Three Pairs of Novel Enantiomeric 8-O-4′ Type Neolignans from Saussurea medusa and Their Anti-inflammatory Effects In Vitro
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of Compounds
2.2. Anti-Inflammatory Effects
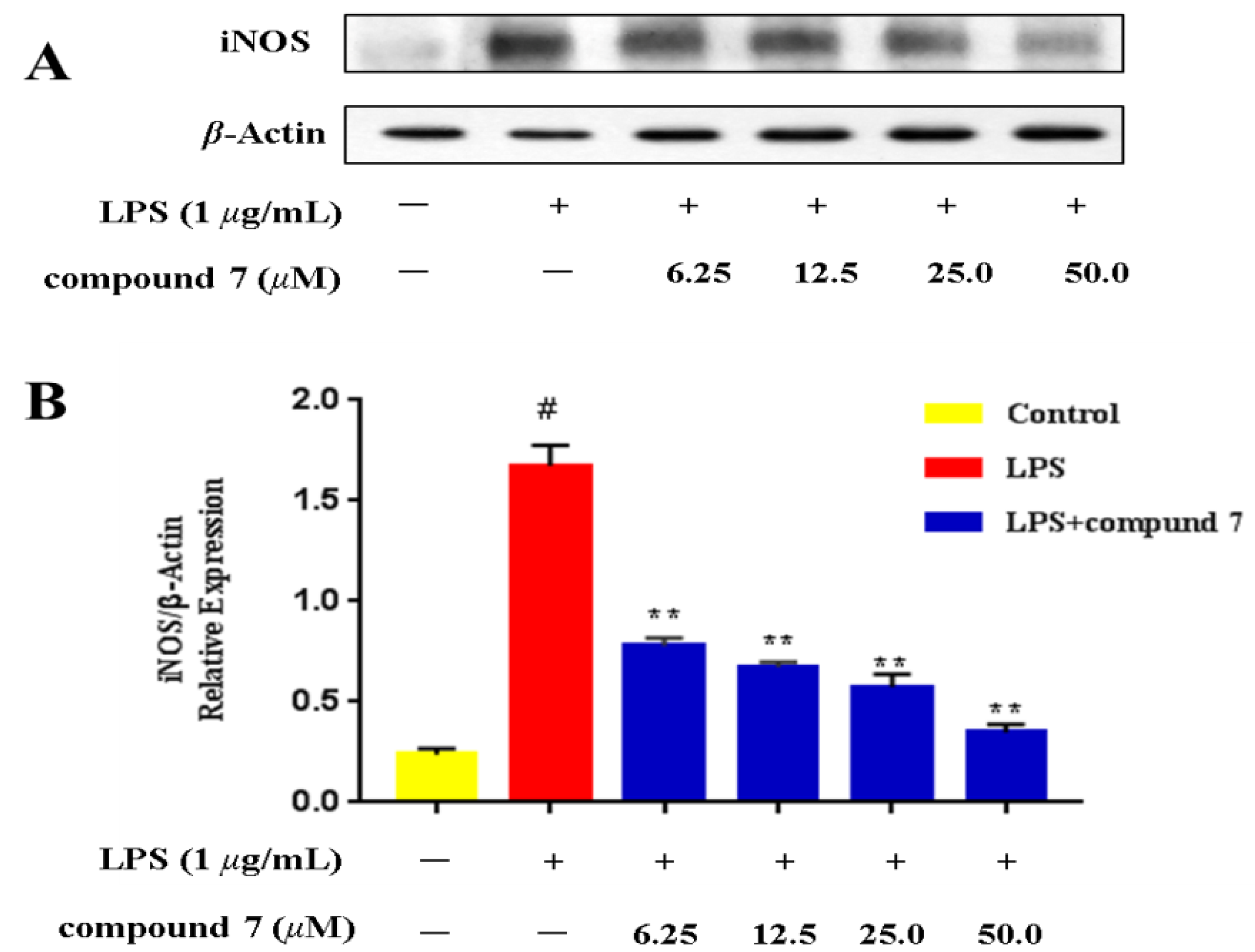
2.3. Effect of the Selected Active Compounds on iNOS Expression
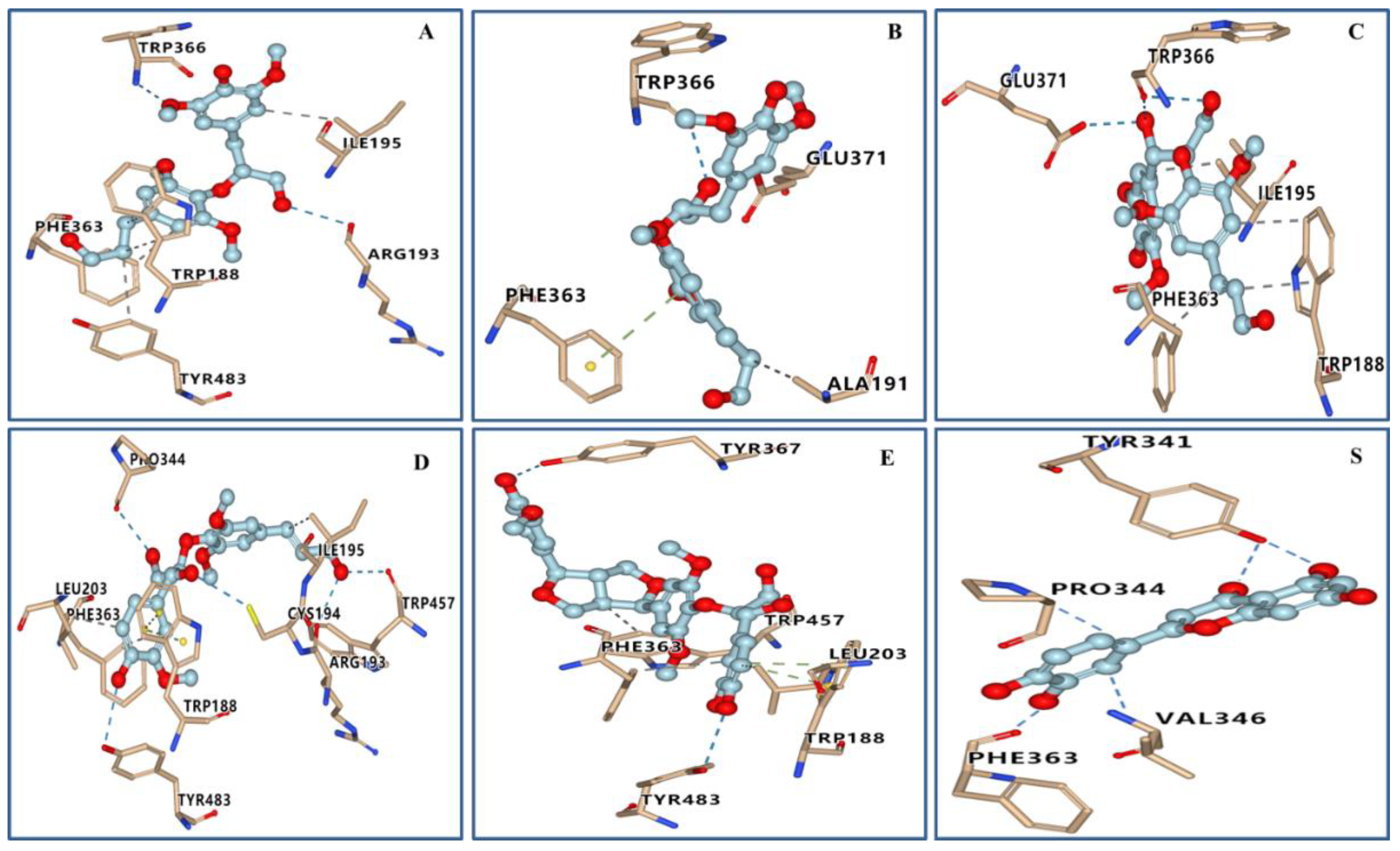
2.4. Molecular Docking Studies
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Details of New Compounds
3.4.1. Medusidine A (1)
3.4.2. Medusidine B (2)
3.4.3. Medusidine C (3)
3.5. ECD Calculations
3.6. Determination of NO Production
3.7. Determination of iNOS Expression
3.8. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, H.H.; Wang, T.; Matsuda, H.; Morikawa, T.; Yoshikawa, M.; Tani, T. Bioactive constituents from Chinese natural medicines. XV. Inhibitory effect on aldose reductase and structures of Saussureosides A and B from Saussurea medusa. Chem. Pharm. Bull. 2005, 53, 1416–1422. [Google Scholar] [CrossRef]
- Committee of Pharmacopoeia of the People’s Repubulic of China. Drug Standard of Ministry of Public Health of the People’s Republic of China; People’s Medical Publinshing House: Beijing, China, 1995; Volume 1, p. 94. [Google Scholar]
- Fan, C.Q.; Yue, J.M. Biologically active phenols from Saussurea medusa. Bioorg. Med. Chem. 2003, 11, 703–708. [Google Scholar] [CrossRef]
- Duan, H.Q.; Takaishi, Y.; Momota, H.; Ohmoto, Y.; Taki, T. Immunosuppressive constituents from Saussurea medusa. Phytochemistry 2002, 59, 85–90. [Google Scholar] [CrossRef]
- Dawa, Z.; Bai, Y.; Zhou, Y.; Gesang, S.; Ding, L. Chemical constituents of the whole plants of Saussurea medusa. J. Nat. Med. 2009, 63, 327–330. [Google Scholar] [CrossRef]
- Cao, J.Y.; Wang, Z.Y.; Dong, Q.; Wang, X.; Yu, R.T.; Tao, Y.D. Saussurenoids A-G, seven new sesquiterpenoids from Saussurea medusa maxim. Tetrahedron 2022, 120, 132850. [Google Scholar] [CrossRef]
- Yi, T.; Zhao, Z.Z.; Yu, Z.L.; Chen, H.B. Comparison of the anti-inflammatory and anti-nociceptive effects of three medicinal plants known as “Snow Lotus” herb in traditional Uighur and Tibetan medicines. J. Ethnopharmacol. 2010, 128, 405–411. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Komatsu, K.; Tokuda, H.; Nishino, H. Anti-tumor-promoting activity of lignans from the aerial part of Saussurea medusa. Cancer Lett. 2000, 158, 53–59. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dong, Q.; Wang, Z.Y.; Zhao, Y.; Ren, Y.; Liu, C.; Dang, J.; Yu, R.T.; Tao, Y.D. Arylnaphthalide lignans from Saussurea medusa and their anti-inflammatory activities. Arab. J. Chem. 2022, 15, 104155. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef]
- Ward, T.J.; Ward, K.D. Chiral separations: A review of current topics and trends. Anal. Chem. 2012, 84, 626–635. [Google Scholar] [CrossRef]
- Tian, A.; Qi, J.; Liu, Y.; Wang, F.; Ito, Y.; Wei, Y. Chiral magnetic microspheres purified by centrifugal field flow fractionation and microspheres magnetic chiral chromatography for benzoin racemate separation. J. Chromatogr. A 2013, 1305, 333–337. [Google Scholar] [CrossRef]
- Kapnissi-Christodoulou, C.P.; Nicolaou, A.G.; Stavrou, I.J. Enantioseparations in open-tubular capillary electrochromatography: Recent advances and applications. J. Chromatogr. A 2016, 1467, 145–154. [Google Scholar] [CrossRef]
- Cuong, T.D.; Hung, T.M.; Kim, J.C.; Kim, E.H.; Woo, M.H.; Choi, J.S.; Lee, J.H.; Min, B.S. Phenolic compounds from Caesalpinia sappan heartwood and their anti-inflammatory activity. J. Nat. Prod. 2012, 75, 2069–2075. [Google Scholar] [CrossRef]
- Hagerling, C.; Casbon, A.J.; Werb, Z. Balancing the innate immune system in tumor development. Trends Cell Biol. 2015, 25, 214–220. [Google Scholar] [CrossRef]
- Garcia, C.; Feve, B.; Ferré, P.; Halimi, S.; Baizri, H.; Bordier, L.; Guiu, G.; Dupuy, O.; Bauduceau, B.; Mayaudon, H. Diabetes and inflammation: Fundamental aspects and clinical implications. Diabetes Metab. 2010, 36, 327–338. [Google Scholar] [CrossRef]
- Perera, W.H.; Shivanagoudra, S.R.; Pérez, J.L.; Kim, D.M.; Sun, Y.X.; Jayaprakasha, G.K.; Patil, B.S. Anti-inflammatory, antidiabetic properties and in silico modeling of cucurbitane-type triterpene glycosides from fruits of an Indian cultivar of Momordica charantia L. Molecules 2021, 26, 1038. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease-a double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef]
- Su, X.M.; Federoff, H.J. Immune responses in Parkinson’s disease: Interplay between central and peripheral immune systems. Biomed. Res. Int. 2015, 2014, 275178. [Google Scholar] [CrossRef]
- Willerson, J.T.; Ridker, P.M. Inflammation as a cardiovascular risk factor. Circulation 2004, 109, 2–10. [Google Scholar] [CrossRef]
- Shivanagoudra, R.; Perera, W.H.; Perez, J.L.; Athrey, G.; Sun, Y.X.; Jayaprakasha, G.K.; Patil, B.S. Cucurbitane-type compounds from Momordica charantia: Isolation, in vitro antidiabetic, anti-inflammatory activities and in silico modeling approaches. Bioorg. Chem. 2019, 87, 31–42. [Google Scholar] [CrossRef]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef]
- Chen, X.; Tang, S.A.; Lee, E.; Qiu, Y.L.; Wang, R.; Duan, H.Q.; Dan, S.; Jin, M.H.; Kong, D.X. IVSE, isolated from Inula japonica, suppresses LPS-induced NO production via NF-κB and MAPK inactivation in RAW 264.7 cells. Life Sci. 2015, 124, 8–15. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.R.; Yin, Y.; Song, K.R.; Long, L.P.; Li, X.Z.; Zhou, B.; Gao, H.Y. New cassane diterpenoids from the seed kernels of Caesalpinia cucullata, exhibit anti-inflammatory effect in vitro by inhibiting iNOS enzymatic activity. Chin. J. Chem. 2021, 39, 1625–1634. [Google Scholar] [CrossRef]
- Hussain, M.S.; Azam, F.; Eldarrat, H.A.; Haque, A.; Khalid, M.; Hassan, M.Z.; Ali, M.; Arif, M.; Ahmad, I.; Zaman, G.; et al. Structural, functional, molecular, and biological evaluation of novel triterpenoids isolated from Helichrysum stoechas (L.) Moench. Collected from Mediterranean Sea bank: Misurata-Libya. Arab. J. Chem. 2022, 15, 103818. [Google Scholar] [CrossRef]
- Wang, Y.H.; Sun, Q.Y.; Yang, F.M.; Long, C.L.; Zhao, F.W.; Tang, G.H.; Niu, H.M.; Wang, H.; Huang, Q.Q.; Xu, J.J.; et al. Neolignans and Caffeoyl Derivatives from Selaginella moellendorffii. Helv. Chim. Acta 2010, 93, 2467–2477. [Google Scholar] [CrossRef]
- Braga, A.C.H.; Zacchino, S.; Badano, H.; Sierra, M.G.; Rúveda, E.A. 13C NMR spectral and conformational analysis of 8-O-4′ neolignans. Phytochemistry 1984, 23, 2025–2028. [Google Scholar] [CrossRef]
- Fang, L.; Du, D.; Ding, G.Z.; Si, Y.K.; Yu, S.S.; Liu, Y.; Wang, W.J.; Ma, S.G.; Xu, S.; Qu, J.; et al. Neolignans and glycosides from the stem bark of Illicium difengpi. J. Nat. Prod. 2010, 73, 818–824. [Google Scholar] [CrossRef]
- Huang, X.X.; Zhou, C.C.; Li, L.Z.; Li, F.F.; Lou, L.L.; Li, D.M.; Ikejima, T.; Peng, Y.; Song, S.J. The cytotoxicity of 8-O-4’ neolignans from the seeds of Crataegus pinnatifida. Bioorg. Med. Chem. Lett. 2013, 23, 5599–5604. [Google Scholar] [CrossRef]
- Zhu, J.X.; Ren, J.; Qin, J.J.; Cheng, X.R.; Zeng, Q.; Zhang, F.; Yan, S.K.; Jin, H.Z.; Zhang, W.D. Phenylpropanoids and lignanoids from Euonymus acanthocarpus. Arch. Pharm. Res. 2012, 35, 1739–1747. [Google Scholar] [CrossRef]
- Cutillo, F.; D’Abrosca, B.; Dellagreca, M.; Fiorentino, A.; Zarrelli, A. Lignans and neolignans from Brassica fruticulosa: Effects on seed germination and plant growth. J. Agric. Food Chem. 2003, 51, 6165–6172. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, C.G.; Li, Y.R.; Tian, Y.; Lin, S.; Yuan, S.P.; Hu, J.F.; Hou, Q.; Chen, N.H.; Yang, Y.C.; et al. Lignans and neolignans from Sinocalamus affinis and their absolute configurations. J. Nat. Prod. 2011, 74, 1188–1200. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Xue, Y.B.; Liu, J.J.; Yao, G.M.; Li, D.Y.; Sun, B.; Zhang, J.W.; Liu, Y.F.; Qi, C.X.; Xiang, M.; et al. (±)-Acortatarinowins A−F, norlignan, neolignan, and lignan enantiomers from Acorus tatarinowii. J. Nat. Prod. 2015, 78, 2205–2214. [Google Scholar] [CrossRef]
- Woo, K.W.; Suh, W.S.; Subedi, L.; Kim, S.Y.; Kim, A.; Lee, K.R. Bioactive lignan derivatives from the stems of Firmiana simplex. Bioorg. Med. Chem. Lett. 2016, 26, 730–733. [Google Scholar] [CrossRef]
- Jeong, E.J.; Cho, J.H.; Sung, S.H.; Kim, S.Y.; Kim, Y.C. Inhibition of nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophage cells by lignans isolated from Euonymus alatus leaves and twigs. Bioorg. Med. Chem. Lett. 2011, 21, 2283–2286. [Google Scholar] [CrossRef]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.J.; Reboul, M.; Xiang, J.Y.; Wang, L.L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 2015, 12, 281–296. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, B.V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schubert, D. Cytotoxic amyloid peptides inhibit cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction by enhancing MTT formazan exocytosis. J. Neurochem. 1997, 69, 2285–2293. [Google Scholar] [CrossRef]
- Sheeba, M.S.; Asha, V.V. Cardiospermum halicacabum ethanol extract inhibits LPS induced COX-2, TNF-α and iNOS expression, which is mediated by NF-kB regulation, in RAW264.7 cells. J. Ethnopharmacol. 2009, 124, 39–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.Z.; Wang, M.Y.; Sun, C.J.; Li, X.B. Five new compounds from Hosta plantaginea flowers and their anti-inflammatory activities. Bioorg. Chem. 2019, 95, 103494. [Google Scholar] [CrossRef] [PubMed]

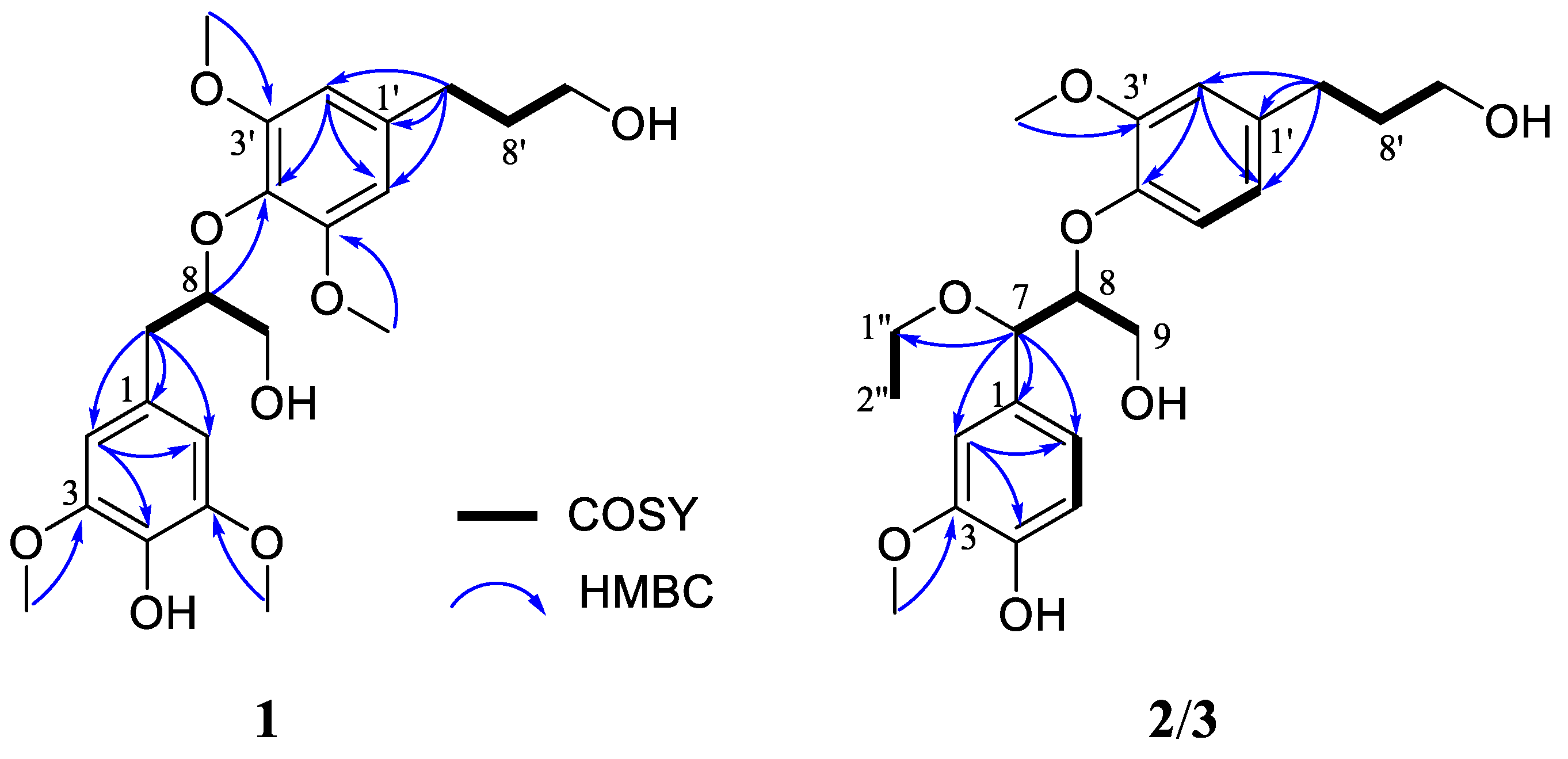

| Position | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | |
| 1 | ― | 129.6, C | ― | 131.8, C | ― | 131.7, C |
| 2 | 6.52, s | 106.3, CH | 6.90, d (1.8) | 112.4, CH | 6.97, d (1.7) | 112.1, CH |
| 3 | ― | 147.0, C | ― | 148.8, C | ― | 149.0, C |
| 4 | ― | 133.3, C | ― | 147.3, C | ― | 147.4, C |
| 5 | ― | 147.0, C | 6.72, d (8.1) | 115.6, CH | 6.77, d (8.0) | 115.9, CH |
| 6 | 6.52, s | 106.3, CH | 6.78, dd (8.1, 1.8) | 122.0, CH | 6.81, dd (8.0, 1.7) | 121.4, CH |
| 7 | a 3.20, dd (13.6, 5.4) | 38.0, CH2 | 4.46, d (6.4) | 81.8, CH | 4.53, d (5.9) | 82.5, CH |
| b 2.97, dd (13.6, 8.6) | ||||||
| 8 | 4.18, m | 84.5, CH | 4.27, ddd (6.4, 5.4, 3.5) | 85.9, CH | 4.26, ddd (5.9, 4.0, 3.5) | 86.5, CH |
| 9 | a 3.56, dd (12.4, 2.4) | 62.5, CH2 | a 3.85, dd (11.9, 5.4) | 62.3, CH2 | a 3.63, dd (11.7, 4.0) | 62.3, CH2 |
| b 3.43, dd (12.4, 3.8) | b 3.80, dd (11.9, 3.5) | b 3.42, dd (11.7, 3.5) | ||||
| 1′ | ― | 138.2, C | ― | 137.9, C | ― | 137.8, C |
| 2′ | 6.44, s | 105.7, CH | 6.74, d (1.9) | 114.1, CH | 6.83, d (1.8) | 114.0, CH |
| 3′ | ― | 153.3, C | ― | 151.7, C | ― | 151.7, C |
| 4′ | ― | 133.7, C | ― | 147.3, C | ― | 148.1, C |
| 5′ | ― | 153.3, C | 6.69, d (8.2) | 119.4, CH | 6.92, d (8.2) | 119.3, CH |
| 6′ | 6.44, s | 105.7, CH | 6.61, dd (8.2, 1.9) | 121.7, CH | 6.69, dd (8.2, 1.8) | 121.9, CH |
| 7′ | 2.66, m | 32.7, CH2 | 2.57, t (7.7) | 32.6, CH2 | 2.62, t (7.7) | 32.7, CH2 |
| 8′ | 1.88, m | 34.4, CH2 | 1.76, m | 35.5, CH2 | 1.81, m | 35.6, CH2 |
| 9′ | 3.69, t (6.4) | 62.3, CH2 | 3.53, t (6.5) | 62.2, CH2 | 3.56, t (6.5) | 62.2, CH2 |
| 1″ | a 3.41, dq (9.3, 7.0) | 65.4, CH2 | a 3.43, dq (9.3, 7.0) | 65.7, CH2 | ||
| b 3.38, dq (9.3, 7.0) | b 3.40, dq (9.3, 7.0) | |||||
| 2″ | 1.15, t (7.0) | 15.6, CH3 | 1.14, t (7.0) | 15.6, CH3 | ||
| OMe-3/3′ | 3.87, s/3.83, s | 56.5,CH3/56.2,CH3 | 3.77, s/3.74, s | 56.5,CH3/56.2,CH3 | 3.83, s/3.83, s | 56.5,CH3/56.4,CH3 |
| OMe-5/5′ | 3.87, s/3.83, s | 56.5,CH3/56.2,CH3 | ||||
| Compound | IC50 (μM) a | Compound | IC50 (μM) |
|---|---|---|---|
| 1a | 26.4 ± 2.1 | 5 | 41.4 ± 3.1 |
| 1b | 23.1 ± 1.8 | 6 | 18.5 ± 1.9 |
| 2a | >50 | 7 | 14.3 ± 1.6 |
| 2b | >50 | 8 | >50 |
| 3a | >50 | 9 | >50 |
| 3b | >50 | 10 | >50 |
| 4 | >50 | Quercetin b | 15.9 ± 1.2 |
| Compound | Docking Scores (kcal/mol) | Hydrogen Bonds | Hydrophobic Interaction |
|---|---|---|---|
| 1a | −7.8 | TRP366, ARG193 | TRP188, ILE195, PHE363, TYR483 |
| 1b | −7.7 | TRP366, GLU371, ARG193, CYS194 | ALA191 |
| 5 | −7.7 | TRP366, GLU371 | TRP188, ILE195, PHE363 |
| 6 | −8.5 | PRO344, TRP457, TYR483 | ILE195, LEU203, PHE363 |
| 7 | −9.4 | TYR367, TYR483 | LEU203, PHE363, TRP457 |
| quercetin | −7.5 | TYR341, PHE363 | PRO344, VAL346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.-Y.; Dong, Q.; Wang, Z.-Y.; Mei, L.-J.; Tao, Y.-D.; Yu, R.-T. Three Pairs of Novel Enantiomeric 8-O-4′ Type Neolignans from Saussurea medusa and Their Anti-inflammatory Effects In Vitro. Int. J. Mol. Sci. 2022, 23, 14062. https://doi.org/10.3390/ijms232214062
Cao J-Y, Dong Q, Wang Z-Y, Mei L-J, Tao Y-D, Yu R-T. Three Pairs of Novel Enantiomeric 8-O-4′ Type Neolignans from Saussurea medusa and Their Anti-inflammatory Effects In Vitro. International Journal of Molecular Sciences. 2022; 23(22):14062. https://doi.org/10.3390/ijms232214062
Chicago/Turabian StyleCao, Jing-Ya, Qi Dong, Zhi-Yao Wang, Li-Juan Mei, Yan-Duo Tao, and Rui-Tao Yu. 2022. "Three Pairs of Novel Enantiomeric 8-O-4′ Type Neolignans from Saussurea medusa and Their Anti-inflammatory Effects In Vitro" International Journal of Molecular Sciences 23, no. 22: 14062. https://doi.org/10.3390/ijms232214062
APA StyleCao, J.-Y., Dong, Q., Wang, Z.-Y., Mei, L.-J., Tao, Y.-D., & Yu, R.-T. (2022). Three Pairs of Novel Enantiomeric 8-O-4′ Type Neolignans from Saussurea medusa and Their Anti-inflammatory Effects In Vitro. International Journal of Molecular Sciences, 23(22), 14062. https://doi.org/10.3390/ijms232214062






