Preparation and Characterization of Nanofibrous Membranes Electro-Spun from Blended Poly(l-lactide-co-ε-caprolactone) and Recombinant Spider Silk Protein as Potential Skin Regeneration Scaffold
Abstract
:1. Introduction
2. Results and Discussions
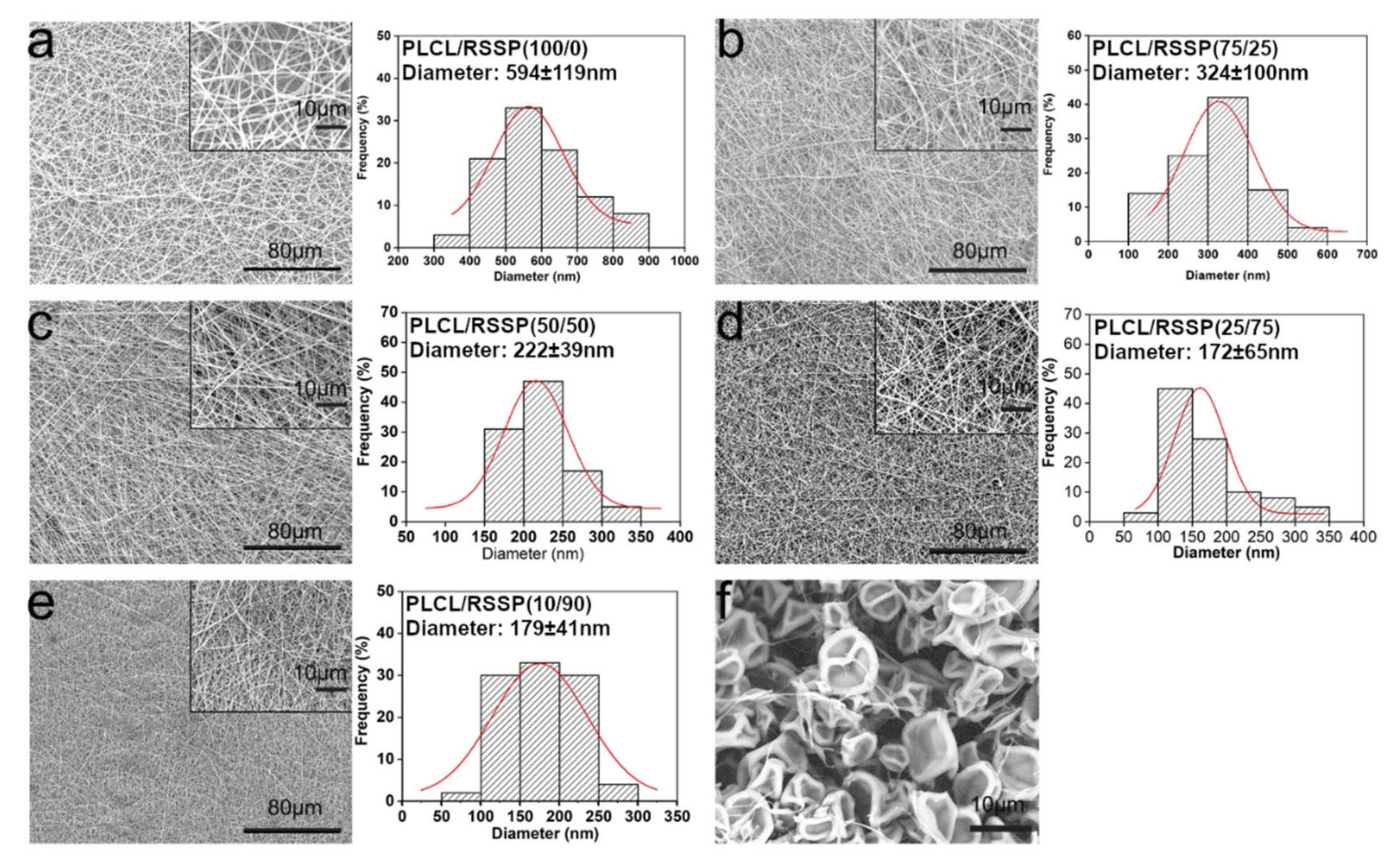
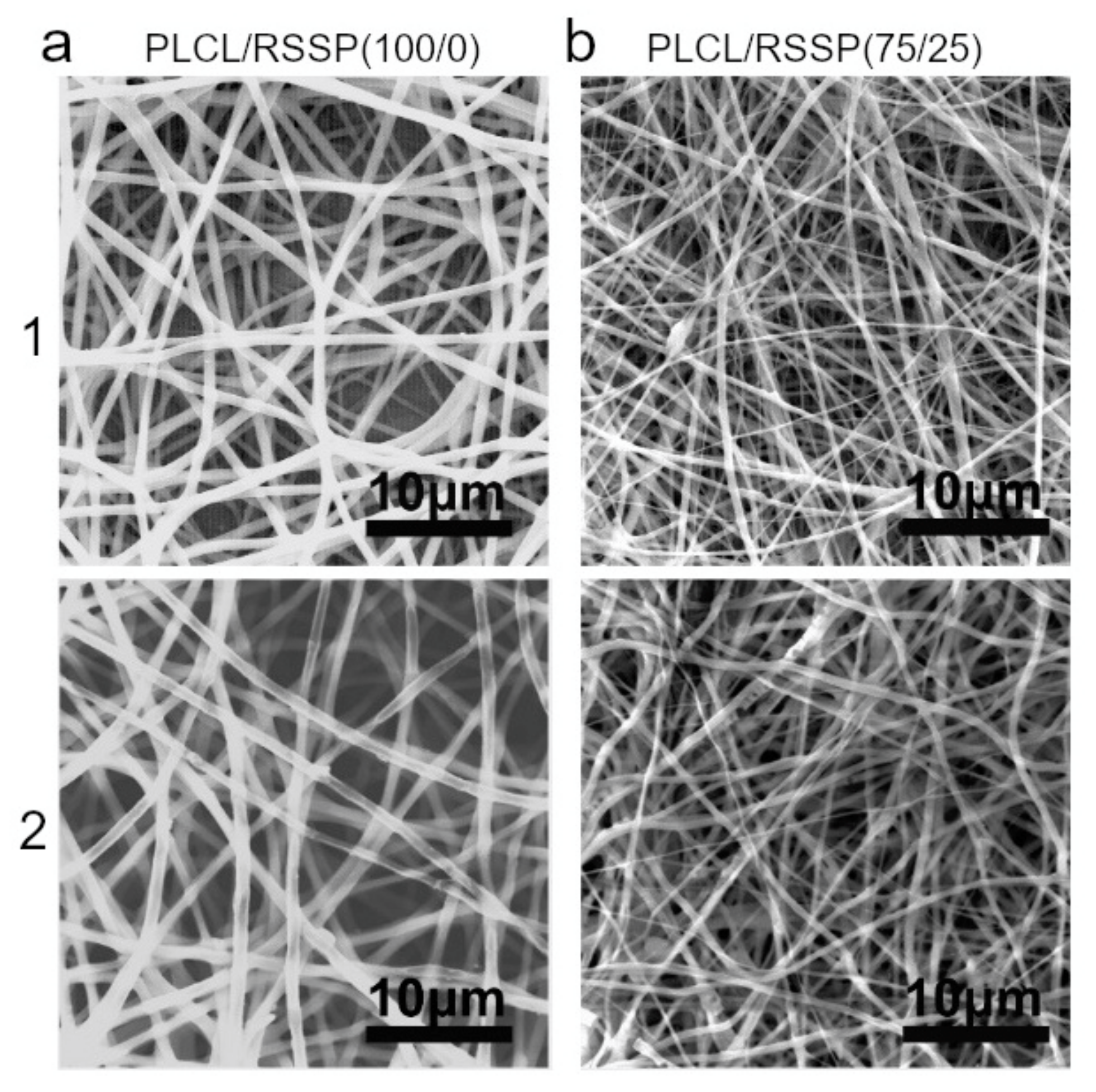
2.1. Fabrication and Morphology of the PLCL/RSSP Nanofibrous Membranes
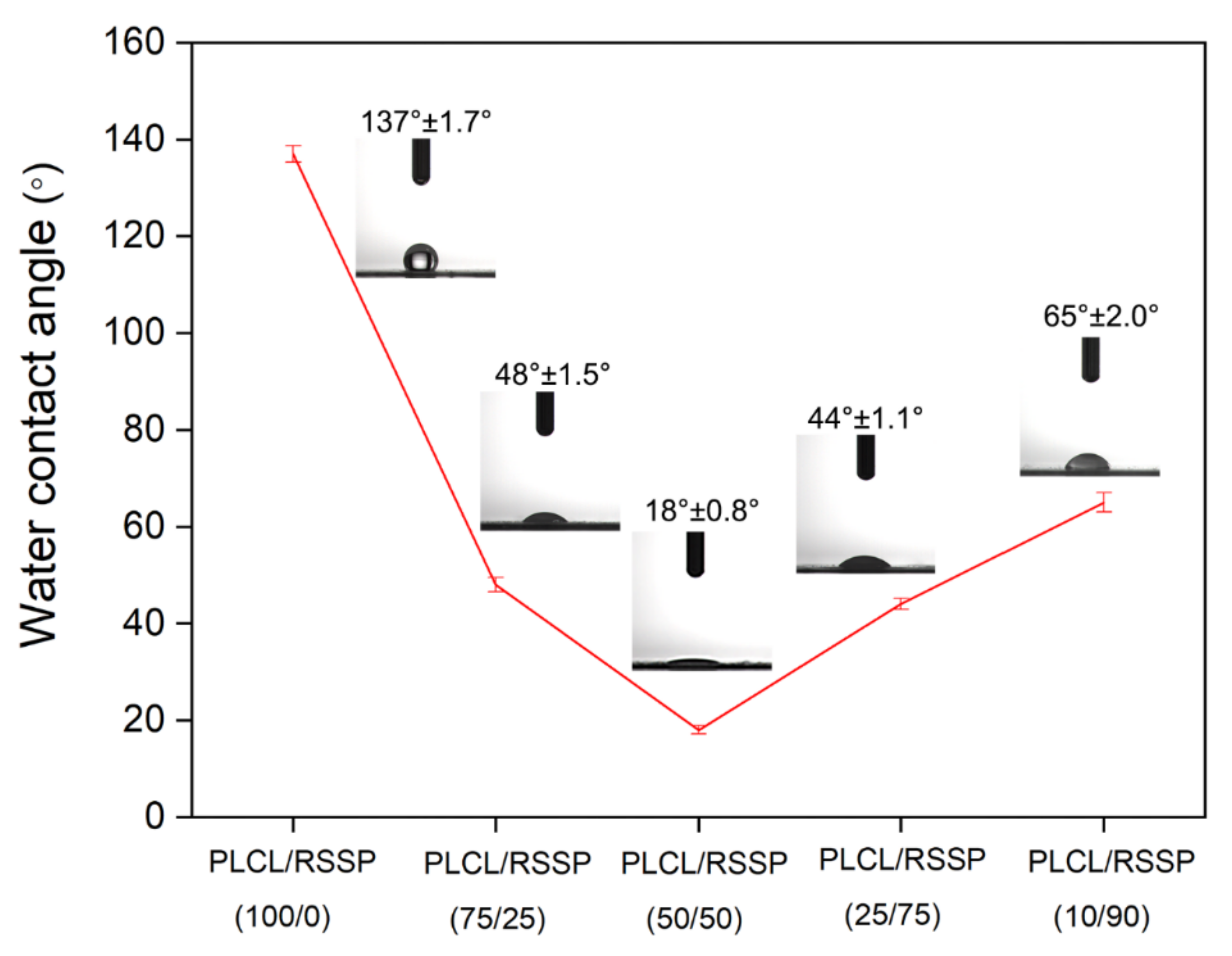
2.2. Hydrophilicity of the PLCL/RSSP Nanofibrous Membranes
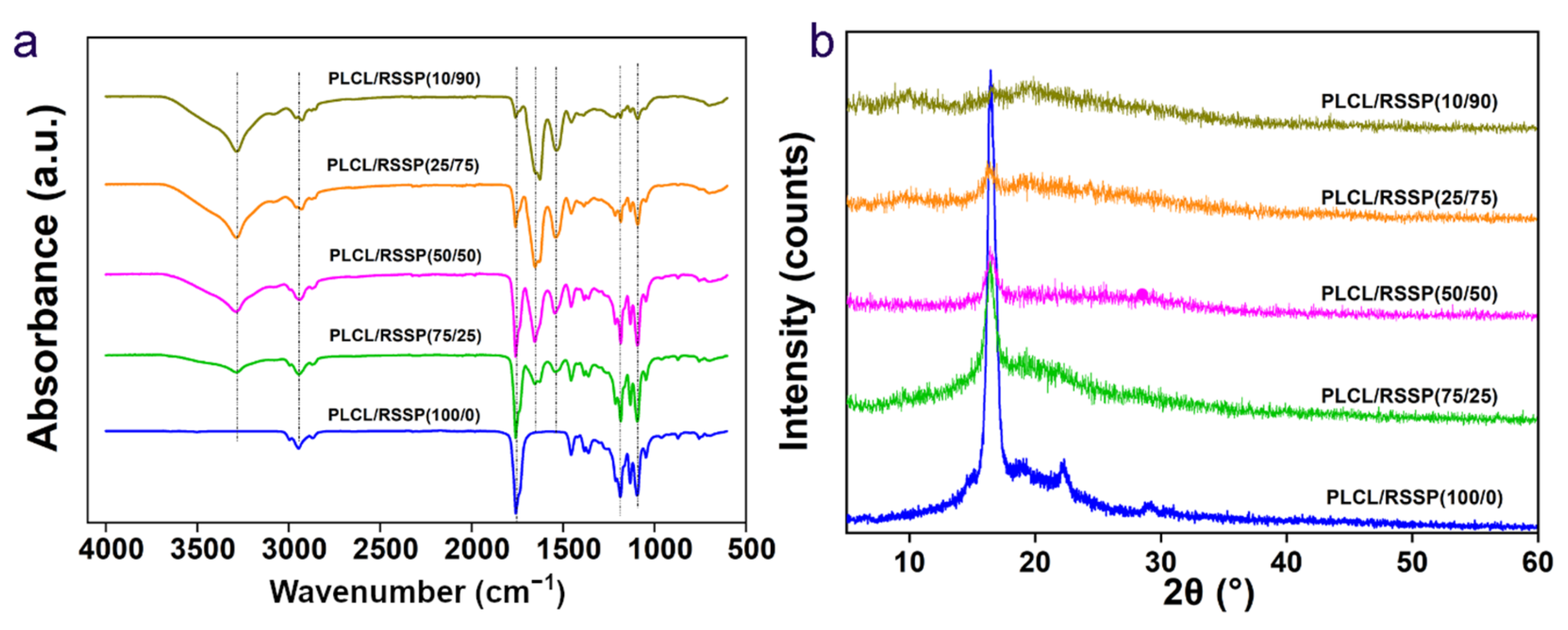
2.3. Structure of the PLCL/RSSP Nanofibrous Membranes
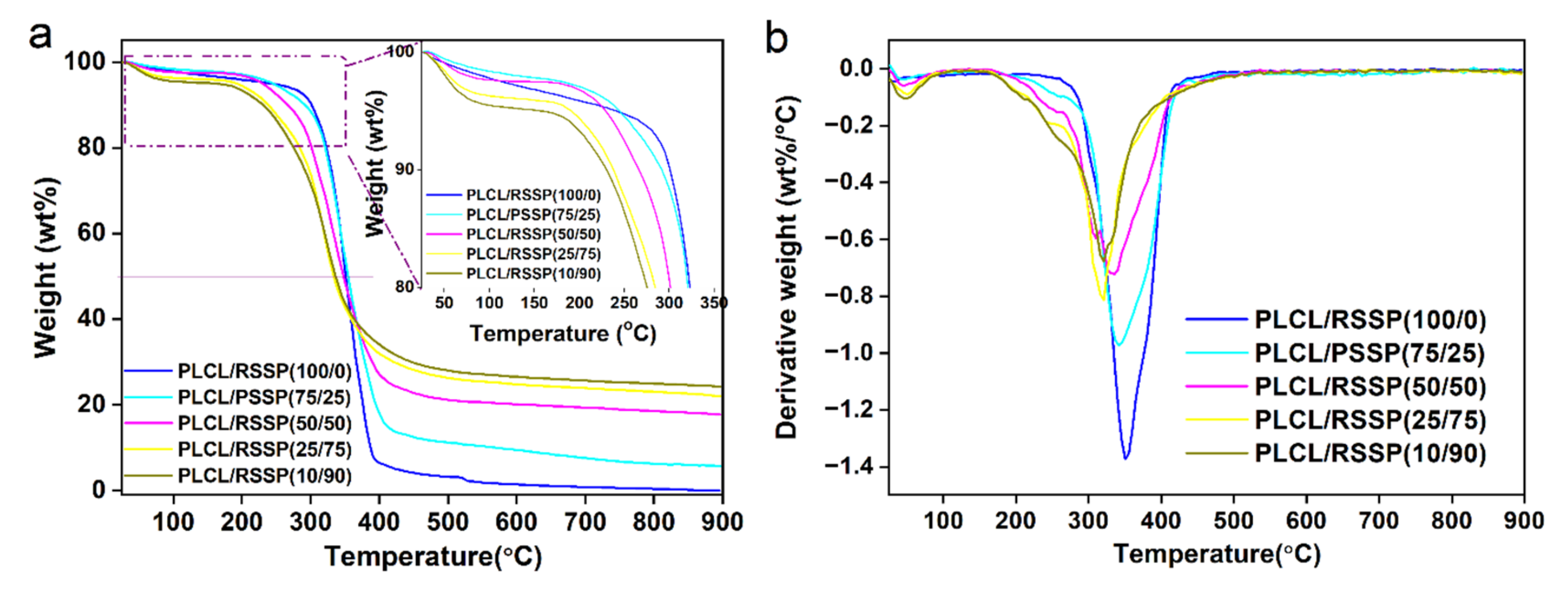
2.4. Thermal Decomposition Properties of the PLCL/RSSP Nanofibrous Membranes
2.5. Mechanical Properties of the PLCL/RSSP Nanofibrous Membranes
2.6. Hemocompatibility of the PLCL/RSSP Nanofibrous Membranes
2.7. Cytocompatibility of the PLCL/RSSP Nanofibrous Membranes
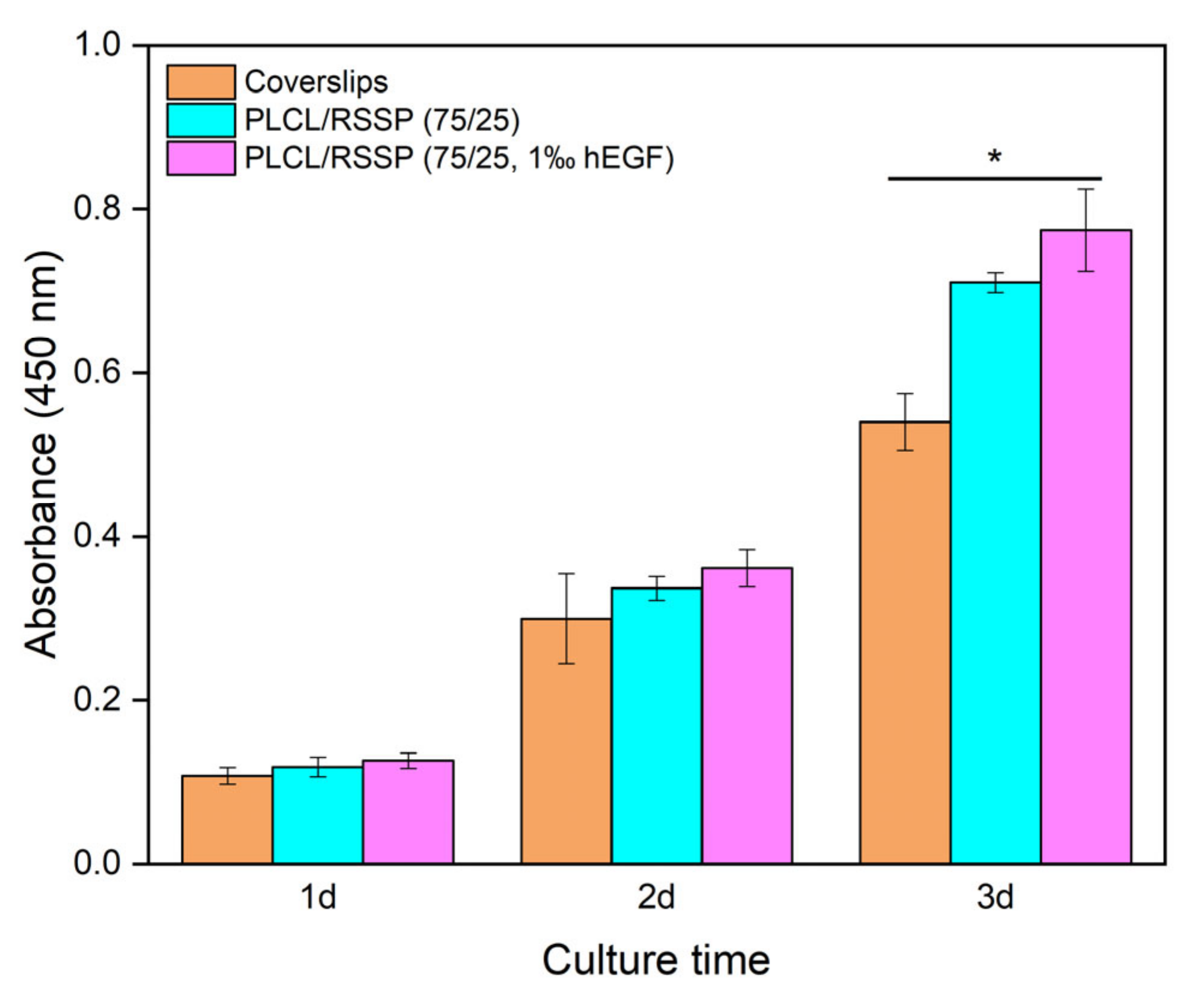
2.8. Cell Proliferation of the PLCL/RSSP Nanofibrous Membranes with hEGF
2.9. Degradability of the PLCL/RSSP Nanofibrous Membranes In Vitro
3. Materials and Methods
3.1. Materials
3.2. Preparation of Nanofibrous Membranes
3.3. Scanning Electron Microscopy (SEM)
3.4. Water Contact Angle Measurement
3.5. Fourier Transform Infrared (FTIR) Spectroscopy
3.6. Thermogravimetric Analysis (TGA)
3.7. Wide-Angle X-ray Diffraction (WAXD)
3.8. Mechanical Testing
3.9. Hemolysis Assays
3.10. Cell Attachment and Proliferation on the Nanofibrous Membranes
3.11. Degradation of the Nanofibrous Membranes
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RSSP | recombinant spider silk protein |
| PLCL | poly(l-lactide-co-ε-caprolactone) |
| SEM | scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| WAXD | wide angle X-ray diffraction |
| TGA | thermo-gravimetric analysis |
| hFFCs | human foreskin fibroblast cells |
| NIH-3T3 | mouse embryonic fibroblast cells (NIH-3T3) |
| hEGF | human epidermal growth factor |
| DTG | derivative thermogravimetry |
| TG | thermogravimetry |
| HR | hemolysis rates |
References
- Edwards, C.; Marks, R. Evaluation of biomechanical properties of human skin. Clin. Dermatol. 1995, 13, 375–380. [Google Scholar] [CrossRef]
- Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release 2010, 142, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.A.; Ong, J.F.; Eriksson, E.; Junker, J.P.; Caterson, E.J. Tissue engineering of skin. J. Am. Coll. Surg. 2013, 217, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.T.; Marques, A.P.; Reis, R.L. Using stem cells in skin regeneration: Possibilities and reality. Stem Cells Dev. 2012, 21, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Wendt, H.; Hillmer, A.; Reimers, K.; Kuhbier, J.W.; Schafer-Nolte, F.; Allmeling, C.; Kasper, C.; Vogt, P.M. Artificial skin--culturing of different skin cell lines for generating an artificial skin substitute on cross-weaved spider silk fibres. PLoS ONE 2011, 6, e21833. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, D.; Lohe, T.U.; Samudrala, P.K.; Mandal, B.B. In situ forming injectable silk fibroin hydrogel promotes skin regeneration in full thickness burn wounds. Adv. Healthc. Mater. 2018, 7, e1801092. [Google Scholar] [CrossRef]
- Blais, M.; Parenteau-Bareil, R.; Cadau, S.; Berthod, F. Concise Review: Tissue-Engineered Skin and Nerve Regeneration in Burn Treatment. Stem Cells Transl. Med. 2013, 2, 545–551. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Pan, J.-f.; Liu, N.-h.; Sun, H.; Xu, F. Preparation and Characterization of Electrospun PLCL/Poloxamer Nanofibers and Dextran/Gelatin Hydrogels for Skin Tissue Engineering. PLoS ONE 2014, 9, e112885. [Google Scholar] [CrossRef]
- Gu, J.; Liu, N.; Yang, X.; Feng, Z.; Qi, F. Adiposed-derived stem cells seeded on PLCL/P123 eletrospun nanofibrous scaffold enhance wound healing. Biomed. Mater. 2014, 9, 035012. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-H.; Pan, J.-F.; Miao, Y.-E.; Liu, T.-X.; Xu, F.; Sun, H. Electrospinning of poly (ε-caprolactone-co-lactide)/Pluronic blended scaffolds for skin tissue engineering. J. Mater. Sci. 2014, 49, 7253–7262. [Google Scholar] [CrossRef]
- Honda, M.; Yada, T.; Ueda, M.; Kimata, K. Cartilage formation by cultured chondrocytes in a new scaffold made of poly(L-lactide-ϵ-caprolactone) sponge. J. Oral Maxillofac. Surg. 2000, 58, 767–775. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, B.S.; Kang, S.W.; Kwon, J.H.; Lee, Y.M.; Kim, S.H.; Kim, Y.H. In vivo biocompatibilty and degradation behavior of elastic poly(l-lactide-co-epsilon-caprolactone) scaffolds. Biomaterials 2004, 25, 5939–5946. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Bei, J.; Wang, S. Synthesis, characterization and degradation of ABA block copolymer of L-lactide and ε-caprolactone. Polym. Degrad. Stab. 2000, 68, 423–429. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, X.; Liang, Q.; Xu, X.; Jing, X. Enzymatic degradation of poly(L-lactide) and poly(epsilon-caprolactone) electrospun fibers. Macromol. Biosci. 2004, 4, 1118–1125. [Google Scholar] [CrossRef]
- De Groot, J.H.; Zijlstra, F.M.; Kuipers, H.W.; Pennings, A.J.; Klompmaker, J.; Veth, R.P.H.; Jansen, H.W.B. Meniscal tissue regeneration in porous 50/50 copoly(l-lactide/epsilon-caprolactone) implants. Biomaterials 1996, 18, 613–622. [Google Scholar] [CrossRef]
- Perego, G.; Cella, G.D.; Aldini, N.; Fini, M.; Giardino, R. Preparation of a new nerve guide from a poly(l-lactide-co-6-caprolactone). Biomaterials 1994, 15, 189–193. [Google Scholar] [CrossRef]
- Honda, M.; Morikawa, N.; Hata, K.; Yada, T.; Morita, S.; Ueda, M.; Kimata, K. Rat costochondral cell characteristics on poly (l-lactide-co-ε-caprolactone) scaffolds. Biomaterials 2003, 24, 3511–3519. [Google Scholar] [CrossRef]
- Marston, W.A.; Usala, A.; Hill, R.S.; Mendes, R.; Minsley, M.-A. Initial report of the use of an injectable porcine collagen-derived matrix to stimulate healing of diabetic foot wounds in humans. Wound Repair Regen. 2005, 13, 243–247. [Google Scholar] [CrossRef]
- Liu, H.; Mao, J.; Yao, K.; Yang, G.; Cui, L.; Cao, Y. A study on a chitosan-gelatin-hyaluronic acid scaffold as artificial skin in vitro and its tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2004, 15, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.A.; Tabata, Y.; Mikos, A.G. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J. Control. Release 2003, 91, 299–313. [Google Scholar] [CrossRef]
- Suganya, S.; Venugopal, J.; Ramakrishna, S.; Lakshmi, B.S.; Dev, V.R. Naturally derived biofunctional nanofibrous scaffold for skin tissue regeneration. Int. J. Biol. Macromol. 2014, 68, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Anulekha, K.H.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Fabrication of chitosan/poly(caprolactone) nanofibrous scaffold for bone and skin tissue engineering. Int. J. Biol. Macromol. 2011, 48, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.; Seifalian, A.M. Skin regeneration scaffolds: A multimodal bottom-up approach. Trends Biotechnol. 2012, 30, 638–648. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Xue, J.; Li, H.; Zhu, C.; Mo, X.; Xia, Y. General method for generating circular gradients of active proteins on nanofiber scaffolds sought for wound closure and related applications. ACS Appl. Mater. Interfaces 2018, 10, 8536–8545. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.H.; Shen, C.Y.; You, M.L.; Xiao, J.F.; Chen, G.Q. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials 2010, 31, 1691–1698. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.W.; Khor, H.L.; Hutmacher, D.W. In vitro characterization of natural and synthetic dermal matrices cultured with human dermal fibroblasts. Biomaterials 2004, 25, 2807–2818. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Fibrin sealant: The only approved hemostat, sealant, and adhesive: A laboratory and clinical perspective. ISRN Surg. 2014, 2014, 203943. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.V. Spider Silk: Ancient Ideas for New Biomaterials. Chem. Rev. 2006, 106, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Rising, A.; Johansson, J. Toward spinning artificial spider silk. Nat. Chem. Biol. 2015, 11, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.X.; Qian, Z.G.; Ki, C.S.; Park, Y.H.; Kaplan, D.L.; Lee, S.Y. Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proc. Natl. Acad. Sci. USA 2010, 107, 14059–14063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-J.; Cho, K.; Kim, A.-Y.; Kim, G.-W. Injectable Click Fibroin Bioadhesive Derived from Spider Silk for Accelerating Wound Closure and Healing Bone Fracture. Materials 2022, 15, 5269. [Google Scholar] [CrossRef]
- Jones, J.A.; Harris, T.I.; Tucker, C.L.; Berg, K.R.; Christy, S.Y.; Day, B.A.; Gaztambide, D.A.; Needham, N.J.; Ruben, A.L.; Oliveira, P.F.; et al. More than just fibers: An aqueous method for the production of innovative recombinant spider silk protein materials. Biomacromolecules 2015, 16, 1418–1425. [Google Scholar] [CrossRef]
- Lewis, R.V.; Hinman, M.; Kothakota, S.; Fournier, M.J. Expression and purification of a spider silk protein: A new strategy for producing repetitive proteins. Protein Expr. Purif. 1996, 7, 400–406. [Google Scholar] [CrossRef]
- Fahnestock, S.R.; Bedzyk, L.A. Production of synthetic spider dragline silk protein in Pichia pastoris. Appl. Microbiol. Biotechnol. 1997, 47, 33–39. [Google Scholar] [CrossRef]
- Scheller, J.; Guhrs, K.H.; Grosse, F.; Conrad, U. Production of spider silk proteins in tobacco and potato. Nat. Biotechnol. 2001, 19, 573–577. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, L.; Day, B.A.; Harris, T.I.; Oliveira, P.; Knittel, C.; Licon, A.L.; Gong, C.; Dion, G.; Lewis, R.V.; et al. CRISPR/Cas9 Initiated Transgenic Silkworms as a Natural Spinner of Spider Silk. Biomacromolecules 2019, 20, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Wen, R.; Meng, Q. Novel highly soluble chimeric recombinant spidroins with high yield. Int. J. Mol. Sci. 2020, 21, 6905. [Google Scholar] [CrossRef] [PubMed]
- Termonia, Y. Molecular modeling of spider silk elasticity. Macromolecules 1994, 27, 7378–7381. [Google Scholar] [CrossRef]
- Müller-Herrmann, S.; Scheibel, T. Enzymatic degradation of films, particles, and nonwoven meshes made of a recombinant spider silk protein. ACS Biomater. Sci. Eng. 2015, 1, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Doblhofer, E.; Heidebrecht, A.; Scheibel, T. To spin or not to spin: Spider silk fibers and more. Appl. Microbiol. Biotechnol. 2015, 99, 9361–9380. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Meinel, L.; Hofmann, S.; Malhotra, A.; Volloch, V.; Kaplan, D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. A 2004, 71, 528–537. [Google Scholar] [CrossRef]
- Bini, E.; Foo, C.W.; Huang, J.; Karageorgiou, V.; Kitchel, B.; Kaplan, D.L. RGD-functionalized bioengineered spider dragline silk biomaterial. Biomacromolecules 2006, 7, 3139–3145. [Google Scholar] [CrossRef]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider silk for tissue engineering applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef] [Green Version]
- Leal-Egaña, A.; Lang, G.; Mauerer, C.; Wickinghoff, J.; Weber, M.; Geimer, S.; Scheibel, T. Interactions of Fibroblasts with Different Morphologies Made of an Engineered Spider Silk Protein. Adv. Eng. Mater. 2012, 14, B67–B75. [Google Scholar] [CrossRef]
- DeSimone, E.; Aigner, T.B.; Humenik, M.; Lang, G.; Scheibel, T. Aqueous electrospinning of recombinant spider silk proteins. Mater. Sci. Eng. C 2020, 106, 110145. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Q.; Lin, Y.; Xu, S.; Meng, Q. Evaluation of the potential of chimeric spidroins/poly(L-lactic-co-ε-caprolactone) (PLCL) nanofibrous scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 111, 110752. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Rising, A.; Johansson, J.; Zhang, X.; Lin, Y.; Zhang, L.; Yi, T.; Mi, J.; Meng, Q. Tensile properties of synthetic pyriform spider silk fibers depend on the number of repetitive units as well as the presence of N- and C-terminal domains. Int. J. Biol. Macromol. 2020, 154, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, B.; Liu, S.; Chen, W.; Zhang, Y.; Wang, C.; Mo, X.; Che, J.; Ouyang, Y.; Yuan, W.; et al. Polymerizing pyrrole coated poly (l-lactic acid-co-ε-caprolactone) (PLCL) conductive nanofibrous conduit combined with electric stimulation for long-range peripheral nerve regeneration. Front. Mol. Neurosci. 2016, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Chittur, K.K. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials 1998, 19, 357–369. [Google Scholar] [CrossRef]
- Heidebrecht, A.; Eisoldt, L.; Diehl, J.; Schmidt, A.; Geffers, M.; Lang, G.; Scheibel, T. Biomimetic fibers made of recombinant spidroins with the same toughness as natural spider silk. Adv. Mater. 2015, 27, 2189–2194. [Google Scholar] [CrossRef]
- Yarger, J.L.; Cherry, B.R.; van der Vaart, A. Uncovering the structure–function relationship in spider silk. Nat. Rev. Mater. 2018, 3, 18008. [Google Scholar] [CrossRef]
- Plaza, G.R.; Pérez-Rigueiro, J.; Riekel, C.; Perea, G.B.; Agulló-Rueda, F.; Burghammer, M.; Guinea, G.V.; Elices, M. Relationship between microstructure and mechanical properties in spider silk fibers: Identification of two regimes in the microstructural changes. Soft Matter 2012, 8, 6015. [Google Scholar] [CrossRef] [Green Version]
- Eisoldt, L.; Smith, A.; Scheibel, T. Decoding the secrets of spider silk. Mater. Today 2011, 14, 80–86. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yun, Y.S.; Lee, S.; Jang, D.; Park, K.Y.; Kim, J.K.; Kim, B.H.; Kang, K.; Kaplan, D.L.; Jin, H.J. Carbonization of a stable beta-sheet-rich silk protein into a pseudographitic pyroprotein. Nat. Commun. 2015, 6, 7145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Mi, J.; Wen, R.; Zhang, J.; Cai, Y.; Meng, Q.; Lin, Y. Wet-Spinning Synthetic Fibers from Aggregate Glue: Aggregate Spidroin 1 (AgSp1). ACS Appl. Bio Mater. 2020, 3, 5957–5965. [Google Scholar] [CrossRef] [PubMed]
- Escoffier, C.; de Rigal, J.; Rochefort, A.; Vasselet, R.; Leveque, J.L.; Agache, P.G. Age-related mechanical properties of human skin: An in vivo study. J. Investig. Derm. 1989, 93, 353–357. [Google Scholar] [CrossRef]
- Sanders, R. Torsional elasticity of human skin in vivo. Pflug. Arch. 1973, 342, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Kakou, A.; Louis, H.; Cattan, V.; Lacolley, P.; Thornton, S. Correlation between arterial mechanical properties, vascular biomaterial and tissue engineering. Clin. Hemorheol. Microcirc. 2007, 37, 71–75. [Google Scholar] [PubMed]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004, 10, 1160–1168. [Google Scholar] [CrossRef]
- Gao, X.; Wen, M.; Liu, Y.; Hou, T.; An, M. Mechanical performance and cyocompatibility of PU/PLCL nanofibrous electrospun scaffolds for skin regeneration. Eng. Regen. 2022, 3, 53–58. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Xin, B.; Li, T. Doxorubicin hydrochloride-loaded electrospun poly(l-lactide-co-ε-caprolactone)/gelatin core–shell nanofibers for controlled drug release. Polym. Int. 2021, 70, 1717–1724. [Google Scholar] [CrossRef]
- Du, J.; Zhu, T.; Yu, H.; Zhu, J.; Sun, C.; Wang, J.; Chen, S.; Wang, J.; Guo, X. Potential applications of three-dimensional structure of silk fibroin/poly(ester-urethane) urea nanofibrous scaffold in heart valve tissue engineering. Appl. Surf. Sci. 2018, 447, 269–278. [Google Scholar] [CrossRef]
- Hou, L.; Li, Z.; Pan, Y.; Du, L.; Li, X.; Zheng, Y.; Li, L. Microstructure, mechanical properties, corrosion behavior and hemolysis of as-extruded biodegradable Mg-Sn-Zn alloy. AIP Conf. Proc. 2016, 1727, 020010. [Google Scholar] [CrossRef]
- Agostini, E.; Winter, G.; Engert, J. Water-based preparation of spider silk films as drug delivery matrices. J. Control. Release 2015, 213, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Oliveira, N.M.; Oliveira, M.B.; da Costa, D.P.S.; Naskar, D.; Mano, J.F.; Kundu, S.C.; Reis, R.L. Fabrication and characterization of Eri silk fibers-based sponges for biomedical application. Acta Biomater. 2016, 32, 178–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.; Zhu, T.; Wu, Y.; Zhou, X.; Yang, X.; Wang, J.; Fang, J.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Incorporation of amoxicillin-loaded organic montmorillonite into poly(ester-urethane) urea nanofibers as a functional tissue engineering scaffold. Colloids Surf. B Biointerfaces 2017, 151, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Huber, A.; Takanari, K.; Amoroso, N.J.; Hashizume, R.; Badylak, S.F.; Wagner, W.R. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber-extracellular matrix hydrogel biohybrid scaffold. Biomaterials 2011, 32, 3387–3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrizales, C.; Pelfrey, S.; Rincon, R.; Eubanks, T.; Kuang, A.; McClure, M.; Bowlin, G.; Macossay, J. Thermal and mechanical properties of electrospun PMMA, PVC, Nylon 6, and Nylon 6,6. Polym. Adv. Technol. 2008, 19, 124–130. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, K.; Bhutto, M.A.; Guo, X.; Shen, W.; Wang, J.; Chen, W.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Synthesis of RGD-peptide modified poly(ester-urethane) urea electrospun nanofibers as a potential application for vascular tissue engineering. Chem. Eng. J. 2017, 315, 177–190. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Kim, D.H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef] [Green Version]


| Stress (MPa) | Strain (%) | Secant Modulus at 5% (MPa) | |
|---|---|---|---|
| PLCL/RSSP (100/0) | 7.6 ± 0.3 | 287.7 ± 26.3 | 6.7 ± 1.0 |
| PLCL/RSSP (75/25) | 16.6 ± 0.9 | 81.9 ± 2.2 | 45.6 ± 0.8 |
| PLCL/RSSP (50/50) | 8.0 ± 0.8 | 46.2 ± 5.2 | 64.2 ± 5.3 |
| PLCL/RSSP (25/75) | 15.4 ± 0.3 | 36.1 ± 4.4 | 205.1 ± 22.9 |
| PLCL/RSSP (10/90) | 6.5 ± 0.3 | 18.0 ± 7.40 | 111.8 ± 9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhu, H.; Meng, Q. Preparation and Characterization of Nanofibrous Membranes Electro-Spun from Blended Poly(l-lactide-co-ε-caprolactone) and Recombinant Spider Silk Protein as Potential Skin Regeneration Scaffold. Int. J. Mol. Sci. 2022, 23, 14055. https://doi.org/10.3390/ijms232214055
Wang S, Zhu H, Meng Q. Preparation and Characterization of Nanofibrous Membranes Electro-Spun from Blended Poly(l-lactide-co-ε-caprolactone) and Recombinant Spider Silk Protein as Potential Skin Regeneration Scaffold. International Journal of Molecular Sciences. 2022; 23(22):14055. https://doi.org/10.3390/ijms232214055
Chicago/Turabian StyleWang, Suyang, Hongnian Zhu, and Qing Meng. 2022. "Preparation and Characterization of Nanofibrous Membranes Electro-Spun from Blended Poly(l-lactide-co-ε-caprolactone) and Recombinant Spider Silk Protein as Potential Skin Regeneration Scaffold" International Journal of Molecular Sciences 23, no. 22: 14055. https://doi.org/10.3390/ijms232214055
APA StyleWang, S., Zhu, H., & Meng, Q. (2022). Preparation and Characterization of Nanofibrous Membranes Electro-Spun from Blended Poly(l-lactide-co-ε-caprolactone) and Recombinant Spider Silk Protein as Potential Skin Regeneration Scaffold. International Journal of Molecular Sciences, 23(22), 14055. https://doi.org/10.3390/ijms232214055






