Effect of Quercetin and Fingolimod, Alone or in Combination, on the Sphingolipid Metabolism in HepG2 Cells
Abstract
1. Introduction
2. Results
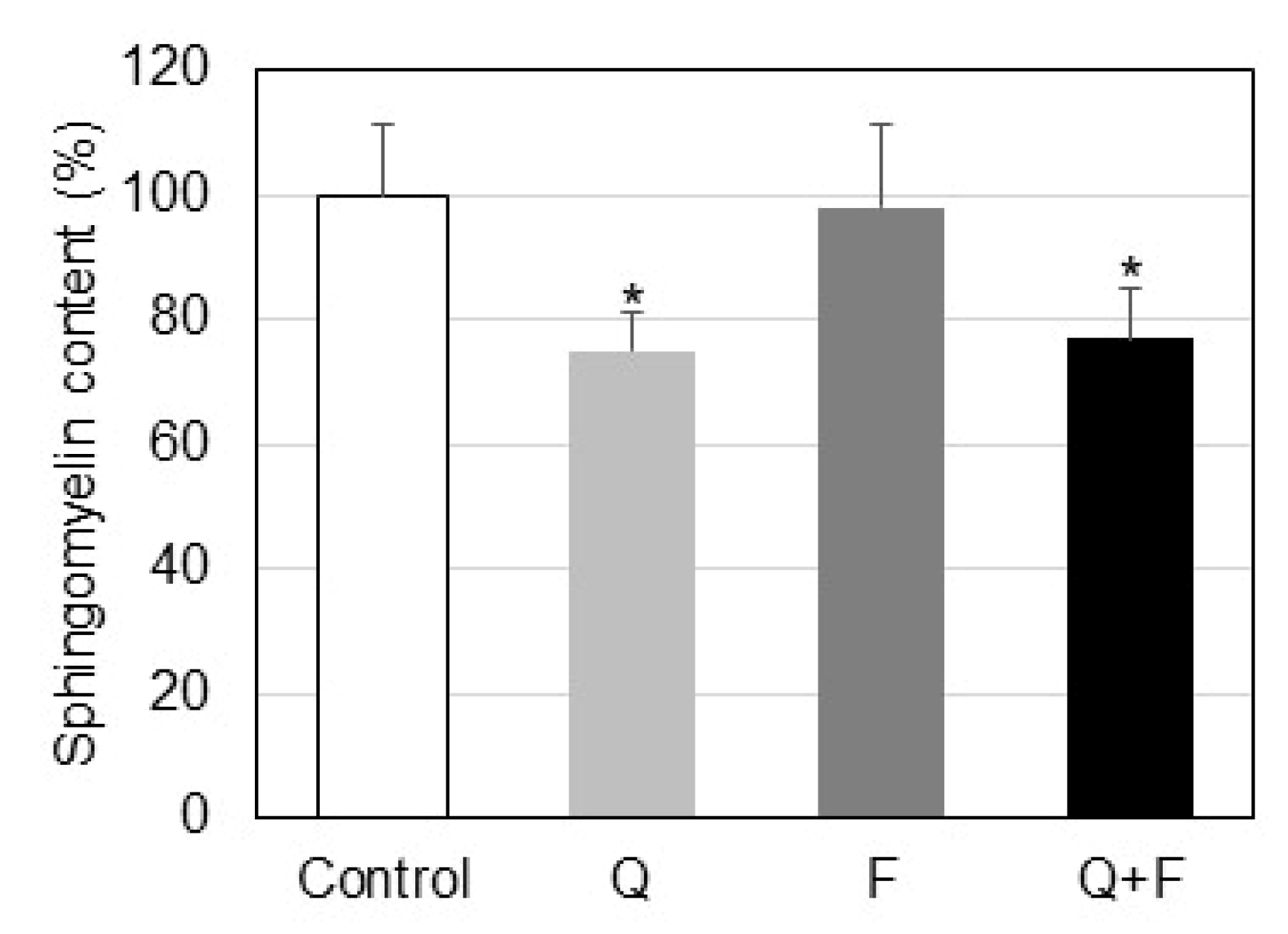
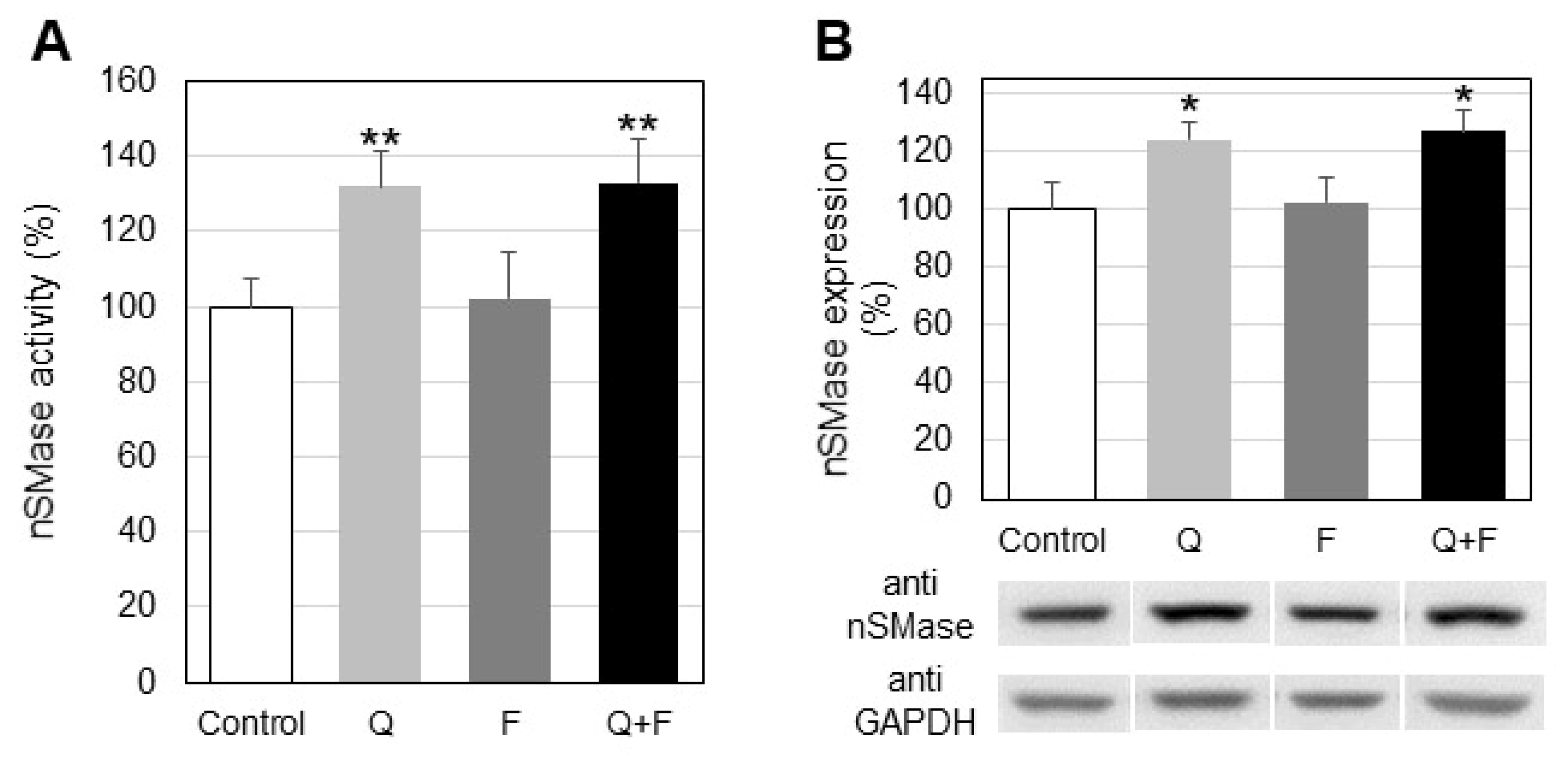
2.1. Effect of Quercetin and Fingolimod on Sphingolipid Levels and Neutral Sphingomyelinase
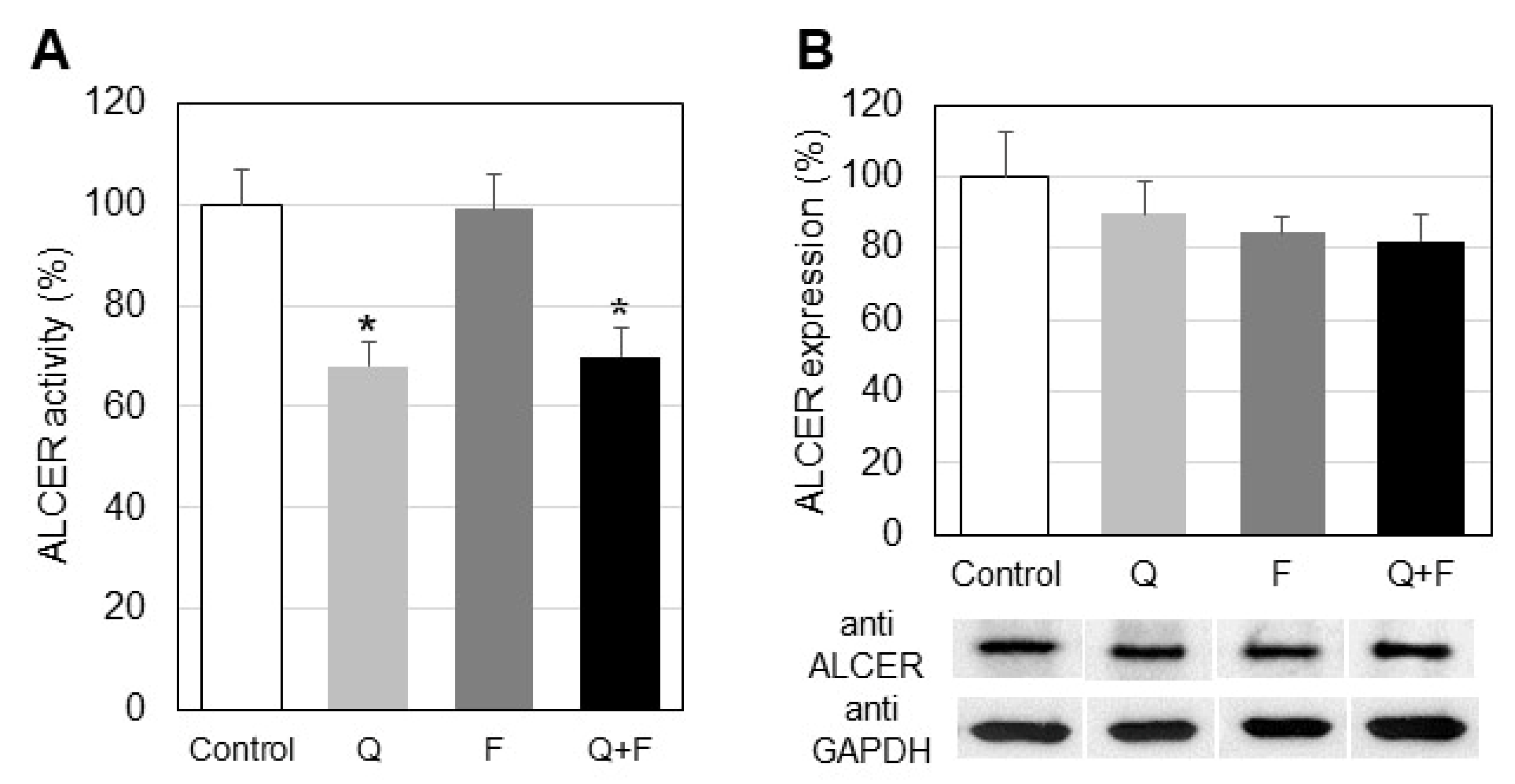
2.2. Effect of Quercetin and Fingolimod on the Enzymes Maintaining Ceramide Levels
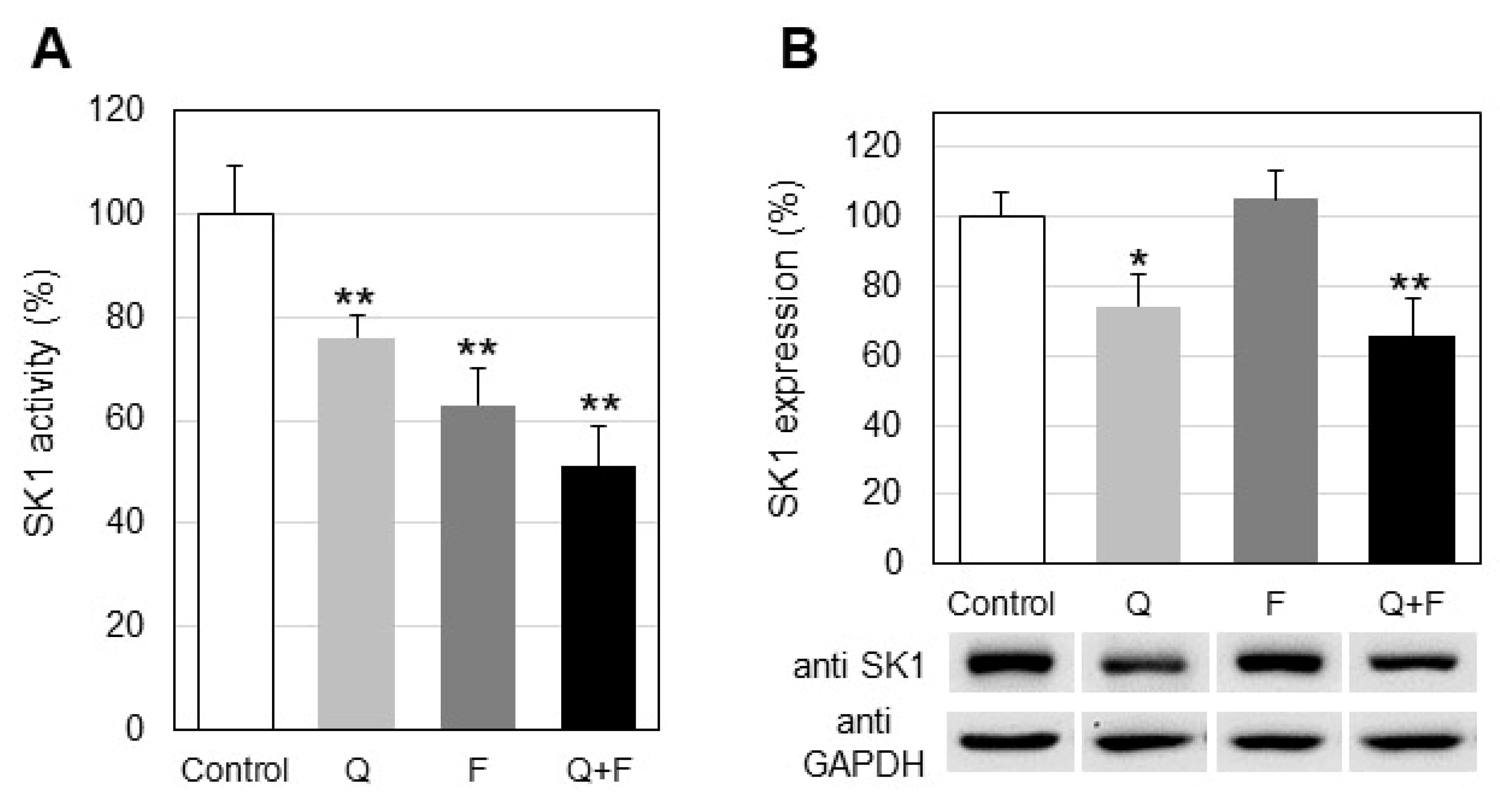
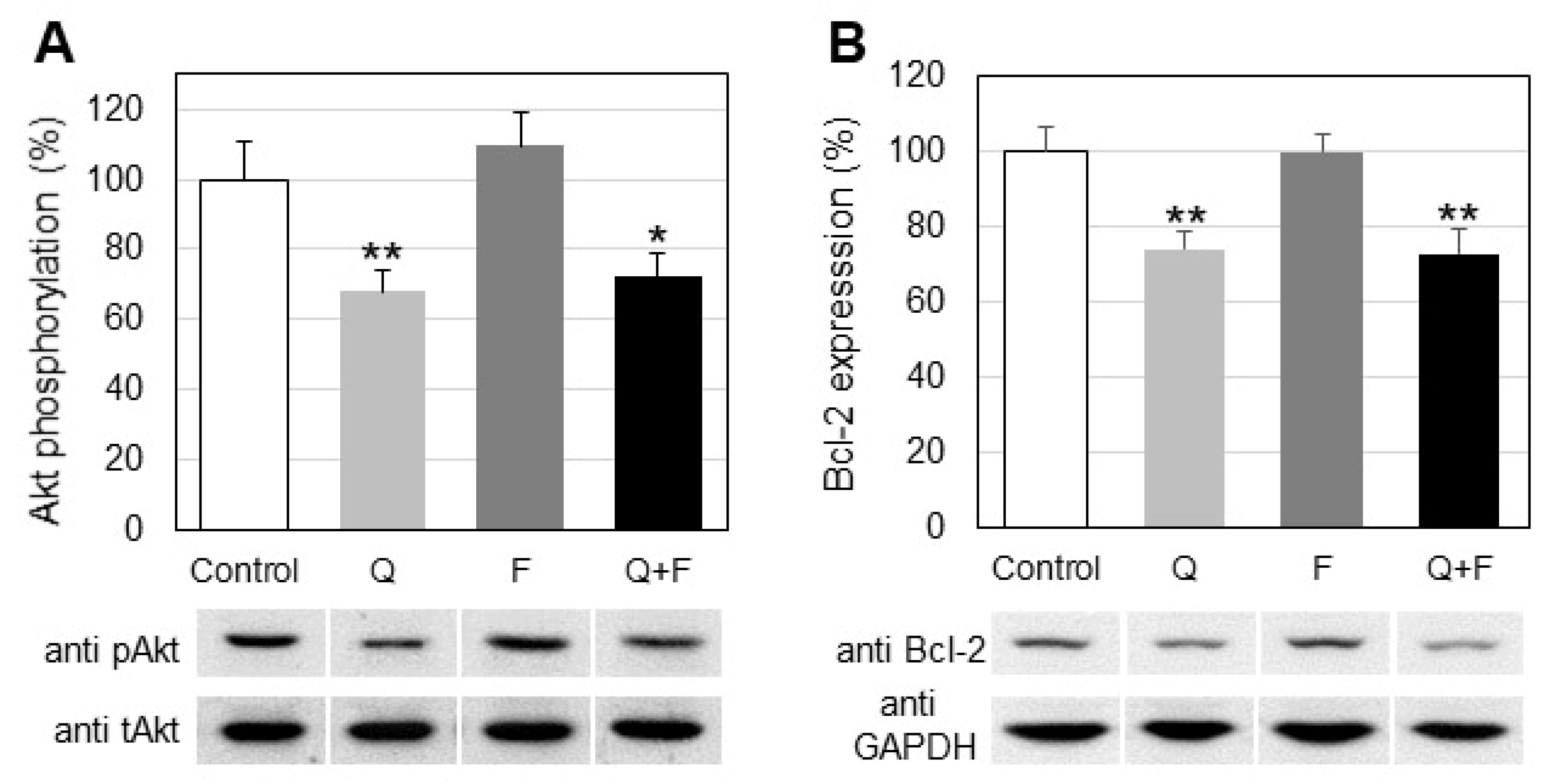
2.3. Influence of Quercetin and Fingolimod on Sphingosine Kinase 1 Activity and Expression
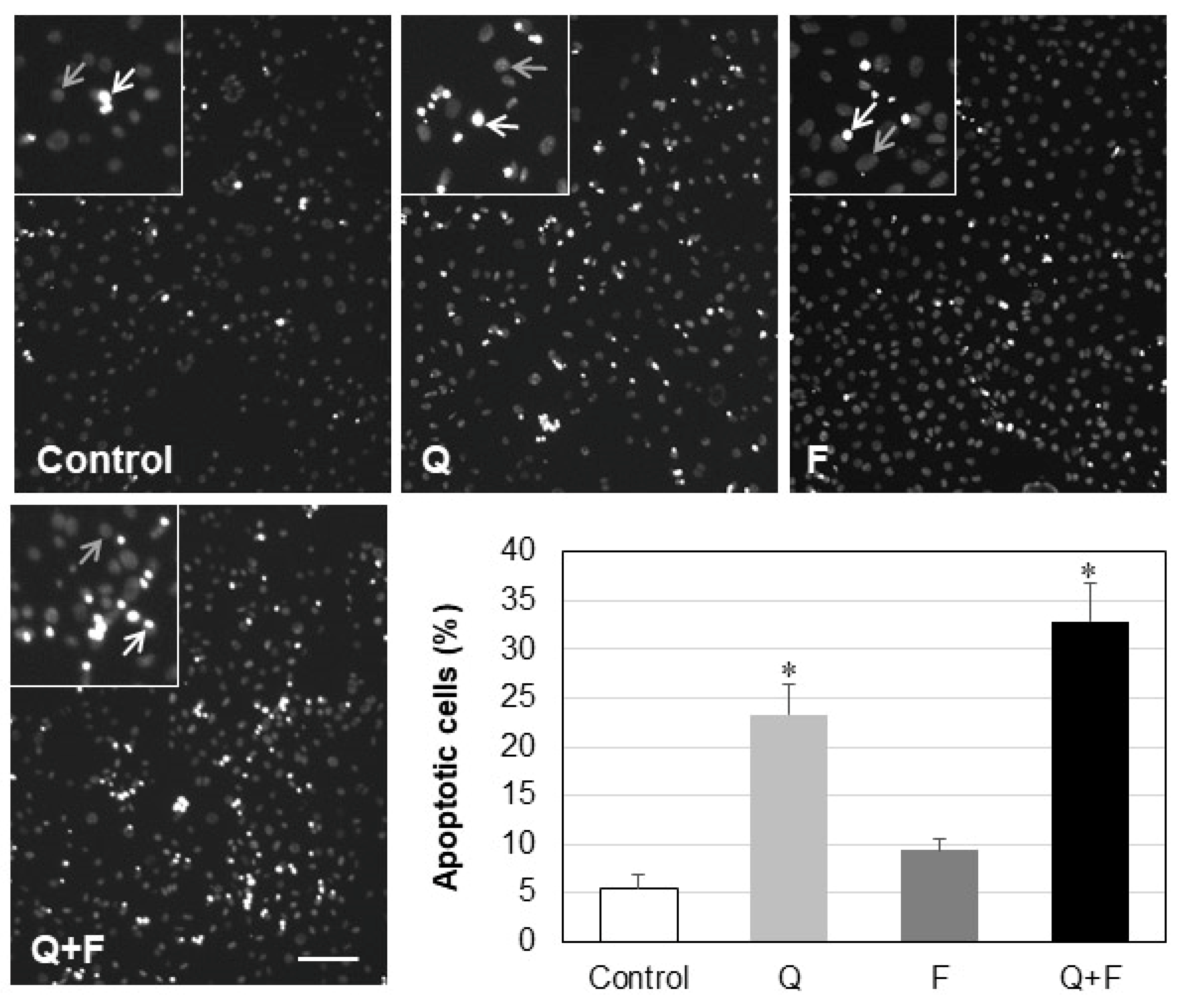
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Incubation with Quercetin and Fingolimod
4.3. Cell Viability Assay after Incubation with Quercetin and Fingolimod
4.4. Determination of Sphingomyelin
4.5. Determination of Ceramide
4.6. Determination of Sphingosine
4.7. Determination of Sphingosine-1-Phosphate
4.8. Neutral Sphingomyelinase Activity Assay
4.9. Alkaline Ceramidase Activity Assay
4.10. Sphingosine Kinase 1 Activity Assay
4.11. Western Blotting and Antibodies
4.12. Apoptosis Assay
4.13. Statistical Analysis
5. Conclusions
- Quercetin, but not fingolimod, down-regulates neutral sphingomyelinase and induces reduction of sphingomyelin in hepatocellular carcinoma HepG2 cells.
- Quercetin reduces the content of ceramide, whereas the combined treatment of quercetin with fingolimod most significantly affects the level of sphingosine-1-phosphate.
- Quercetin treatment down-regulates the activity, but not protein expression, of alkaline ceramidase in HepG2 cells.
- Combined treatment of HepG2 cells with quercetin and fingolimod induces a stronger down-regulation of sphingosine kinase 1, compared to individual treatments with each one of them.
- Treatment with quercetin, but not with fingolimod, reduced the phosphorylation of Akt and Bcl-2 expression, leading to increased apoptosis in HepG2 cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terry, K.; Copur, M.S. Molecular targeted therapy of hepatocellular carcinoma. J. Cancer Ther. 2013, 4, 426–439. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol. Biol. Rep. 2015, 42, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Das, A.S.; Majumder, M.; Mukhopadhyay, R. Antiatherogenic roles of dietary flavonoids chrysin, quercetin, and luteolin. J. Cardiovasc. Pharmacol. 2016, 68, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci. Biotechnol. Biochem. 2008, 72, 797–804. [Google Scholar] [CrossRef]
- Ferrer, P.; Asensi, M.; Priego, S.; Benlloch, M.; Mena, S.; Ortega, A.; Obrador, E.; Esteve, J.M.; Estrela, J.M. Nitric oxide mediates natural polyphenol-induced Bcl-2 down-regulation and activation of cell death in metastatic B16 melanoma. J. Biol. Chem. 2007, 282, 2880–2890. [Google Scholar] [CrossRef]
- Torres, F.; Quintana, J.; Estévez, F. 5,7,30-Trihydroxy-3,40-dimethoxyflavone Inhibits the Tubulin Polymerization and Activates the Sphingomyelin Pathway. Mol. Carcinog. 2011, 50, 113–122. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Harasim-Symbor, E.; Polak, A.; Drygalski, K.; Berk, K.; Chabowski, A.; Konstantynowicz-Nowicka, K. Influence of Resveratrol on Sphingolipid Metabolism in Hepatocellular Carcinoma Cells in Lipid Overload State. Anti-Cancer Agents Med. Chem. 2019, 19, 121–129. [Google Scholar] [CrossRef]
- Cowart, L.A. Sphingolipids: Players in the pathology of metabolic disease. Trends Endocrinol. Metab. 2009, 20, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Larrauri, A.; Presa, N.; Dominguez-Herrera, A.; Ouro, A.; Trueba, M.; Gomez-Muñoz, A. Role of bioactive sphingolipids in physiology and pathology. Essays Biochem. 2020, 64, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Meyers-Needham, M.; Senkal, C.E.; Saddoughi, S.A.; Sentelle, D.; Selvam, S.P.; Salas, A.; Ogretmen, B. Sphingolipids and cancer: Ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010, 6, 1603–1624. [Google Scholar] [CrossRef] [PubMed]
- Maeng, H.J.; Song, J.H.; Kim, G.T.; Song, Y.J.; Lee, K.; Kim, J.Y.; Park, T.S. Celecoxib-mediated activation of endoplasmic reticulum stress induces de novo ceramide biosynthesis and apoptosis in hepatoma HepG2 cells mobilization. BMB Rep. 2017, 50, 144–149. [Google Scholar] [CrossRef]
- Wang, S.W.; Hojabrpour, P.; Zhang, P.; Kolesnick, R.N.; Steinbrecher, U.P.; Gómez-Muñoz, A.; Duronio, V. Regulation of ceramide generation during macrophage apoptosis by ASMase and de novo synthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 1482–1489. [Google Scholar] [CrossRef]
- Yildiz-Ozer, M.; Oztopcu-Vatan, P.; Kus, G. The investigation of ceranib-2 on apoptosis and drug interaction with carboplatin in human non small cell lung cancer cells in vitro. Cytotechnology 2018, 70, 387–396. [Google Scholar] [CrossRef]
- Suzuki, M.; Cao, K.; Kato, S.; Komizu, Y.; Mizutani, N.; Tanaka, K.; Arima, C.; Tai, M.C.; Yanagisawa, K.; Togawa, N.; et al. Targeting ceramide synthase 6-dependent metastasis-prone phenotype in lung cancer cells. J. Clin. Investig. 2016, 126, 254–265. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signaling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Lim, K.G.; Tonelli, F.; Li, Z.; Lu, X.; Bittman, R.; Pyne, S.; Pyne, N.J. FTY720 analogues as sphingosine kinase 1 inhibitors: Enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangement in MCF-7 breast cancer cells. J. Biol. Chem. 2011, 286, 18633–18640. [Google Scholar] [CrossRef]
- Pchejetski, D.; Bohler, T.; Brizuela, L.; Sauer, L.; Doumerc, N.; Golzio, M.; Salunkhe, V.; Teissié, J.; Malavaud, B.; Waxman, J.; et al. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res. 2010, 70, 8651–8661. [Google Scholar] [CrossRef]
- Svinka, J.; Mikulits, W.; Eferl, R. STAT3 in hepatocellular carcinoma: New perspectives. Hepat Oncol. 2014, 1, 107–120. [Google Scholar] [CrossRef]
- Zhao, Y.; Butler, E.B.; Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013, 4, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Van Blitterswijk, W.J.; Verheij, M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.; Sordillo, P.; Helson, L. Sphingosine kinase inhibitors as maintenance therapy of glioblastoma after ceramide-induced response. Anticancer Res. 2016, 36, 2085–2095. [Google Scholar]
- Brito, A.F.; Ribeiro, M.; Abrantes, A.M.; Mamede, A.C.; Laranjo, M.; Casalta-Lopes, J.E.; Gonçalves, A.C.; Ribeiro, A.B.S.; Tralhão, J.G.; Botelho, M.F. New approach for treatment of primary liver tumors: The role of quercetin. Nutr. Cancer 2016, 68, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Romero, A.; Gehin, C.; Riezman, H. Sphingolipid homeostasis in the web of metabolic routes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Husain, N.; Gupta, S. A critical study on chemistry and distribution of phenolic compounds in plants, and their role in human health. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 1, 57–60. [Google Scholar]
- Hagiwara, A.; Kokubo, Y.; Takesada, Y.; Tanaka, H.; Tamano, S.; Hirose, M.; Shirai, T.; Ito, N. Inhibitory effects of phenolic compounds on development of naturally occurring preneoplastic hepatocytic foci in long-term feeding studies using male F344 rats. Teratog. Carcinog. Mutagen. 1996, 16, 317–325. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Momchilova, A.; Markovska, T.; Georgiev, G.; Pankov, S.; Staneva, G.; Petkova, D.; Krastev, P.; Pinkas, A.; Pankov, R. Quercetin affects membrane lipids and apoptosis in three-dimensional fibroblast cultures. Biotechnol. Biotechnol. Equip. 2019, 35, 943–952. [Google Scholar] [CrossRef]
- Momchilova, A.; Pankov, R.; Staneva, G.; Pankov, S.; Krastev, P.; Vassileva, E.; Hazarosova, R.; Krastev, N.; Robev, B.; Nikolova, B.; et al. Resveratrol Affects Sphingolipid Metabolism in A549 Lung Adenocarcinoma Cells. Int. J. Mol. Sci. 2022, 23, 10870. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, F.; Chen, K.; Tian, M.-L.; Zhao, D.-L. Sphingosine kinase 1 as an anticancer therapeutic target. Drug Des. Dev. Ther. 2015, 9, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.P.; Ogretmen, B. Sphingosine kinase/sphingosine 1-phosphate signaling in cancer therapeutics and drug resistance. Hand Exp. Pharmacol. 2013, 216, 3–27. [Google Scholar] [CrossRef]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef]
- Song, L.; Xiong, H.; Li, J.; Liao, W.; Wang, L.; Wu, J.; Li, M. Sphingosine Kinase-1 Enhances Resistance to Apoptosis through Activation of PI3K/Akt/NFκB Pathway in Human Non-small Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 1740. [Google Scholar] [CrossRef]
- Lightle, A.; Oakley, J.; Nikolova-Karakashian, M. Activation of sphingolipid turnover and chronic generation of ceramide and sphingosine in liver during aging. Mech. Ageing Dev. 2000, 120, 111–125. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momchilova, A.; Nikolaev, G.; Pankov, S.; Vassileva, E.; Krastev, N.; Robev, B.; Krastev, D.; Pinkas, A.; Pankov, R. Effect of Quercetin and Fingolimod, Alone or in Combination, on the Sphingolipid Metabolism in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 13916. https://doi.org/10.3390/ijms232213916
Momchilova A, Nikolaev G, Pankov S, Vassileva E, Krastev N, Robev B, Krastev D, Pinkas A, Pankov R. Effect of Quercetin and Fingolimod, Alone or in Combination, on the Sphingolipid Metabolism in HepG2 Cells. International Journal of Molecular Sciences. 2022; 23(22):13916. https://doi.org/10.3390/ijms232213916
Chicago/Turabian StyleMomchilova, Albena, Georgi Nikolaev, Stefan Pankov, Evgenia Vassileva, Nikolai Krastev, Bozhil Robev, Dimo Krastev, Adriana Pinkas, and Roumen Pankov. 2022. "Effect of Quercetin and Fingolimod, Alone or in Combination, on the Sphingolipid Metabolism in HepG2 Cells" International Journal of Molecular Sciences 23, no. 22: 13916. https://doi.org/10.3390/ijms232213916
APA StyleMomchilova, A., Nikolaev, G., Pankov, S., Vassileva, E., Krastev, N., Robev, B., Krastev, D., Pinkas, A., & Pankov, R. (2022). Effect of Quercetin and Fingolimod, Alone or in Combination, on the Sphingolipid Metabolism in HepG2 Cells. International Journal of Molecular Sciences, 23(22), 13916. https://doi.org/10.3390/ijms232213916






