Proteomics of Salt Gland–Secreted Sap Indicates a Pivotal Role for Vesicle Transport and Energy Metabolism in Plant Salt Secretion
Abstract
:1. Introduction
2. Results
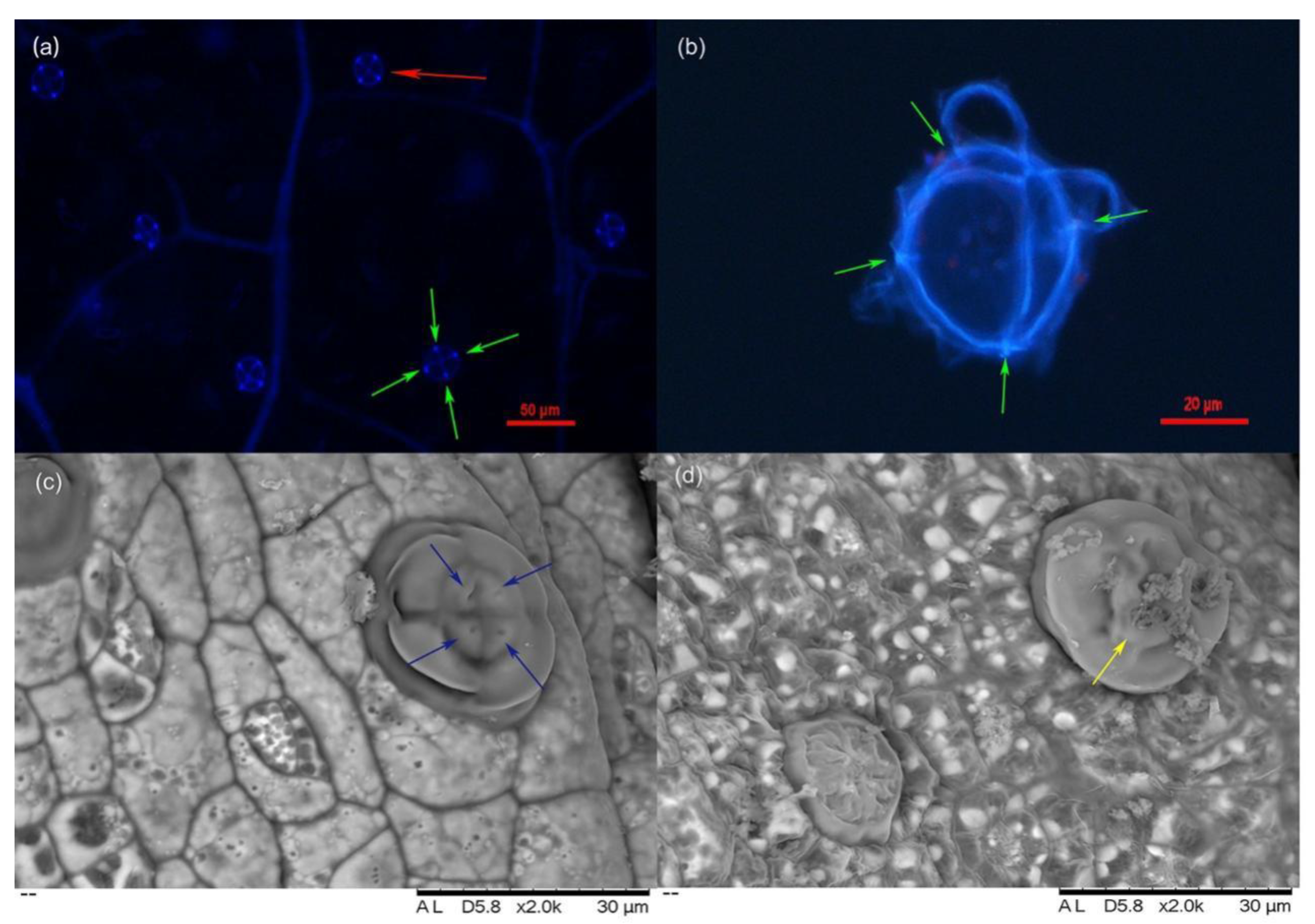
2.1. Characteristics of L. bicolor Salt Glands
2.2. SDS-PAGE Analysis of Proteins in the Secretion Liquids
2.3. Protein Identification by Mass Spectrometry
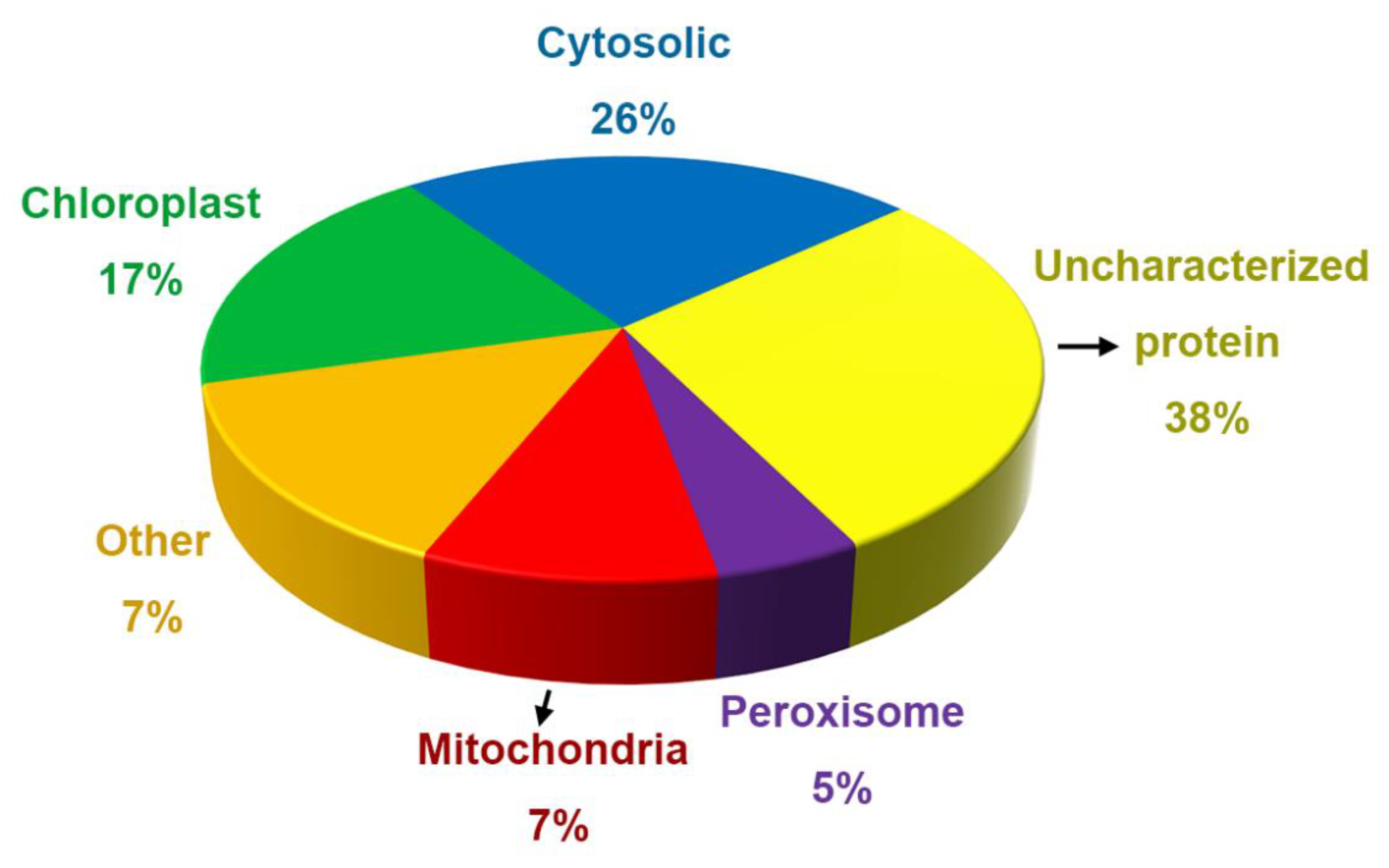
2.4. Annotation of Proteins and Peptides
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Salt Gland Observations
4.3. Extraction of Proteins from the Secretion Liquid of Salt Glands
4.4. Protein Identification, Annotation, and Characterization
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Z.; Sun, Q.; Deng, Y.; Sun, S.; Zhang, J.; Wang, B. Study on pathway and characteristics of ion secretion of salt glands of Limonium bicolor. Acta Physiol. Plant. 2014, 36, 2729–2741. [Google Scholar] [CrossRef]
- Rodriguez, H.G.; Roberts, J.; Jordan, W.R.; Drew, M.C. Growth, Water Relations, and Accumulation of Organic and Inorganic Solutes in Roots of Maize Seedlings during Salt Stress. Plant Physiol. 1997, 113, 881–893. [Google Scholar] [CrossRef] [Green Version]
- Lutts, S.; Lefevre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 509–528. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Yuan, F.; Leng, B.; Wang, B. Progress in Studying Salt Secretion from the Salt Glands in Recretohalophytes: How Do Plants Secrete Salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Shi, W.; Liu, R.; Xu, Y.; Sui, N.; Zhou, J.; Feng, G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Species Biol. 2017, 32, 107–114. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, B.; Guo, H.; Zhang, X. Preliminary study on physiologic response of salt-dilution halophyte Salicornia europaea L. to salt stress. Xinjiang Agric. Sci. 2012, 49, 694–700. [Google Scholar]
- Dassanayake, M.; Larkin, J.C. Making Plants Break a Sweat: The Structure, Function, and Evolution of Plant Salt Glands. Front. Plant Sci. 2017, 8, 724. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Yuan, F.; Guo, J.; Han, G.; Wang, C.; Chen, M.; Wang, B. Current Understanding of Role of Vesicular Transport in Salt Secretion by Salt Glands in Recretohalophytes. Int. J. Mol. Sci. 2021, 22, 2203. [Google Scholar] [CrossRef]
- Caperta, A.D.; Róis, A.S.; Teixeira, G.; Garcia-Caparros, P.; Flowers, T.J. Secretory structures in plants: Lessons from the Plumbaginaceae on their origin, evolution and roles in stress tolerance. Plant Cell Environ. 2020, 43, 2912–2931. [Google Scholar] [CrossRef]
- Yuan, F.; Leng, B.; Zhang, H.; Wang, X.; Han, G.; Wang, B. A WD40-Repeat Protein from the Recretohalophyte Limonium bicolor Enhances Trichome Formation and Salt Tolerance in Arabidopsis. Front. Plant Sci. 2019, 10, 1456. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Wang, X.; Yuan, F.; Zhang, H.; Lu, C.; Chen, M.; Wang, B. Heterologous expression of the Limonium bicolor MYB transcription factor LbTRY in Arabidopsis thaliana increases salt sensitivity by modifying root hair development and osmotic homeostasis. Plant Sci. 2021, 302, 110704. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, H.; Lüttge, U. Die Salzdrüsen von Limonium vulgare. Planta 1967, 74, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Levering, C.A.; Thomson, W.W. The ultrastructure of the salt gland of Spartina foliosa. Planta 1971, 97, 183–196. [Google Scholar] [CrossRef]
- Shimony, C.; Fahn, A. Light- and electron-microscopical studies on the structure of salt glands of Tamarix aphylla L. Bot. J. Linn. Soc. 1968, 60, 283–288. [Google Scholar] [CrossRef]
- Semenova, G.A.; Fomina, I.R.; Biel, K.Y. Structural features of the salt glands of the leaf of Distichlis spicata ‘Yensen 4a’ (Poaceae). Protoplasma 2010, 240, 75–82. [Google Scholar] [CrossRef]
- Arisz, W.; Camphuis, I.; Heikens, H.; Van Tooren, A.V. The secretion of the salt glands of Limonium Latifolium Ktze. Acta Botánica Neerl. 1955, 4, 322–338. [Google Scholar] [CrossRef]
- Kobayashi, H.; Masaoka, Y.; Takahashi, Y.; Ide, Y.; Sato, S. Ability of salt glands in Rhodes grass (Chloris gayana Kunth) to secrete Na+ and K+. Soil Sci. Plant Nutr. 2007, 53, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Laursen, M.; Gregersen, J.L.; Yatime, L.; Nissen, P.; Fedosova, N.U. Structures and characterization of digoxin- and bufalin-bound Na+,K+-ATPase compared with the ouabain-bound complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1755–1760. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.-T.; Deng, Y.-Q.; Zhang, S.-C.; Liang, X.; Yuan, F.; Hao, J.-L.; Zhang, J.-C.; Sun, S.-F.; Wang, B.-S. K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Sci. 2015, 238, 286–296. [Google Scholar] [CrossRef]
- Yuan, F.; Lyu, M.J.A.; Leng, B.Y.; Zheng, G.Y.; Feng, Z.T.; Li, P.H.; Zhu, X.G.; Wang, B.S. Comparative transcriptome analysis of developmental stages of the Limonium bicolor leaf generates insights into salt gland differentiation. Plant Cell Environ. 2015, 38, 1637–1657. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Glenn, E.P.; Volkov, V. Could vesicular transport of Na+ and Cl− be a feature of salt tolerance in halophytes? Ann. Bot. 2019, 123, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Feng, Z.; Yuan, F.; Han, G.; Guo, J.; Chen, M.; Wang, B. The SNARE protein LbSYP61 participates in salt secretion in Limonium bicolor. Environ. Exp. Bot. 2020, 176, 104076. [Google Scholar] [CrossRef]
- Tan, W.K.; Lin, Q.; Lim, T.M.; Kumar, P.; Loh, C.S. Dynamic secretion changes in the salt glands of the mangrove tree species Avicennia officinalis in response to a changing saline environment. Plant Cell Environ. 2013, 36, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Tyerman, S.D. The devil in the detail of secretions. Plant Cell Environ. 2013, 36, 1407–1409. [Google Scholar] [CrossRef]
- Sookbirsingh, R.; Castillo, K.; Gill, T.E.; Chianelli, R.R. Salt Separation Processes in the Saltcedar Tamarix ramosissima (Ledeb.). Commun. Soil Sci. Plant Anal. 2010, 41, 1271–1281. [Google Scholar] [CrossRef]
- Hill, A.E. Ion and water transport in Limonium: II. Short-circuit analysis. Biochim. Biophys. Acta 1967, 135, 461–465. [Google Scholar] [CrossRef]
- Ding, F.; Yang, J.; Yuan, F.; Wang, B.S. Progress in mechanism of salt excretion in recretohalopytes. Front. Biol. 2010, 5, 164–170. [Google Scholar] [CrossRef]
- Pollak, G.; Waisel, Y. Salt Secretion in Aeluropus litoralis (Willd.) Parl. Ann. Bot. 1970, 34, 879–888. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, Z.; Yuan, F.; Guo, J.; Suo, S.; Wang, B. Identification and functional analysis of the autofluorescent substance in Limonium bicolor salt glands. Plant Physiol. Biochem. 2015, 97, 20–27. [Google Scholar] [CrossRef]
- Leng, B.Y.; Yuan, F.; Dong, X.X.; Wang, B.S. Salt gland distribution in Limonium bicolor at the individual level. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 3rd International Conference on Advances in Energy Resources and Environment Engineering, Harbin, China, 8–10 December 2017; Volume 113, p. 012202. [Google Scholar]
- Spreitzer, R.J.; Thow, G.; Zhu, G. Pseudoreversion substitution at large-subunit residue 54 influences the CO2/O2 specificity of chloroplast ribulose-bisphosphate carboxylase/oxygenase. Plant Physiol. 1995, 109, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Smalle, J.A. Oxidative stress-induced formation of covalently linked ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit dimer in tobacco plants. BMC Res. Notes 2019, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Grabsztunowicz, M.; Gorski, Z.; Lucinski, R.; Jackowski, G. A reversible decrease in ribulose 1,5-bisphosphate carboxylase/oxygenase carboxylation activity caused by the aggregation of the enzyme’s large subunit is triggered in response to the exposure of moderate irradiance-grown plants to low irradiance. Physiol. Plant. 2015, 154, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.R.; Rosenbusch, J.P. A functionally active tryptic fragment of Escherichia coli elongation factor Tu. Biochemistry 1976, 15, 5105–5110. [Google Scholar] [CrossRef]
- Slobin, L.I.; Clark, R.V.; Olson, M.O. Functional and structural studies on a tryptic fragment of eucaryotic elongation factor Tu from rabbit reticulocytes. Biochemistry 1981, 20, 5761–5767. [Google Scholar] [CrossRef]
- Hofmann, K.; Bucher, P. The PCI domain: A common theme in three multiprotein complexes. Trends Biochem. Sci. 1998, 23, 204–205. [Google Scholar] [CrossRef]
- Deshmukh, K.; Anamika, K.; Srinivasan, N. Evolution of domain combinations in protein kinases and its implications for functional diversity. Prog. Biophys. Mol. Biol. 2010, 102, 1–15. [Google Scholar] [CrossRef]
- Cao, Z.; He, Q.; Wang, P.; Yan, J.; Awais, M.M.; Liu, Z.; Yan, H.; Sun, J. Functional characteristics of a calcium-dependent protein kinase (MaCDPK1) enduring stress tolerance from Morus atropurpurea Roxb. Plant Cell Tissue Organ Cult. 2020, 141, 131–143. [Google Scholar] [CrossRef]
- Krishna, S.S.; Aravind, L. The bridge-region of the Ku superfamily is an atypical zinc ribbon domain. J. Struct. Biol. 2010, 172, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Van Ooijen, G.; Mayr, G.; Kasiem, M.M.A.; Albrecht, M.; Cornelissen, B.J.C.; Takken, F.L.W. Structure-function analysis of the NB-ARC domain of plant disease resistance proteins. J. Exp. Bot. 2008, 59, 1383–1397. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Moon, S.-J.; Park, S.R.; Kim, B.-G.; Byun, M.-O. Elongation factor 1 alpha from A. thaliana functions as molecular chaperone and confers resistance to salt stress in yeast and plants. Plant Sci. 2009, 177, 156–160. [Google Scholar] [CrossRef]
- Chivasa, S.; Tome, D.F.A.; Hamilton, J.M.; Slabas, A.R. Proteomic Analysis of Extracellular ATP-Regulated Proteins Identifies ATP Synthase beta-Subunit as a Novel Plant Cell Death Regulator. Mol. Cell. Proteom. 2011, 10, M110.003905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, H.; Shen, H.; Pan, Y.; Guo, B.; Lv, C.; Xu, R. Elucidating the hypoxic stress response in barley (Hordeum vulgare L.) during waterlogging: A proteomics approach. Sci. Rep. 2018, 8, 9655. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, W.; Zhang, B.; Zhou, J.; Wang, Y.; Yang, Q.; Ke, Y.; He, H. Phosphoproteins regulated by heat stress in rice leaves. Proteome Sci. 2011, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Rabey, H.A.; Al-Malki, A.L.; Abulnaja, K.O.; Rohde, W. Proteome Analysis for Understanding Abiotic Stress (Salinity and Drought) Tolerance in Date Palm (Phoenix dactylifera L.). Int. J. Genom. 2015, 2015, 407165. [Google Scholar]
- Parrine, D.; Greco, T.M.; Muhammad, B.; Wu, B.-S.; Zhao, X.; Lefsrud, M. Color-Specific Recovery to Extreme High-Light Stress in Plants. Life 2021, 11, 812. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Zhang, H.; Wang, Y.; Li, T.; Che, Y.; Wang, J.; Guo, D.; Sun, G.; Li, X. Thioredoxin-like protein CDSP32 alleviates Cd-induced photosynthetic inhibition in tobacco leaves by regulating cyclic electron flow and excess energy dissipation. Plant Physiol. Biochem. 2021, 167, 831–839. [Google Scholar] [CrossRef]
- Rosquete, M.R.; Drakakaki, G. Plant TGN in the stress response: A compartmentalized overview. Curr. Opin. Plant Biol. 2018, 46, 122–129. [Google Scholar] [CrossRef]
- Gu, X.; Brennan, A.; Wei, W.; Guo, G.; Lindsey, K. Vesicle Transport in Plants: A Revised Phylogeny of SNARE Proteins. Evol. Bioinform. 2020, 16, 1176934320956575. [Google Scholar] [CrossRef]
- Davidson, H.W.; Balch, W.E. Differential inhibition of multiple vesicular transport steps between the endoplasmic reticulum and trans Golgi network. J. Biol. Chem. 1993, 268, 4216–4226. [Google Scholar] [CrossRef]
- Chidambaram, S.; Mullers, N.; Wiederhold, K.; Haucke, V.; von Mollard, G.F. Specific interaction between SNARES and epsin N-terminal homology (ENTH) domains of epsin-related proteins in trans-Golgi network to endosome transport. J. Biol. Chem. 2004, 279, 4175–4179. [Google Scholar] [CrossRef] [Green Version]
- Smaoui, A.; Barhoumi, Z.; Rabhi, M.; Abdelly, C. Localization of potential ion transport pathways in vesicular trichome cells of Atriplex halimus L. Protoplasma 2011, 248, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.; Barr, F.A.; Stinchcombe, J.C.; Binacchi, C.; Huttner, W.B. Brefeldin A inhibits the formation of constitutive secretory vesicles and immature secretory granules from the trans-Golgi network. Eur. J. Cell Biol. 1992, 59, 265–274. [Google Scholar] [PubMed]
- Satiat-Jeunemaitre, B.; Cole, L.; Bourett, T.; Howard, R.; Hawes, C. Brefeldin A effects in plant and fungal cells: Something new about vesicle trafficking? J. Microsc. 1996, 181, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Cai, Y.; Tse, Y.C.; Wang, J.; Law, A.H.; Pimpl, P.; Chan, H.Y.; Xia, J.; Jiang, L. BFA-induced compartments from the Golgi apparatus and trans-Golgi network/early endosome are distinct in plant cells. Plant J. 2009, 60, 865–881. [Google Scholar] [CrossRef]
- Hamaji, K.; Nagira, M.; Yoshida, K.; Ohnishi, M.; Oda, Y.; Uemura, T.; Goh, T.; Sato, M.H.; Morita, M.T.; Tasaka, M.; et al. Dynamic Aspects of Ion Accumulation by Vesicle Traffic Under Salt Stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2023–2033. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, C.P.; Dilokpimol, A.; Mouille, G.; Burow, M.; Geshi, N. Arabinogalactan Glycosyltransferases Target to a Unique Subcellular Compartment That May Function in Unconventional Secretion in Plants. Traffic 2014, 15, 1219–1234. [Google Scholar] [CrossRef] [Green Version]
- Barhomi, Z.; Djebali, W.; Smaoui, A.; Chaibi, W.; Abdelly, C. Contribution of NaCl excretion to salt resistance of Aeluropus littoralis (Willd) Parl. J. Plant Physiol. 2007, 164, 842–850. [Google Scholar] [CrossRef]
- Yun, H.S.; Kwon, C. Vesicle trafficking in plant immunity. Curr. Opin. Plant Biol. 2017, 40, 34–42. [Google Scholar] [CrossRef]
- Wilson, H.; Mycock, D.; Weiersbye, I.M. The salt glands of Tamarix usneoides E. Mey. ex Bunge (South African Salt Cedar). Int. J. Phytoremediation 2017, 19, 587–595. [Google Scholar] [CrossRef]
- Gondim, F.A.; Gomes-Filho, E.; Costa, J.H.; Mendes Alencar, N.L.; Prisco, J.T. Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiol. Biochem. 2012, 56, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Tiika, R.J.; Wei, J.; Cui, G.; Ma, Y.; Yang, H.; Duan, H. Transcriptome-wide characterization and functional analysis of Xyloglucan endo-transglycosylase/hydrolase (XTH) gene family of Salicornia europaea L. under salinity and drought stress. BMC Plant Biol. 2021, 21, 491. [Google Scholar] [CrossRef] [PubMed]
- Otulak-Koziel, K.; Koziel, E.; Bujarski, J.J. Spatiotemporal Changes in Xylan-1/Xyloglucan and Xyloglucan Xyloglucosyl Transferase (XTH-Xet5) as a Step-In of Ultrastructural Cell Wall Remodelling in Potato-Potato Virus Y (PVYNTN) Hypersensitive and Susceptible Reaction. Int. J. Mol. Sci. 2018, 19, 2287. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.C.; Durso, N.A.; Cyr, R.J. Elongation factor-1alpha stabilizes microtubules in a calcium/calmodulin-dependent manner. Cell Motil. Cytoskelet. 1998, 41, 168–180. [Google Scholar] [CrossRef]
- Chien, P.-S.; Nam, H.G.; Chen, Y.-R. A salt-regulated peptide derived from the CAP superfamily protein negatively regulates salt-stress tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 5301–5313. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Yu, Z.; Zhang, S.; Wu, C.; Yang, G.; Yan, K.; Zheng, C.; Huang, J. CYSTM3 negatively regulates salt stress tolerance in Arabidopsis. Plant Mol. Biol. 2019, 99, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, F.; Liu, Y.; Zhang, M.; Liu, Y.; Zhao, Y.; Wang, B.; Chen, M. Exogenous melatonin enhances salt secretion from salt glands by upregulating the expression of ion transporter and vesicle transport genes in Limonium bicolor. BMC Plant Biol. 2020, 20, 493. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Ahmad, M.; Zafar, M.; Malik, K.; Rashid, N.; Ullah, F.; Zaman, W.; Ali, M. A light and scanning electron microscopic diagnosis of leaf epidermal morphology and its systematic implications in Dryopteridaceae: Investigating 12 Pakistani taxa. Micron 2018, 111, 36–49. [Google Scholar] [CrossRef]
- Gaff, F.; Okong’O-Ogola, O. The Use of Non-permeating Pigments for Testing the Survival of Cells. J. Exp. Bot. 1971, 22, 756–758. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate Proteome-wide Label-free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [PubMed]

| Accession | Description | MW (kDa) | Control | NaCl |
|---|---|---|---|---|
| A0A0J8DU34 | Tubulin_C domain-containing protein (Fragment) OS = Beta vulgaris subsp. vulgaris | 31.849 | + | − |
| A0A1L5JKA9 | Actin 11 OS = Sesuvium portulacastrum | 41.67 | + | − |
| A0A1L5JKC2 | Tubulin alpha chain OS = Sesuvium portulacastrum | 49.656 | + | − |
| M4WH59 | Actin 1 OS = Dionaea muscipula | 41.721 | + | − |
| A0A0K9Q9G9 | Protein kinase domain-containing protein (Fragment) OS = Spinacia oleracea | 24.543 | + | − |
| A0A0K9QDZ4 | Protein kinase domain-containing protein OS = Spinacia oleracea | 108.3 | + | − |
| A0A0K9QE47 | GTD-binding domain-containing protein OS = Spinacia oleracea | 97.611 | + | − |
| Q9MB82 | Plasma membrane H+-ATPase (Fragment) OS = Nepenthes alata | 20.592 | + | − |
| Accession | Description | MW (kDa) | Control | NaCl | Function |
|---|---|---|---|---|---|
| A0A0F7G6K5 | Ribulose bisphosphate carboxylase large chain (Fragment) OS = Paronychia herniarioides | 47.9 | + | + | Influence the Vmax of carboxylation and CO2/O2, specificity factor in Chlamydomonas reinhardtii [32] |
| A0A068ELN9 | Ribulose bisphosphate carboxylase large chain (Fragment) OS = Limonium dragonericum | 50.151 | + | + | |
| A0A3S9XK16 | Ribulose bisphosphate carboxylase large chain (Fragment) OS = Plumbago zeylanica | 46.635 | + | − | |
| A0A411JQT5 | Ribulose bisphosphate carboxylase large chain OS = Dioncophyllum thollonii | 54.893 | + | − | |
| A0A650AK20 | Ribulose bisphosphate carboxylase large chain OS = Limonium aureum | 52.67 | + | + | |
| F2WAU2 | Ribulose bisphosphate carboxylase large chain (Fragment) OS = Tecticornia disarticulata | 49.537 | + | − | |
| I3XLX8 | Ribulose bisphosphate carboxylase large chain (Fragment) | 45.152 | + | − | |
| I6WY50 | Ribulose bisphosphate carboxylase large chain (Fragment) OS = Limonium sinense | 46.023 | + | − | |
| O47047 | Ribulose bisphosphate carboxylase large chain (Fragment) OS = Eriogonum flavum | 49.73 | + | − | |
| P28410 | Ribulose bisphosphate carboxylase large chain (Fragment) OS = Drosera petiolaris | 48.899 | + | − | |
| A0A0H3Y5V0 | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (Fragment) OS = Delosperma peglerae | 8.7523 | + | − | Covalently linked to form dimer under oxidative stress and reduce photosynthesis in tobacco [33]; triggered in response to the exposure of low irradiance condition and reduce the activity of Rubisco in Arabidopsis [34] |
| A0A140ETK2 | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (Fragment) OS = Aerva lanata | 17.336 | + | − | |
| A0A346TN31 | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (Fragment) OS = Atriplex vesicaria | 9.2556 | + | − | |
| Q6A161 | Ribulose 1,5 bisphosphate carboxylase/oxygenase, large subunit OS = Limonium gibertii | 53.413 | + | − | |
| A0A410S7A2 | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (Fragment) OS = Achyranthes aspera | 12.178 | + | − | |
| G0WXT9 | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (Fragment) OS = Amaranthus deflexus | 33.481 | + | − | |
| A0A0J7YLM1 | Elongation factor Tu (Fragment) OS = Beta vulgaris subsp. vulgaris | 44.306 | + | − | Proved to be an independent structural and functional unit of Tryptic fragment and could bind with GDP in Escherichia coli and rabbit [35,36] |
| A0A0K9Q7G6 | Elongation factor Tu OS = Spinacia oleracea | 49.602 | + | − | |
| A0A0K9Q895 | PCI domain-containing protein (Fragment) OS = Spinacia oleracea | 43.288 | + | − | Could be believed have catalytic activity [37] |
| A0A0K9Q9G9 | Protein kinase domain-containing protein (Fragment) OS = Spinacia oleracea | 24.543 | + | − | Protein biosynthesis; signal transmission [38]; proved to response to biotic and abiotic stress [39] |
| A0A0K9QJ83 | Ku domain-containing protein (Fragment) OS = Spinacia oleracea | 93.807 | + | − | DNA-binding domain [40] |
| A0A0K9RD36 | NB-ARC domain-containing protein (Fragment) OS = Spinacia oleracea | 105.01 | + | − | Regulate plant disease resistance protein [41] |
| A0A249Y703 | Elongation factor 1-alpha OS = Hylocereus polyrhizus | 49.869 | + | − | As molecular chaperone under salt stress in yeast and plants [42]; play a role of response to heat stress in wheat [41] |
| A0A5B8WHU7 | Elongation factor 1-alpha OS = Hylocereus undatus | 49.373 | + | − | |
| A0A411JPW9 | ATP synthase subunit beta OS = Ceratostigma willmottianum | 53.874 | + | + | As a plant cell death regulator [43]; response to waterlogging stress in barley (Hordeum vulgare L.) [44]; response to heat stress in rice (Oryza sativa L.) [45]; response to salt and drought stress [46] |
| A0A411JS23 | ATP synthase subunit beta OS = Limonium tenellum | 53.218 | + | − | |
| G8A3M7 | ATP synthase subunit beta (Fragment) OS = Alluaudia procera | 50.753 | + | − | |
| A0A0K9RQ86 | ATP synthase subunit beta OS = Spinacia oleracea | 59.387 | + | − | |
| B0L803 | 33 kDa OEC protein (Fragment) OS = Salicornia veneta | 18.289 | + | − | Related to the recovery of high-Light stress [47]; response to heat stress in Zea mays L. |
| G4WNY3 | NAD(P)H-quinone oxidoreductase subunit 5, chloroplastic (Fragment) OS = Stegnosperma halimifolium | 57.819 | + | − | Alleviated photosynthetic inhibition in tobacco leaves under Cd stress [48] |
| G5DVX3 | Phosphoglycerate kinase (Fragment) OS = Silene latifolia | 51.21 | + | − | Could be believed response to salt stress [46] |
| A0A411JPX2 | ATP synthase subunit alpha OS = Ceratostigma willmottianum | 55.662 | + | + | Response to salt and drought stress [46] |
| A0A411JPX2 | ATP synthase subunit alpha OS = Ceratostigma willmottianum | 55.662 | + | + | Response to salt and drought stress [46] |
| A0A411JRY5 | ATP synthase subunit alpha OS = Limonium tenellum | 55.584 | + | − | |
| A0A411L2V2 | ATP synthase subunit alpha, chloroplastic OS = Froelichia latifolia | 55.562 | + | − | |
| D3WEW2 | ATP synthase subunit alpha OS = Plumbago auriculata | 55.647 | + | + | |
| H8Y672 | ATP synthase subunit alpha OS = Silene vulgaris | 58.181 | + | − | |
| P29409 | Phosphoglycerate kinase, chloroplastic (Fragment) OS = Spinacia oleracea | 45.572 | + | − | Response to salt stress [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Zhang, Y.; Mi, P.; Guo, X.; Wen, Y.; Han, G.; Wang, B. Proteomics of Salt Gland–Secreted Sap Indicates a Pivotal Role for Vesicle Transport and Energy Metabolism in Plant Salt Secretion. Int. J. Mol. Sci. 2022, 23, 13885. https://doi.org/10.3390/ijms232213885
Lu C, Zhang Y, Mi P, Guo X, Wen Y, Han G, Wang B. Proteomics of Salt Gland–Secreted Sap Indicates a Pivotal Role for Vesicle Transport and Energy Metabolism in Plant Salt Secretion. International Journal of Molecular Sciences. 2022; 23(22):13885. https://doi.org/10.3390/ijms232213885
Chicago/Turabian StyleLu, Chaoxia, Yuanyuan Zhang, Ping Mi, Xueying Guo, Yixuan Wen, Guoliang Han, and Baoshan Wang. 2022. "Proteomics of Salt Gland–Secreted Sap Indicates a Pivotal Role for Vesicle Transport and Energy Metabolism in Plant Salt Secretion" International Journal of Molecular Sciences 23, no. 22: 13885. https://doi.org/10.3390/ijms232213885





