The Impact of Duodenal Mucosal Vulnerability in the Development of Epigastric Pain Syndrome in Functional Dyspepsia
Abstract
1. Introduction
2. Results
2.1. Study Population
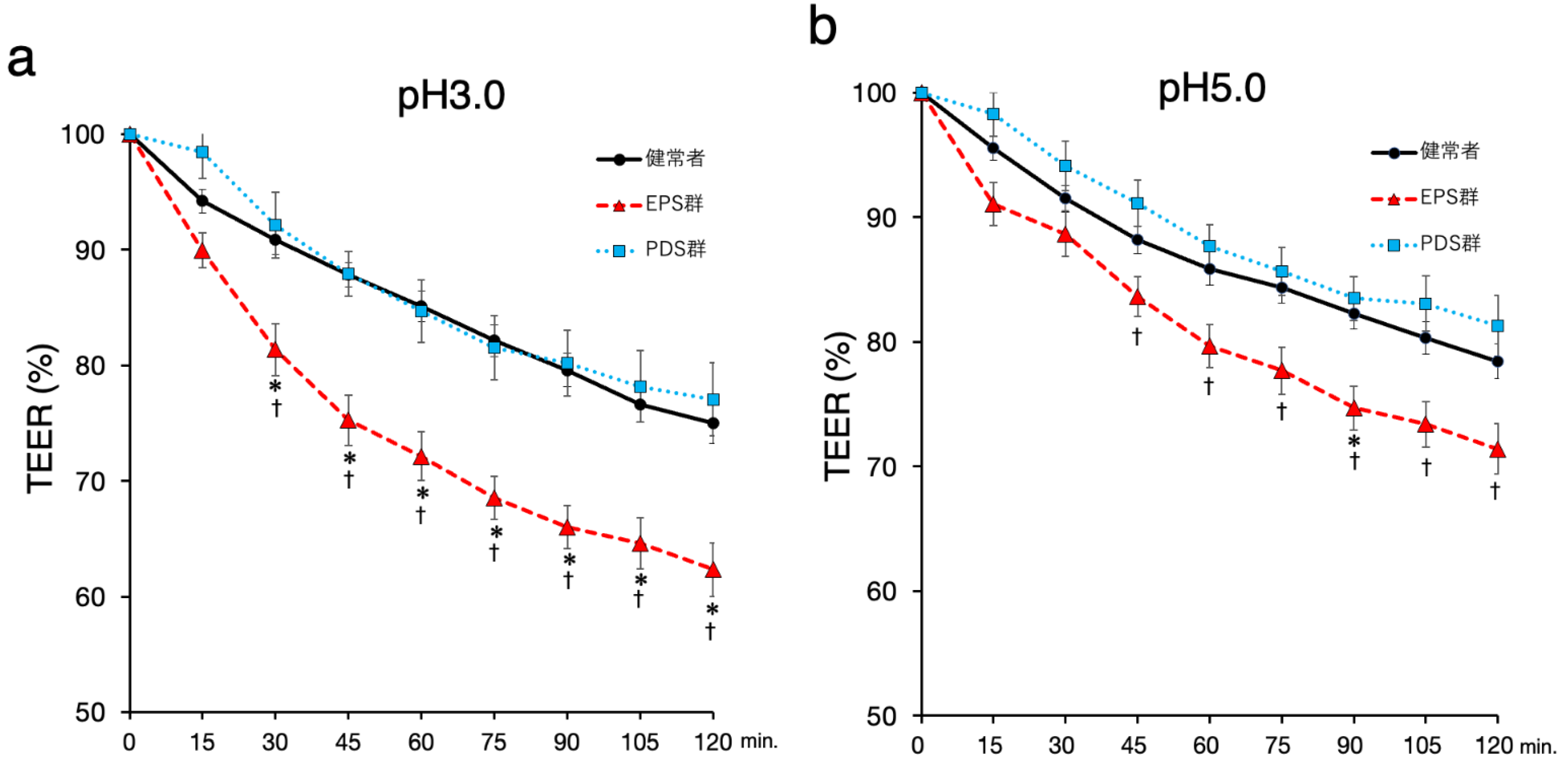
2.2. Duodenal Mucosal Barrier Function against Acid Is Impaired in FD Patients with EPS
2.3. Symptoms in the Patients with EPS Are Related to Duodenal Mucosal Stimulation with A pH 5.0 Acidic Solution but Not a pH 3.0 Acidic Solution
2.4. Duodenal Mucosa in Females Is Susceptible to Acid
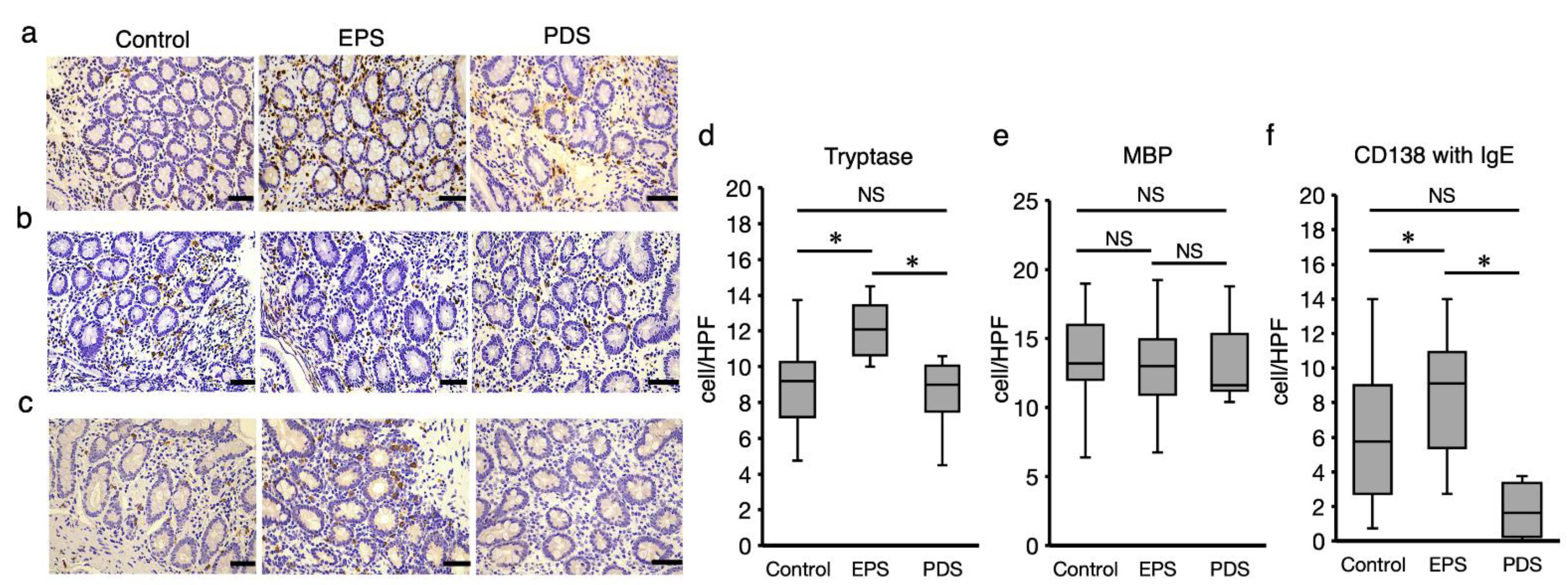
2.5. Development of EPS Is Related to Mucosal Infiltration of Mast Cells in the Duodenum
3. Discussion
4. Materials and Methods
4.1. Subject
4.2. Endoscopic Examination
4.3. Electrophysiological Measurement of Transepithelial Electrical Resistance (TEER)
4.4. Immunohistochemistry
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- van Oudenhove, L.; Holvoet, L.; Vandenberghe, J.; Vos, R.; Tack, J. Do we have an alternative for the Rome III gastroduodenal symptom-based subgroups in functional gastroduodenal disorders? A cluster analysis approach. Neurogastroenterol. Motil. 2011, 23, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Bisschops, R.; Sarnelli, G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology 2004, 127, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Halling, K.; Kulich, K.; Carlsson, J.; Wiklund, I. An international comparison of the burden of illness in patients with dyspepsia. Dig. Dis. 2008, 26, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J. What Causes Functional Gastrointestinal Disorders? A Proposed Disease Model Am. J. Gastroenterol. 2020, 115, 41–48. [Google Scholar] [CrossRef]
- Stanghellini, V.; Chan, F.K.; Hasler, W.L.; Malagelada, J.R.; Suzuki, H.; Tack, J.; Talley, N.J. Gastroduodenal Disorders. Gastroenterology 2016, 150, 1380–1392. [Google Scholar] [CrossRef]
- Talley, N.J.; Meineche-Schmidt, V.; Pare, P.; Duckworth, M.; Räisänen, P.; Pap, A.; Kordecki, H.; Schmid, V. Efficacy of omeprazole in functional dyspepsia: Double-blind, randomized, placebo-controlled trials (the Bond and Opera studies). Aliment Pharmacol. Ther. 1998, 12, 1055–1065. [Google Scholar] [CrossRef]
- van Zanten, S.V.; Armstrong, D.; Chiba, N.; Flook, N.; White, R.J.; Chakraborty, B.; Gasco, A. Esomeprazole 40 mg once a day in patients with functional dyspepsia: The randomized, placebo-controlled “ENTER” trial. Am. J. Gastroenterol. 2006, 101, 2096–2106. [Google Scholar] [CrossRef]
- Moayyedi, P.; Soo, S.; Deeks, J.; Delaney, B.; Innes, M.; Forman, D. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst. Rev. 2006, 18, CD001960. [Google Scholar]
- Suzuki, H.; Kusunoki, H.; Kamiya, T.; Futagami, S.; Yamaguchi, Y.; Nishizawa, T.; Iwasaki, T.; Matsuzaki, J.; Takahashi, S.; Sakamoto, C.; et al. Effect of lansoprazole on the epigastric symptoms of functional dyspepsia (ELF study): A multicentre, prospective, randomized, double-blind, placebo-controlled clinical trial. United Eur. Gastroenterol. J. 2013, 1, 445–452. [Google Scholar] [CrossRef]
- Asaoka, D.; Nagahara, A.; Hojo, M.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Izumi, K.; Takeda, T.; Komori, H.; Akazawa, Y.; et al. Efficacy of a potassium-competitive acid blocker for improving symptoms in patients with reflux esophagitis, non-erosive reflux disease, and functional dyspepsia. Biomed. Rep. 2017, 6, 175–180. [Google Scholar] [CrossRef]
- Lee, K.J.; Demarchi, B.; Demedts, I.; Sifrim, D.; Raeymaekers, P.; Tack, J. A pilot study on duodenal acid exposure and its relationship to symptoms in functional dyspepsia with prominent nausea. Am. J. Gastroenterol. 2004, 99, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Bratten, J.; Jones, M.P. Prolonged recording of duodenal acid exposure in patients with functional dyspepsia and controls using a radiotelemetry pH monitoring system. J. Clin. Gastroenterol. 2009, 43, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, J.H.; Cho, S.W. Dyspeptic symptoms associated with hypersensitivity to gastric distension induced by duodenal acidification. J. Gastroenterol. Hepatol. 2006, 21, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Kusunoki, H.; Manabe, N.; Kamada, T.; Sato, M.; Imamura, H.; Shiotani, A.; Hata, J.; Haruma, K. Evaluation of duodenal hypersensitivity induced by duodenal acidification using transnasal endoscopy. J. Gastroenterol. Hepatol. 2010, 25, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Vanheel, H.; Vicario, M.; Vanuytsel, T.; Van Oudenhove, L.; Martinez, C.; Keita, A.V.; Pardon, N.; Santos, J.; Söderholm, J.D.; Tackm, J.; et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014, 63, 262–271. [Google Scholar] [CrossRef]
- Svedlund, J.; Sjodin, I.; Dotevall, G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- Sakurai, Y.; Mori, Y.; Okamoto, H.; Nishimura, A.; Komura, E.; Araki, T.; Shiramoto, M. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects—A randomised open-label cross-over study. Aliment. Pharm. Ther. 2015, 42, 719–730. [Google Scholar] [CrossRef]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef]
- Vanheel, H.; Vicario, M.; Boesmans, W.; Vanuytsel, T.; Salvo-Romero, E.; Tack, J.; Farré, R. Activation of Eosinophils and Mast Cells in Functional Dyspepsia: An Ultrastructural Evaluation. Sci. Rep. 2018, 8, 5383. [Google Scholar] [CrossRef]
- Walker, M.M.; Talley, N.J.; Prabhakar, M.; Pennaneac’h, C.J.; Aro, P.; Ronkainen, J.; Storskrubb, T.; Harmsen, W.S.; Zinsmeister, A.R.; Agreus, L. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment. Pharm. Ther. 2009, 29, 765–773. [Google Scholar] [CrossRef]
- Cheng, E.; Zhang, X.; Huo, X.; Yu, C.; Zhang, Q.; Wang, D.H.; Spechler, S.J.; Souza, R.F. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut 2013, 62, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, N.; Ishihara, S.; Kinoshita, Y. Sustained Acid Suppression by Potassium-Competitive Acid Blocker (P-CAB) May Be An Attractive Treatment Candidate for Patients with Eosinophilic Esophagitis. Am. J. Gastroenterol. 2016, 111, 1203–1204. [Google Scholar] [CrossRef] [PubMed]
- Welen, K.; Faresjo, A.; Faresjo, T. Functional dyspepsia affects women more than men in daily life: A case-control study in primary care. Gend. Med. 2008, 5, 62–73. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, N.; Lee, J.Y.; Park, K.S.; Shin, J.E.; Nam, K.; Kim, H.J.; Song, H.J.; Joo, Y.E.; Myung, D.S.; et al. Prevalence and Risk Factors of Functional Dyspepsia in Health Check-up Population: A Nationwide Multicenter Prospective Study. J. Neurogastroenterol. Motil. 2018, 24, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Zagari, R.M.; Law, G.R.; Fuccio, L.; Cennamo, V.; Gilthorpe, M.S.; Forman, D.; Bazzoli, F. Epidemiology of functional dyspepsia and subgroups in the Italian general population: An endoscopic study. Gastroenterology 2010, 138, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Takemoto, T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy 1969, 1, 87–97. [Google Scholar] [CrossRef]
- Norita, K.; Asanuma, K.; Koike, T.; Okata, T.; Fujiya, T.; Abe, Y.; Nakagawa, K.; Hatta, W.; Uno, K.; Nakamura, T.; et al. Impaired Mucosal Integrity in Proximal Esophagus Is Involved in Development of Proton Pump Inhibitor-Refractory Nonerosive Reflux Disease. Digestion 2020, 102, 404–414. [Google Scholar] [CrossRef] [PubMed]

| Control (n = 23) | EPS (n = 16) | PDS (n = 11) | p | |
|---|---|---|---|---|
| Gender (male:female) | 13:10 | 6:10 | 2:9 | NS b |
| Age (average ± SD) | 51.7 ± 3.2 | 52.4 ± 3.8 | 54.3 ± 4.8 | NS a |
| BMI (average ± SD) | 22.5 ± 0.6 | 22.0 ± 0.8 | 20.7 ± 0.7 | NS a |
| Smoke (no:yes) | 20:3 | 13:3 | 9:2 | NS b |
| Drink (no:yes) | 11:12 | 9:7 | 9:2 | NS b |
| Gastric atrophy (none~mild/moderate/severe) | 21/2/0 | 11/3/2 | 8/2/1 | NS b |
| H. pylori (none:eradicated) | 21:2 | 11:5 | 8:3 | NS b |
| Medication | ||||
| PPI or P-cab alone | NA | 4 | 4 | |
| PPI or P-cab + acotiamide | NA | 9 | 5 | NS c |
| PPI or P-cab + others | NA | 3 | 2 |
| EPS | PDS | p | |
|---|---|---|---|
| abdominal pain | 10.1 ± 1.0 | 5.7 ± 0.8 | 0.005 |
| indigestion | 9.3 ± 0.7 | 12.4 ± 1.7 | 0.08 |
| reflux | 5.4 ± 0.6 | 3.9 ± 0.5 | 0.09 |
| constipation | 6.4 ± 0.7 | 5.7 ± 0.9 | 0.57 |
| diarrhea | 4.9 ± 0.5 | 6.2 ± 0.8 | 0.12 |
| total | 36.2 ± 2.4 | 33.9 ± 2.9 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okata, T.; Asanuma, K.; Nakagawa, K.; Hatta, W.; Koike, T.; Imatani, A.; Masamune, A. The Impact of Duodenal Mucosal Vulnerability in the Development of Epigastric Pain Syndrome in Functional Dyspepsia. Int. J. Mol. Sci. 2022, 23, 13947. https://doi.org/10.3390/ijms232213947
Okata T, Asanuma K, Nakagawa K, Hatta W, Koike T, Imatani A, Masamune A. The Impact of Duodenal Mucosal Vulnerability in the Development of Epigastric Pain Syndrome in Functional Dyspepsia. International Journal of Molecular Sciences. 2022; 23(22):13947. https://doi.org/10.3390/ijms232213947
Chicago/Turabian StyleOkata, Tomoki, Kiyotaka Asanuma, Kenichiro Nakagawa, Waku Hatta, Tomoyuki Koike, Akira Imatani, and Atsushi Masamune. 2022. "The Impact of Duodenal Mucosal Vulnerability in the Development of Epigastric Pain Syndrome in Functional Dyspepsia" International Journal of Molecular Sciences 23, no. 22: 13947. https://doi.org/10.3390/ijms232213947
APA StyleOkata, T., Asanuma, K., Nakagawa, K., Hatta, W., Koike, T., Imatani, A., & Masamune, A. (2022). The Impact of Duodenal Mucosal Vulnerability in the Development of Epigastric Pain Syndrome in Functional Dyspepsia. International Journal of Molecular Sciences, 23(22), 13947. https://doi.org/10.3390/ijms232213947







