - | - | - | - | - | - |
| [L-MetOiPr][NAP] | 7.5 ± 0.3 | - | - | - | - | - | - | - |
| SA | 12.6 ± 0.7 | 8.6 ± 0.3 | 7.0 ± 0.2 | 6.2 ± 0.1 | - | - | - | - |
| [L-ValOiPr][SA] | 10.7 ± 0.3 | 8.4 ± 0.3 | 6.7 ± 0.3 | - | - | - | - | - |
| [L-IleOiPr][SA] | 16.6 ± 0.5 | 10.5 ± 0.3 | 7.3 ± 0.4 | - | - | - | - | - |
| [L-ThrOiPr][SA] | - | - | - | - | - | - | - | - |
| [L-MetOiPr][SA] | 10.7 ± 0.6 | 9.2 ± 0.5 | 7.5 ± 0.2 | 7.1 ± 0.1 | 6.6 ± 0.2 | - | - | - |
| Compound | Concentration of Solution Transferred to Disc [mg cm−3] | |||||||
|---|---|---|---|---|---|---|---|---|
| 1000 | 800 | 600 | 400 | 200 | 100 | 50 | 25 | |
| IBU | 13.4 ± 0.1 | 12.7 ± 0.2 | 11.5 ± 0.2 | 10.6 ± 0.1 | 10.2 ± 0.1 | 9.7± 0.2 | 9.4 ± 0.2 | 8.6 ± 0.3 |
| [L-ValOiPr][IBU] | 13.2 ± 0.2 | 12.6 ± 0.3 | 11.7 ± 0.2 | 11.2 ± 0.2 | 10.5 ± 0.2 | 9.6 ± 0.1 | 9.1 ± 0.2 | 8.4 ± 0.3 |
| [L-IleOiPr][IBU] | 12.3 ± 0.2 | 11.6 ± 0.1 | 11.2 ± 0.1 | 10.7 ± 0.1 | 9.4 ± 0.2 | 8.6 ± 0.1 | 7.6 ± 0.1 | 7.2 ± 0.2 |
| [L-ThrOiPr][IBU] | 19.3 ± 0.2 | 18.2 ± 0.2 | 17.5 ± 0.1 | 17.0 ± 0.2 | 16.3 ± 0.1 | 15.5 ± 0.2 | 10.4 ± 0.2 | 7.3 ± 0.2 |
| [L-MetOiPr][IBU] | 14.6 ± 0.3 | 14.3 ± 0.1 | 14.1 ± 0.1 | 12.6 ± 0.1 | 10.4 ± 0.1 | 8.5 ± 0.2 | 7.3 ± 0.2 | - |
| KETO | 17.3 ± 0.2 | 14.2 ± 0.1 | 13.3 ± 0.2 | 12.5 ± 0.2 | 11.1 ± 0.4 | 10.2 ± 0.2 | 9.6 ± 0.2 | 8.3 ± 0.3 |
| [L-ValOiPr][KETO] | 15.1 ± 0.2 | 14.5 ± 0.2 | 11.7 ± 0.1 | 10.3 ± 0.2 | 9.1 ± 0.3 | 8.5 ± 0.3 | 7.4 ± 0.1 | 6.3 ± 0.2 |
| [L-IleOiPr][KETO] | 9.4 ± 0.2 | 8.4 ± 0.3 | 8.3 ± 0.2 | 7.3 ± 0.1 | 7.1 ± 0.2 | 6.6 ± 0.3 | 6.3 ± 0.2 | - |
| [L-ThrOiPr][KETO] | 17.4 ± 0.1 | 15.4 ± 0.2 | 14.3 ± 0.2 | 9.6 ± 0.1 | 7.4 ± 0.3 | - | - | - |
| [L-MetOiPr][KETO] | 14.3 ± 0.2 | 12.4 ± 0.2 | 9.6 ± 0.2 | 9.2 ± 0.1 | 8.5 ± 0.3 | 8.2 ± 0.2 | 7.2 ± 0.2 | - |
| NAP | 8.7 ± 0.1 | 8.5 ± 0.2 | 7.6 ± 0.2 | 7.3 ± 0.2 | 6.8 ± 0.1 | 6.7 ± 0.1 | 6.4 ± 0.1 | 6.2 ± 0.1 |
| [L-ValOiPr][NAP] | 8.6 ± 0.2 | 8.2 ± 0.2 | 7.4 ± 0.2 | 7.3 ± 0.1 | 7.2 ± 0.1 | 6.4 ± 0.1 | 6.3 ± 0.1 | 6.2 ± 0.1 |
| [L-IleOiPr][NAP] | 9.5 ± 0.1 | 8.7 ± 0.1 | 8.1 ± 0.6 | 7.5 ± 0.2 | 7.3 ± 0.1 | 7.0 ± 0.1 | 6.7 ± 0.1 | 6.4 ± 0.1 |
| [L-ThrOiPr][NAP] | 10.8 ± 0.5 | 9.6 ± 0.1 | 8.8 ± 0.2 | 8.5 ± 0.1 | 7.6 ± 0.1 | 6.9 ± 0.1 | 6.6 ± 0.1 | 6.2 ± 0.1 |
| [L-MetOiPr][NAP] | 7.8 ± 0.2 | 7.5 ± 0.1 | 7.1 ± 0.2 | 6.4 ± 0.1 | - | - | - | - |
| SA | 13.1 ± 1.9 | 9.4 ± 0.8 | 8.7 ± 0.8 | 7.4 ± 0.4 | - | - | - | - |
| [L-ValOiPr][SA] | 17.4 ± 1.4 | 15.9 ± 1.2 | 8.6 ± 0.3 | - | - | - | - | - |
| [L-IleOiPr][SA] | 23.5 ± 2.2 | 12.4 ± 0.4 | 10.8 ± 0.5 | - | - | - | - | - |
| [L-ThrOiPr][SA] | 12.7 ± 1.0 | 10.2 ± 0.7 | 8.7 ± 0.5 | 8.1 ± 0.1 | 7.6 ± 0.2 | 7.0 ± 0.1 | 6.6 ± 0.2 | 6.3 ± 0.1 |
| [L-MetOiPr][SA] | 11.1 ± 0.9 | 10.5 ± 1.0 | 8.1 ± 0.9 | 7.3 ± 0.4 | 6.3 ± 0.1 | 6.1 ± 0.1 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klebeko, J.; Krüger, O.; Dubicki, M.; Ossowicz-Rupniewska, P.; Janus, E. Isopropyl Amino Acid Esters Ionic Liquids as Vehicles for Non-Steroidal Anti-Inflammatory Drugs in Potential Topical Drug Delivery Systems with Antimicrobial Activity. Int. J. Mol. Sci. 2022, 23, 13863. https://doi.org/10.3390/ijms232213863
Klebeko J, Krüger O, Dubicki M, Ossowicz-Rupniewska P, Janus E. Isopropyl Amino Acid Esters Ionic Liquids as Vehicles for Non-Steroidal Anti-Inflammatory Drugs in Potential Topical Drug Delivery Systems with Antimicrobial Activity. International Journal of Molecular Sciences. 2022; 23(22):13863. https://doi.org/10.3390/ijms232213863
Chicago/Turabian StyleKlebeko, Joanna, Oliver Krüger, Mateusz Dubicki, Paula Ossowicz-Rupniewska, and Ewa Janus. 2022. "Isopropyl Amino Acid Esters Ionic Liquids as Vehicles for Non-Steroidal Anti-Inflammatory Drugs in Potential Topical Drug Delivery Systems with Antimicrobial Activity" International Journal of Molecular Sciences 23, no. 22: 13863. https://doi.org/10.3390/ijms232213863






